Abstract
Background
Macitentan blocks endothelin receptors in order to control the pulmonary arterial hypertension (PAH). Oral administration of macitentan is associated with painful urination and troubled breathing.
Objectives
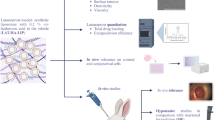
Formulated macitentan hydrogel film was used for examining the control of intraocular pressure, and the effect of surfactant and cosurfactant was studied.
Methods
Macitentan ocular film formulation has been prepared in hydroxypropyl methylcellulose (HPMC) matrix system using different surfactant/co-surfactant system, and intraocular pressure was monitored on normotensive rabbit eyes after application in the cul-de-sac.
Results
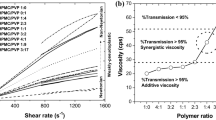
The solid state characterization of the film indicated amorphisation of macitentan and no issues regarding major incompatibility was observed. Combination of surfactant, co-surfactant and hydrophilic co-solvent systems in the said films markedly improved the drug release and mucosal tissue permeation. Presence of PEG and Transcutol significantly improved ex vivo corneal permeation of MP and MT respectively compared to other films. Transcutol (MT) exhibited greatest difference among the formulations by improving the vesicular bilayer fluidity and reducing the mucosal tissue barrier facilitating the transcorneal diffusion. A combination of diffusion and erosion control behavior was observed in drug release and corneal permeation of the films due to the balanced liquid penetration and polymeric chain relaxation rate. MP and MT films were used for further in vivo studies to achieve possible effective and prolonged control of intraocular pressure. In vivo study has revealed the reduction in intraocular pressure upto about 23 % when tested on normotensive rabbit model. The films has managed to lower the IOP upto 3 h.
Conclusion
Developed macitentan hydrogel film containing Transcutol (MT) could have a high potential for the control and management of ocular hypertension after topical application.
Graphical abstract







Similar content being viewed by others
Availability of data and material
The authors confirm that the data supporting the findings of this study are available within the article
References
Garg A, Gupta V, Tomar R, Arora MK. Experimental models for vascular endothelial dysfunction. Bangladesh J Pharmacol. 2021;16:65–83.
Krishnamoorthy RR, Minton AZ, He S, McGrady NR, Stankowska DL. Endothelin receptor mediated neurodegeneration in a rodent model of glaucoma. Investig Ophthalmol Vis Sci. 2016;26:57.
Opsumit (macitentan). https://reference.medscape.com/drug/opsumit-macitentan-999880#4. Accessed: 12th July 2021.
Jansook P, Muankaew C, Stefánsson E, Loftsson T. Development of eye drops containing antihypertensive drugs: formulation of aqueous irbesartan/γCD eye drops. Pharm Dev Technol. 2015;20:626–32.
Duxfield L, Sultana R, Wang R, Englebretsen V, Deo S, Swift S, Rupenthal I, Al-Kassas R. Development of gatifloxacin-loaded cationic polymeric nanoparticles for ocular drug delivery. Pharm Dev Technol. 2016;21:172–9.
Kapanigowda UG, Nagaraja SH, Ramaiah B, Boggarapu PR. Improved intraocular bioavailability of ganciclovir by mucoadhesive polymer based ocular microspheres: development and simulation process in Wistar rats. DARU J Pharm Sci. 2015. https://doi.org/10.1186/s40199-015-0132-7.
Phan TQ, Tran PH, Tran TT. The relationship between mucoadhesive polymers and surface coating in tablets for the controlled colonic delivery of a poorly water-soluble drug. DARU J Pharm Sci. 2020;28:545–53.
Deshpande PB, Dandagi P, Udupa N, Gopal SV, Jain SS, Vasanth SG. Controlled release polymeric ocular delivery of acyclovir. Pharm Dev Technol. 2010;15:369–78.
Nandi S, Ojha A, Nanda A, Sahoo R, Swain R, Pattnaik K, Mallick S. Vildagliptin plasticized hydrogel film in the control of ocular inflammation after topical application: study of hydration and erosion behaviour. Z Phys Chem. 2021. https://doi.org/10.1515/zpch-2021-3081.
Pramanik A, Sahoo RN, Nanda A, Pattnaik KP, Mallick S. Swelling kinetics and corneal hydration level of Kaolinin-HPMC hydrogel film. Indian J Pharm Sci. 2020;82:306–14.
Akhtar MF, Ranjha NM, Hanif M. Effect of ethylene glycol dimethacrylate on swelling and on metformin hydrochloride release behavior of chemically crosslinked pH–sensitive acrylic acid–polyvinyl alcohol hydrogel. DARU J Pharm Sci. 2015. https://doi.org/10.1186/s40199-015-0123-8.
Johnson BA, Kreuter J, Zografi G. Effects of surfactants and polymers on advancing and receding contact angles. Colloids Surf. 1986;17:325–42.
Butler L, Fellows C, Gilbert R. Effect of surfactant systems on the water sensitivity of latex films. J Appl Polym Sci. 2004;92:1813–23.
Joshi SC. Sol-gel behavior of hydroxypropyl methylcellulose (HPMC) in ionic media including drug release. Materials. 2011;4:1861–905.
Vuddanda PR, Montenegro-Nicolini M, Morales JO, Velaga S. Effect of surfactants and drug load on physico-mechanical and dissolution properties of nanocrystalline tadalafil-loaded oral films. Eur J Pharm Sci. 2017;109:372–80.
Mohapatra R, Mallick S, Nanda A, Sahoo RN, Pramanik A, Bose A, Das D, Pattnaik L. Analysis of steady state and non-steady state corneal permeation of diclofenac. RSC Adv. 2016;6:31976–87.
Pramanik A, Sahoo RN, Nanda A, Mohapatra R, Singh R, Mallick S. Ocular permeation and sustained anti-inflammatory activity of dexamethasone from kaolin nanodispersion hydrogel system. Curr Eye Res. 2018;43:828–38.
Nandi S, Mishra SA, Sahoo RN, Swain R, Mallick S. Influence of TiO2 on mucosal permeation of aceclofenac: Analysis of crystal strain and dislocation density. Acta Chim Slov. 2020;67:1227–32.
Shah M, Cabrera-Ghayouri S, Christie LA, Held KS, Viswanath V. Translational preclinical pharmacologic disease models for ophthalmic drug development. Pharm Res. 2019;36:1–34.
Stevens S. How to measure intraocular pressure: Schiötz tonometry. Community Eye Health. 2008;21:34.
Hussain SA, Mohammed HM, Jwaied AH, Abdulrazzaq MH. Effect of silibinin in lowering the intraocular pressure in normotensive rabbits: Interaction with betaxolol. Iraqi J Pharm Sci. 2007;16:39–44.
Pattanaik S, Nandi S, Sahoo RN, Nanda A, Swain R, Das S, Mallick S. Budesonide-cyclodextrin in hydrogel system: impact of quaternary surfactant on in vitro-in vivo assessment of mucosal drug delivery. Rev Chim. 2020;71:332–45.
Mura P, Corti G, Cirri M, Maestrelli F, Mennini N, Bragagni M. Development of mucoadhesive films for buccal administration of flufenamic acid: effect of cyclodextrin complexation. J Pharma Sci. 2010;99:3019–29.
Bertolini G, Feliciani L, Ferrando I, inventors; Olon SpA, assignee. Amorphous form and new crystalline forms of macitentan. United States patent application US 15/325, 2017;907.
Alkrad JA, Shukla A, Mrestani Y, Neubert RH. Investigation in W/O developed microemulsions with DMSO as a cosurfactant. Pharmazie. 2016;71:258–62.
Yeom DW, Chae BR, Kim JH, Chae JS, Shin DJ, Kim CH, Kim SR, Choi JH, Song SH, Oh D, Sohn SI. Solid formulation of a supersaturable self-microemulsifying drug delivery system for valsartan with improved dissolution and bioavailability. Oncotarget. 2017;8:94297–316.
Mandić J, Pobirk AZ, Vrečer F, Gašperlin M. Overview of solidification techniques for self-emulsifying drug delivery systems from industrial perspective. Int J Pharm. 2017;533:335–45.
Manconi M, Caddeo C, Sinico C, Valenti D, Mostallino MC, Lampis S, Monduzzi M, Fadda AM. Penetration enhancer-containing vesicles: composition dependence of structural features and skin penetration ability. Eur J Pharm Biopharm. 2012;82:352–9.
Roy A, Ghosh A, Datta S, Das S, Mohanraj P, Deb J, Rao MEB. Effects of plasticizers and surfactants on the film forming properties of hydroxypropyl methylcellulose for the coating of diclofenac sodium tablets. Saudi Pharm J. 2009;17:233–41.
Honary S, Orafai H. The effect of different plasticizer molecular weights and concentrations on mechanical and thermomechanical properties of free films. Drug Dev Ind Pharm. 2002;28:711–5.
Gelatt KM, Mackay EO. Effect of different dose schedules of bimatoprost on intraocular pressure and pupil size in the glaucomatous beagle. J Occul Pharmacol Ther. 2002;18:525–35.
Gum GG, Kingsbury S, Whitley RD, Garcia A, Gelatt KN. Effect of topical prostaglandin PGA2, PGA2 isopropyl ester, and PGF2α isopropyl ester on intraocular pressure in normotensive and glaucomatous canine eyes. J Ocul Pharmacol Ther. 1991;7:107–16.
Vaajanen A, Vapaatalo H, Kautiainen H, Oksala O. Angiotensin (1–7) reduces intraocular pressure in the normotensive rabbit eye. Investig Ophthalmol Vis Sci. 2008;49:2557–62.
Andrews GP, Laverty TP, Jones DS. Mucoadhesive polymeric platforms for controlled drug delivery. Eur J Pharm Biopharm. 2009;71:505–18.
Handbook of Pharmaceutical Excipients. London: Washington, DC :Pharmaceutical Press, American Pharmaceutical Association, 2017.
CAPTEX® MEDIUM CHAIN TRIGLYCERIDES. https://www.abiteccorp.com/en/product-repository/captex-medium-chain-triglycerides/. (Accessed at 25th November, 2021)
Varela-Garcia A, Concheiro A, Alvarez-Lorenzo C. Soluplus micelles for acyclovir ocular delivery: Formulation and cornea and sclera permeability. Int J Pharm. 2018;552:39–47.
Liu Z, Nie S, Guo H, Pan W, Li J. Effects of Transcutol P on the corneal permeability of drugs and evaluation of its ocular irritation of rabbit eyes. J Pharm Pharmacol. 2006;58:45–50.
Kakkar S, Karuppayil SM, Raut JS, Giansanti F, Papucci L, Schiavone N, Kaur IP. Lipid-polyethylene glycol based nano-ocular formulation of ketoconazole. Int J Pharm. 2015;495:276–89.
Acknowledgements
The authors are grateful to the Department of Science & Technology, Ministry of Science & Technology, New Delhi, India, for providing INSPIRE fellowship to Souvik Nandi (IF 180534). The authors are grateful to the Honorable Prof. Manojranjan Nayak, President, Siksha ‘O’ Anusandhan (Deemed to be University) for facilitating and encouraging the research. The authors are also thankful to Dr. (Ms) Barsha Dash, Senior Scientist, CSIR-Institute of Minerals and Materials Technology, Bhubaneswar, for facilitating the other analytical facilities for the research.
Author information
Authors and Affiliations
Contributions
SSH was responsible for the laboratory works and in preparing the primary manuscript. SN took care of the software analysis and made the final draft of the manuscript. SM was responsible for the study design and final approval of the manuscript as the supervisor.
Corresponding author
Ethics declarations
Ethical approval
The animal studies has been approved by the institutional ethical committee (Regd. No.: 1171/PO/RE/S/08/CPCSEA) bearing protocol number IAEC/SPS/SOA/13/2018.
Consent to participate
Not Applicable
Consent to publish
All authors have approved the manuscript and given consent for consideration for publication in the journal.
Competing interests
Authors declare no competing of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hota, S.S., Nandi, S. & Mallick, S. Management and control of intraocular pressure applying macitentan hydrogel film formulation: improved effect of surfactant and cosurfactant system. DARU J Pharm Sci 30, 39–47 (2022). https://doi.org/10.1007/s40199-021-00428-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40199-021-00428-2