Abstract
Purpose of Review
Cancer is a leading cause of death, accounting for nearly one in six deaths worldwide. Surgery is a critical intervention in cancer care since it provides a chance of cure or can be used to relieve symptoms in patients with advanced malignancies. Anesthesia (general, regional, or local) and analgesia in any of its delivery modes play critical roles in the treatment and palliation of cancers.
Recent Findings
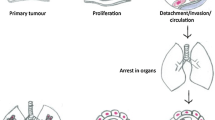
Experimental data demonstrate that surgery itself and anesthetics can promote the growth of micro-metastatic diseases and the seeding of circulating cancer cells, thereby facilitating cancer progression. While this theory originates from in vitro and animal studies, evidence in humans has remained controversial until recently.
Summary
In this report, we summarize current published evidence on the impact of anesthetics and opioids on cancer progression. We focus our discussion on published human studies with special emphasis on randomized controlled trials.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Sung H, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Tohme S, Simmons RL, Tsung A. Surgery for cancer: a trigger for metastases. Cancer Res. 2017;77(7):1548–52.
Hiller JG, et al. Perioperative events influence cancer recurrence risk after surgery. Nat Rev Clin Oncol. 2018;15(4):205–18.
Melamed R, et al. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: mediating mechanisms and prophylactic measures. Anesthesia Analgesia. 2003;97(5):1331–1339.
Bar-Yosef S, et al. Attenuation of the tumor-promoting effect of surgery by spinal blockade in rats. Anesthesiology. 2001;94(6):1066–73.
Yap A, et al. Anesthetic technique and cancer outcomes: a meta-analysis of total intravenous versus volatile anesthesia. Can J Anaesth. 2019;66(5):546–61.
Zhu M, et al. Isoflurane enhances the malignant potential of glioblastoma stem cells by promoting their viability, mobility in vitro and migratory capacity in vivo. Br J Anaesth. 2016;116(6):870–7.
Xu W, et al. Propofol inhibits Wnt signaling and exerts anticancer activity in glioma cells. Oncol Lett. 2018;16(1):402–8.
Pang Q-Y, et al. Comparison of outcomes after breast cancer surgery between inhalational and propofol-based intravenous anaesthesia: a systematic review and meta-analysis. J Pain Res. 2021;14:2165–77.
•• Wigmore TJ, Mohammed K, Jhanji S. Long-term survival for patients undergoing volatile versus IV anesthesia for cancer surgery: a retrospective analysis. Anesthesiology, 2016;124(1): 69–79. Although a retrospective study, this was a large study which after prospective matching and adjustment for known confounding factors demonstrated improved survival in patients undergoing elective cancer surgery with propofol based TIVA compared to volatile anesthesia. This biological plausibility generated great interest in the study of the effects of anesthetic agents on cancer outcomes.
Makito KMK, Fushimi K, Yasunaga H. Volatile versus total intravenous anesthesia for cancer prognosis in patients having digestive cancer surgery: a nationwide retrospective cohort study. Anesthesiology. 2020;133(4):764–73.
Cao S-J, et al. Long-term survival in older patients given propofol or sevoflurane anaesthesia for major cancer surgery: follow-up of a multicentre randomised trial. Br J Anaesth. 2023;131(2):266–75.
Lovett-Carter D, et al. The effect of systemic lidocaine on post-operative opioid consumption in ambulatory surgical patients: a meta-analysis of randomized controlled trials. Perioper Med. 2021;10(1):11.
Cata JP, et al. Lidocaine stimulates the function of natural killer cells in different experimental settings. Anticancer Res. 2017;37(9):4727–32.
Ramirez MF, Tran P, Cata JP. The effect of clinically therapeutic plasma concentrations of lidocaine on natural killer cell cytotoxicity. Reg Anesth Pain Med. 2015;40(1):43–8.
Galoş EV, et al. Neutrophil extracellular trapping and angiogenesis biomarkers after intravenous or inhalation anaesthesia with or without intravenous lidocaine for breast cancer surgery: a prospective, randomised trial. Br J Anaesth. 2020;125(5):712–21.
Li CY, et al. Lidocaine attenuates monocyte chemoattractant protein-1 production and chemotaxis in human monocytes: possible mechanisms for its effect on inflammation. Anesth Analg. 2003;97(5):1312–6.
Zhang H, et al. Effects of intravenous infusion of lidocaine on short-term outcomes and survival in patients undergoing surgery for ovarian cancer: a retrospective propensity score matching study. Front Oncol 2022;11:689832.
Zhang H, et al. Intraoperative lidocaine infusion in patients undergoing pancreatectomy for pancreatic cancer: a mechanistic, multicentre randomised clinical trial. Br J Anaesth. 2022;129(2):244–53.
Zhang H, et al. Association between intraoperative intravenous lidocaine infusion and survival in patients undergoing pancreatectomy for pancreatic cancer: a retrospective study. British J Anaesth. 2020;125(2):141–8.
Lavon H, et al. Dexmedetomidine promotes metastasis in rodent models of breast, lung, and colon cancers. Br J Anaesth. 2018;120(1):188–96.
Chen W, et al. Dexmedetomidine provides type-specific tumour suppression without tumour-enhancing effects in syngeneic murine models. Br J Anaesth. 2023;130(2):142–53.
Cata J, et al. Intraoperative use of dexmedetomidine is associated with decreased overall survival after lung cancer surgery. J Anaesthesiol Clin Pharmacol. 2017;33(3):317–23.
Hu J, et al. Association between intraoperative dexmedetomidine and all-cause mortality and recurrence after laparoscopic resection of colorectal cancer: follow-up analysis of a previous randomized controlled trial. Front Oncol 2023;13:906514.
Xing M-W, et al. Effect of intraoperative dexmedetomidine on long-term survival in older patients after major noncardiac surgery: 3-year follow-up of a randomized trial. J Clin Anesthesia 2023;86.
Connolly JG, et al. Intraoperative opioid exposure, tumour genomic alterations, and survival differences in people with lung adenocarcinoma. Br J Anaesth. 2021;127(1):75–84.
Forget P, et al. Do intraoperative analgesics influence breast cancer recurrence after mastectomy? A retrospective analysis. Anesth Analg. 2010;110(6):1630–5.
Cho JS, et al. The immunomodulatory effect of ketamine in colorectal cancer surgery: a randomized-controlled trial. Canadian J Anesthesia/J Canadien d'Anesthésie. 2021;68(5):683–92.
•• Sessler DI, et al. Recurrence of breast cancer after regional or general anaesthesia: a randomised controlled trial. Lancet 2019;394(10211): 1807–1815. This was the first large multicenter randomized controlled trial performed to evaluate the effect of regional anesthesia-analgesia with paravertebral blocks and propofol sedation on recurrence rates after breast cancer surgery.
Xu Z-Z, et al. Epidural anesthesia–analgesia and recurrence-free survival after lung cancer surgery: a randomized trial. Anesthesiology. 2021;135(3):419–32.
Aloia TA, et al. A randomized controlled trial of postoperative thoracic epidural analgesia versus intravenous patient-controlled analgesia after major hepatopancreatobiliary surgery. Ann Surg. 2017;266(3):545–54.
Vicente D, et al. Impact of epidural analgesia on the systemic biomarker response after hepatic resection. Oncotarget. 2019;10(5):584–94.
Xing W, et al. Lidocaine induces apoptosis and suppresses tumor growth in human hepatocellular carcinoma cells in vitro and in a xenograft model in vivo. Anesthesiology. 2017;126(5):868–81.
Sessler DI. Does regional analgesia reduce the risk of cancer recurrence? A hypothesis. Eur J Cancer Prev. 2008;17(3):269–72.
Illias AM, et al. Association of regional anesthesia with oncological outcomes in patients receiving surgery for bladder cancer: a meta-analysis of observational studies. Front Oncol 2023;13:1097637.
Cata JP, et al. The impact of paravertebral block analgesia on breast cancer survival after surgery. Reg Anesth Pain Med. 2016;41(6):696–703.
Exadaktylos AK, et al. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105(4):660–4.
Christopherson R, et al. Long-term survival after colon cancer surgery: a variation associated with choice of anesthesia. Anesth Analg. 2008;107(1):325–32.
Myles PS, et al. Perioperative epidural analgesia for major abdominal surgery for cancer and recurrence-free survival: randomised trial. BMJ. 2011;342: d1491.
Yu L, et al. Perioperative pectoral nerve block type II and postoperative recurrence in breast cancer: a randomized controlled trial. BMC Surg. 2022;22(1):447.
Karmakar MK, et al. Survival analysis of patients with breast cancer undergoing a modified radical mastectomy with or without a thoracic paravertebral block: a 5-year follow-up of a randomized controlled trial. Anticancer Res. 2017;37(10):5813–20.
Du YT, et al. Long-term survival after combined epidural-general anesthesia or general anesthesia alone: follow-up of a randomized trial. Anesthesiology. 2021;135(2):233–45.
Falk W, et al. Comparison between epidural and intravenous analgesia effects on disease-free survival after colorectal cancer surgery: a randomised multicentre controlled trial. Br J Anaesth. 2021;127(1):65–74.
Badwe RA, et al. Effect of peritumoral infiltration of local anesthetic before surgery on survival in early breast cancer. J Clin Oncol. 2023;41(18):3318–28.
Gorur A, et al. Mu-opioid receptor activation promotes in vitro and in vivo tumor growth in head and neck squamous cell carcinoma. Life Sci. 2021;278:119541.
Cata JP, et al. The µ‐opioid receptor in cancer and its role in perineural invasion: a short review and new evidence. Adv Biol 2022;2200020.
Zylla D, et al. Association of opioid requirement and cancer pain with survival in advanced non-small cell lung cancer. Br J Anaesth. 2014;113:i109–16.
Zylla D, et al. Opioid requirement, opioid receptor expression, and clinical outcomes in patients with advanced prostate cancer. Cancer. 2013;119(23):4103–10.
Zhang H, et al. Increased mu-opioid receptor expression is associated with reduced disease-free and overall survival in laryngeal squamous cell carcinoma. Br J Anaesth. 2020;125(5):722–9.
Zhang H, et al. Association of mu-opioid receptor(MOR) expression and opioids requirement with survival in patients with stage I-III pancreatic ductal adenocarcinoma. Front Oncol. 2021;11: 686877.
Diaz-Cambronero O, et al. Mu opioid receptor 1 (MOR-1) expression in colorectal cancer and oncological long-term outcomes: a five-year retrospective longitudinal cohort study. Cancers (Basel). 2020;12(1):134.
Paice JA, et al. Management of chronic pain in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34(27):3325–45.
Cata JP, et al. Intraoperative opioids use for laryngeal squamous cell carcinoma surgery and recurrence: a retrospective study. J Clin Anesth. 2015;27(8):672–9.
Cata JP, et al. A retrospective analysis of the effect of intraoperative opioid dose on cancer recurrence after non-small cell lung cancer resection. Cancer Med. 2014;3(4):900–8.
Nelson DB, et al. Persistent opioid use is associated with worse survival after lobectomy for stage i non-small cell lung cancer. Pain. 2019;160(10):2365–73.
Steele GL, et al. Impact of pain, opioids, and the mu-opioid receptor on progression and survival in patients with newly diagnosed stage IV pancreatic cancer. Am J Clin Oncol. 2020;43:591–7.
Cronin-Fenton D. Opioids and breast cancer recurrence. Curr Opin Support Palliat Care. 2019;13(2):88–93.
Patino MA, et al. The impact of intraoperative opioid use on survival after oral cancer surgery. Oral Oncol. 2017;74:1–7.
Owusu-Agyemang P, et al. Assessing the survival impact of perioperative opioid consumption in children and adolescents undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Paediatr Anaesth. 2017;27(6):648–56.
Janku F, et al. Treatment with methylnaltrexone is associated with increased survival in patients with advanced cancer. Ann Oncol. 2016;27(11):2032–8.
Vijayakumar J, et al. An open label phase II study of safety and clinical activity of naltrexone for treatment of hormone refractory metastatic breast cancer. Invest New Drugs. 2022;41(1):70–5.
Author information
Authors and Affiliations
Contributions
All authors contributed to the manuscript.
No datasets were generated or analysed during the current study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics Approval and Consent to Participate
Not applicable
Competing Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oliver, JA., Oliver, LA., Cata, J.P. et al. Anesthetic Techniques and Long-Term Oncological Outcomes. Curr Anesthesiol Rep 14, 50–56 (2024). https://doi.org/10.1007/s40140-023-00605-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40140-023-00605-w