Abstract
Introduction
Few qualitative studies have explored the patient experience of daily life with proliferative diabetic retinopathy (PDR) and associated treatments. Herein, a conceptual model was developed to comprehensively examine symptoms, functional impacts, and treatment experiences in PDR.
Methods
A qualitative, mixed-methods study comprising a literature search and semi-structured interviews with clinicians and patients was conducted. Published literature and online patient resources were searched to identify concepts relevant to patients, including symptoms, functional impacts, and treatment experiences of PDR. Semi-structured interviews with experienced clinicians were conducted to identify symptoms and impacts reported by patients with PDR and to receive feedback regarding concepts identified from the literature search. A preliminary conceptual model was then developed based on findings from the literature search and clinician interviews. Patients with PDR participated in two rounds of semi-structured interviews to identify additional concepts relevant to the patient experience in PDR and associated treatments, which informed revisions to the conceptual model. Saturation of patient interviews was assessed.
Results
Findings from the literature search and clinician interviews yielded 109 concepts that were included in a preliminary conceptual model with three overarching domains: symptoms, impacts, and managing the disease. Clinicians confirmed concepts identified from the literature search. During interviews, patients reported a broad spectrum of symptoms (e.g., red vision); functional impacts relating to activities of daily living (e.g., reading), emotional functioning (e.g., loss of independence), and social functioning (e.g., problems recognizing faces); and treatment experiences (e.g., improves eye problems, no change) associated with PDR. Additional concepts elicited in patient interviews informed revisions to the conceptual model. Saturation was achieved in the patient sample.
Conclusions
A wide variety of symptoms, functional impacts, and treatment experiences that significantly affect health-related quality of life were identified in patients with PDR. These insights are critical for understanding PDR symptomology and assessing treatment response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out the study? |
Visual impairment in diabetic retinopathy (DR), particularly in advanced disease (proliferative diabetic retinopathy [PDR]), substantially impacts overall health-related quality of life including activities of daily living and physical, emotional, and social functioning. |
Although previous studies have evaluated DR symptoms, functional impacts, and efficacy and safety of panretinal photocoagulation (PRP) and intravitreal anti-vascular endothelial growth factor (VEGF) therapies, there is a dearth of qualitative evidence from patients that comprehensively characterizes the patient experience of daily life with DR and associated treatments. |
This study explored the full spectrum of patient experiences with PDR and its treatment via in-depth qualitative interviews to examine symptoms and functional impacts of PDR on the daily lives of patients as well as treatment experiences with PRP and the intravitreal anti-VEGF agent aflibercept. |
What was learned from the study? |
A broad range of symptoms, functional impacts, and treatment experiences that significantly affect health-related quality of life were identified in patients with PDR. |
This study used a holistic approach to capture experiences with PDR that are important to patients and highlighted significant burdens, including those from treatment, indicating that unmet needs remain in disease management. |
Introduction
Diabetic retinopathy (DR), a common complication of diabetes mellitus [1], is a progressive condition that presents with a variety of symptoms and is a common cause of blindness worldwide [2, 3]. Hyperglycemia promotes microvascular damage in the retina, leading to ischemia and subsequent upregulation of proangiogenic factors such as vascular endothelial growth factor (VEGF) that can cause hemorrhage, retinal detachment, and substantial vision loss [2, 4]. DR is diagnosed using a comprehensive eye exam and comprises two stages: nonproliferative DR (NPDR), an early stage of DR characterized by microaneurysms and intraretinal hemorrhages; and proliferative DR (PDR), an advanced stage of DR characterized by neovascularization and increased risk of vitreous hemorrhage, retinal detachment, and blindness [3,4,5]. Most patients with NPDR are asymptomatic in the absence of diabetic macular edema (DME) [6]; however, patients with PDR often experience symptoms including blurry vision, flashes of light, floaters, and impaired night vision [7, 8]. In 2020, DR was estimated to affect > 100 million individuals globally, and prevalence is expected to increase [1].
Visual impairment in DR, particularly in advanced disease, substantially impacts overall health-related quality of life (HRQoL), including activities of daily living and physical, emotional, and social functioning [6, 9,10,11]. In a cross-sectional, population-based cohort study, increasing DR severity in adult patients correlated with worse vision-related quality of life and general HRQoL [9]. Patients with symptomatic NPDR and PDR reported the most difficulty with activities of daily living, including reading, cooking, housekeeping, and getting dressed [7, 12]. DR is also associated with poor psychosocial outcomes such as anxiety and depression [13]. Vision loss or decline has led patients to limit social interactions and suffer from isolation because of increased dependency on others [7, 14].
Recommended treatments for advanced DR include panretinal photocoagulation (PRP) and intravitreal anti-VEGF therapy [3, 15]. PRP has effectively improved severe vision loss and neovascularization and is considered the standard treatment for PDR [15, 16]. However, PRP is associated with complications such as loss of peripheral visual field, impaired night vision, and decreased contrast sensitivity [4, 17, 18]. Although intravitreal anti-VEGF therapy is recommended as an adjunctive or alternative treatment, anti-VEGF agents such as aflibercept [19] and ranibizumab [20] have substantially improved best-corrected visual acuity, promoted regression of retinal neovascularization in patients with PDR, and received US Food and Drug Administration approval for the treatment of all stages of DR [15, 18,19,20,21,22,23]. Anti-VEGF agents are also associated with reduced risk of peripheral visual field loss, DME, and vitrectomy compared with PRP [18, 22]. Despite benefits of both treatments, patients have experienced treatment-related burden due to factors including cost, potential complications (e.g., macular edema [17] and pain [19, 20]), and frequency of treatment visits [12, 18, 24].
Although previous studies explored DR symptoms, impacts, and efficacy and safety of PRP and anti-VEGF therapies [18, 22, 25], there is a dearth of qualitative evidence from patients that explores the patient experience of daily life with DR and associated treatments in depth. In this report, findings from a qualitative study that examined symptoms and impacts of PDR on the daily life of patients, as well as patient experiences with PRP and aflibercept, are presented.
Methods
Literature Search
Search Strategy
To identify all key symptoms, impacts, and treatment experiences in PDR, a targeted literature search was performed for articles relevant to DR. PubMed searches were conducted using combinations and variations of terms including qualitative, interview, symptom, activities of daily living, quality of life, well-being, diabetic retinopathy, peripheral vision, night vision, treatment experience, outcome, complication, side effect, aflibercept, panretinal photocoagulation, and anti-vascular endothelial growth factor (literature search terms and results presented in Tables S1 and S2 in the electronic supplementary material). Articles were screened by title and abstract, and the full texts were subsequently reviewed.
Eligibility Criteria
Following full-text review, articles were included if they were considered qualitative research; related to patients’ symptoms, impacts, or treatment experiences of DR; related to treatments of interest; and included research conducted in adult participants. Identified articles pertaining to treatment experience could have presented qualitative or quantitative data relating to treatment experience, burden, and outcomes.
Website Review
Searches were performed on websites of patient advocacy organizations, medical associations, and online forums to supplement findings from the literature regarding the patient experience of DR. Websites that provided quotes from patients describing their experience with DR were selected. No specific search terms were used during website review.
Data Extraction
The following data were extracted from selected full-text articles: title, authors, journal, year, population, sample, methods, analysis, concepts, and limitations of the concepts that were identified. Concepts that were specific to symptoms, impacts, and treatment experiences of patients with DR were also extracted. Patient-generated information on symptoms, impacts, and relevant treatment experiences in DR were extracted from websites, with an emphasis on the PDR patient experience.
Qualitative In-Depth Interviews with Clinicians and Patients
Study Design
To supplement findings from the literature with data received from a clinical perspective, researchers trained in qualitative methods conducted semi-structured interviews with experienced clinicians. Researchers used an interview guide informed by findings from the literature search to elicit information regarding symptoms, impacts, and treatment experiences of patients with PDR as well as feedback on concepts identified from the literature search.
Trained recruiters used a screener form to evaluate inclusion and exclusion criteria in patients. Full inclusion and exclusion criteria are provided in Supplementary Methods in the electronic supplementary material. Patients then completed an electronic consent form and interviews were scheduled following confirmation of eligibility. Researchers who were trained in qualitative methods conducted two rounds of semi-structured interviews with patients (Waves 1 and 2). Semi-structured interview guides, informed by findings from the literature search and clinician interviews, were used to guide the conduct of each interview. Interview guides included open-ended questions to elicit spontaneous patient input regarding PDR symptoms, their impacts on daily life (e.g., disruption to daily activities, emotional impacts, and social interactions), and treatment experience (e.g., improvement or worsening of symptoms and treatment preferences) with PRP and aflibercept. If concepts of interest were not spontaneously elicited, targeted probes were used to collect specific information on symptoms, impacts, and treatment experiences.
Findings from Wave 1 interviews were used to develop the interview guide for Wave 2 interviews, some of which were conducted with patients who had received both PRP and aflibercept to compare experiences between treatments. All interviews were audio-recorded, transcribed verbatim, and anonymized.
Participants
Retina specialists, two of which had ≥ 10 years of experience treating patients with PDR, were recruited for interviews. Eligible patients were ≥ 18 years old with clinician-confirmed diagnoses of type 1 or type 2 diabetes mellitus and PDR (Diabetic Retinopathy Severity Scale [DRSS] score of 61, 65, 71, or 75). All patients must have received PRP, aflibercept, or any combination of these treatments in the 6 months preceding the study. Patients also must have been fluent in English. Patients with DME (central subfield thickness ≥ 305 µm and ≥ 320 µm for females and males, respectively) in the affected eye before PRP; a history of intra- or periocular corticosteroid treatment; a history of intraocular sustained-release treatment, implantable device, or gene therapy in the affected eye; a history of vitreoretinal surgery or intraocular pressure ≥ 25 mmHg in the affected eye; evidence of active infectious blepharitis, keratitis, scleritis, or conjunctivitis in either eye; and substantial visual impairment were excluded. If both eyes were eligible, the eye with the worse DRSS score was classified as the affected eye. If both eyes had the same DRSS score, the recruiting clinician selected the affected eye.
Patients in the New Orleans metropolitan area were recruited from October 2020 to January 2021 by a healthcare market research firm using resources such as recruiter databases, patient associations, clinician referrals, and social media. Recruiters invited patients to participate via e-mail or telephone, and patients were permitted to ask questions before recruitment.
Sampling
To ensure elicited concepts were related to patients’ lived experiences and treatment with PDR, qualitative interviews utilized a purposive quota sampling of adult patients who were diagnosed with PDR and treated with PRP or aflibercept. Patients with variable levels of PDR severity and a range of prior treatments received, time since treatment, and levels of glycemic control were actively recruited to assess a broad range of patient experiences in PDR. A preliminary recruitment target of n = 30 patients was set to allow for subsequent quantitative analyses.
In qualitative research, adequacy of sample size to elicit all potential symptoms and impacts from a population is often determined by the principle of data saturation, defined as the point at which no new concepts are elicited from further individual interviews or focus groups [26]. There was an option to continue recruitment if data saturation had not yet been reached. Saturation analysis was not performed for data collected from clinician interviews.
Clinician and Patient Interviews
To gather information regarding symptoms, impacts, and treatment experiences reported by patients with PDR, trained researchers conducted telephone interviews with three clinicians between June and September 2020 that lasted approximately 1 h. Clinicians were also asked to provide feedback regarding the literature search findings.
Patient interviews during Wave 1 each lasted approximately 1 h and were conducted via phone by trained researchers from November 2020 to January 2021. All patients from Wave 1 interviews were recontacted to participate in Wave 2 interviews in March 2021 upon completion of the Wave 1 interview analysis. Patients who received both PRP and aflibercept were prioritized for Wave 2 interviews to further evaluate treatment preferences, understand any differences in treatment experience during and after treatment, assess the impact of treatment, and understand reasons for switching treatments.
Ethics
This study was conducted in accordance with the principles of the Declaration of Helsinki and all applicable regulatory requirements. All study documents—including the protocol, interview guides, demographic and health information form, screener form, and informed consent form—were approved by the Western Institutional Review Board-Copernicus Group Independent Review Board (reference number: 20202896) before study initiation. All patients provided written informed consent before participation in this study, and all researchers were trained in qualitative methods and Good Clinical Practice.
Data Analysis
Verbatim transcripts were analyzed using thematic analysis and detailed open and inductive coding with ATLAS.ti software [27,28,29]. The first two transcripts were independently coded by two researchers to ascertain consistency among individual coders; any inconsistencies were discussed and resolved to reach coding agreement. Coding guidelines were revised as required and after new concepts in the remaining transcripts were identified. If parallel coders disagreed, a senior researcher was consulted to resolve the disagreement. Multiple researchers analyzed data and compared findings from the first transcripts to maintain consistency in coding. Upon resolution of all issues, a codebook was developed in which code organization and the coding hierarchy were based on a clinically meaningful perspective to establish a clinically meaningful catalog of patient experiences in PDR (Table S3 in the electronic supplementary material). This perspective prioritized concepts indicative of patient feel, function, or life with PDR. Quintiles of transcripts were compared to confirm whether data saturation was reached. No assessment of reflexivity was performed.
Conceptual Model Development
A conceptual model is a visual representation of the impacts related to a condition that facilitates the identification of relationships between concepts [30]. Once coding was complete, a preliminary conceptual model was developed to illustrate aspects of the patient experience of PDR. Proximal and distal concepts were ordered based on experiences of PDR that were perceived most immediately by patients and, thus, most likely to be affected by treatment. Following Wave 1 interviews, the preliminary conceptual model was revised using standard analytical techniques [27, 29, 31]. Codes and quotations were compared with the rest of the data and inductively categorized into higher-order overarching categories classified as concepts, subdomains, and domains that reflected their conceptual content underpinning. This iterative process cross-referenced and compared different analytical categories (concepts, subdomains, and domains), which were reviewed and adjusted accordingly. Coding was targeted to symptoms, impacts, and treatment experiences of PDR to collate these experiences and clearly identify interrelationships among them. The final conceptual model was generated based on patient feedback from Wave 2 interviews.
Results
Literature Search
The targeted literature search yielded a total of 1754 articles (patient experience with DR: n = 1294; DR treatment experience: n = 460) from PubMed (Fig. 1). Of these, 41 articles (patient experience with DR: n = 39; DR treatment experience: n = 2) met eligibility criteria based on title and abstract. Following full-text review, 16 articles (patient experience with DR: n = 15; DR treatment experience: n = 1) met eligibility criteria and were used for data extraction, yielding 84 unique concepts.
Twenty-two unique concepts were identified and extracted from review of the National Eye Institute, American Diabetes Association: Eye Complications, Diabetic Retinopathy Helpline, and American Diabetes Association Support Community websites. These concepts included visual problems (e.g., floaters), impacts (e.g., difficulty reading), and facets of disease management (e.g., burden of self-monitoring). Overall, 106 concepts were identified from the literature search and informed development of a preliminary conceptual model.
Clinician Interviews
Three clinicians were interviewed, and full clinician backgrounds are provided in Table 1. Clinicians reported that vision loss complaints—including floaters, blurry vision, peripheral vision loss, distorted vision, and problems with near and distance vision—were generally similar among patients with PDR. Clinicians reported three unique symptoms not represented in the literature search: contrast sensitivity, double vision, and light/dark adaptation issues.
PDR symptoms were reported to affect daily functioning in patients, particularly with reading and driving (e.g., night driving, loss of side vision while driving, driving to work). Visual distortions, including blurriness and an inability to focus with glasses, were reported to vary from minimal or trivial to high impact (i.e., preventing driving, reading, or functioning). Clinicians also verified DR symptoms and impacts identified during the literature search.
When PDR treatment was discussed, clinicians described PRP in terms of balancing benefits and disadvantages. According to clinicians, benefits of PRP included reduction of floaters, perceived durability compared with aflibercept, delay of disease progression, cost-effectiveness, and decreased impact of patient compliance on treatment success. Reported disadvantages included peripheral vision loss, light/dark adaptation issues, contrast sensitivity issues, and decreased light perception.
All clinicians agreed that aflibercept had many benefits and few disadvantages. Benefits included symptomatic improvement regarding floaters, distortion, non–DME-related blurriness, and vision loss. Disadvantages included side effects such as post-injection burning, grittiness, irritation, cost and burden of returning for multiple visits, and concerns pertaining to compliance with aflibercept injections.
Patient Interviews
Forty-three patients were recruited; however, the last three patients were not enrolled because the recruitment target was reached. Therefore, 40 patients with PDR completed Wave 1 interviews. Of these, 30 patients (75.0%) also completed Wave 2 interviews. Overall, patient demographic and clinical characteristics were comparable in both interview groups (Table 2). The overall mean (standard deviation [SD]) age of patients in Wave 1 and 2 interviews was 53.7 (14.7) years and 53.0 (14.5) years, respectively. In both interview groups, most patients were Black or African American (Wave 1: n = 21 [52.5%]; Wave 2: n = 16 [53.3%]). Mean (SD) time since PDR diagnosis was 6 (7.3) and 8 (8.0) years in Waves 1 and 2, respectively.
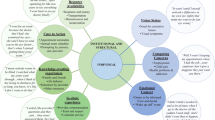
Interviews yielded three overarching domains: symptoms, impacts, and managing the disease with PRP or aflibercept. Saturation analysis showed no new concepts were identified in the eighth and final group of interviews, indicating data saturation was achieved. The preliminary conceptual model was revised based on feedback from both rounds of patient interviews to generate the final conceptual model (Fig. 2).
Symptoms
Patients reported symptoms related to vision, eye sensations, headaches, and dizziness that were organized into three subdomains: vision problems, eye problems, and headaches and dizziness (Fig. 2). Vision problems were blurred vision, cloudy/hazy/foggy vision, depth perception issues, difficulty adapting to lighting changes, difficulty distinguishing color or colorless vision, difficulty focusing, difficulty seeing in bright light/glare, difficulty with distance vision, double vision, flashes of light, floaters (black spots, spiderwebs, or streaks), night blindness (difficulty seeing in the dark or in dim light), peripheral vision issues, red vision, and vision loss (Fig. 3a). Of these, difficulty seeing in bright light/glare, flashes of light, and red vision were symptoms not represented in the literature search. Many vision problems reported by patients, such as blurred vision, double vision, and light/dark adaptation issues, were also reported by clinicians. Eye problems such as burning and itching, dry eye, eye fatigue, eye pressure, pain, scratchy/irritated, and strain/discomfort were not represented in the literature search. In relation to eye fatigue, one patient stated:
“I don’t actually feel tired, but my eyes feel tired, like I need to close my eyes when I’m reading, and it happens, I don’t know, certain times of the day, like after I’ve been doing maybe a lot of reading or something.” Male, 27 years old.
Detailed conceptual model describing a symptoms, b impacts, and c aspects of disease management in PDR. PDR proliferative diabetic retinopathy. Concepts that were added to the conceptual model following patient interviews are indicated in fuchsia and bold. aSpontaneously elicited and probed during patient interviews; bLiterature-based framework only
Headaches and dizziness, both previously unrecognized symptoms, were grouped separately.
Impacts on Daily Life
Patients with PDR reported many impacts of decreased visual functioning on daily life that related to daily activities, emotions, and social activities (Figs. 2 and 3b). Impacted activities of daily living included caretaking, grocery shopping, hobbies, household activities, navigating obstacles, self-care, using a phone or computer, walking, working, and writing. Consistent with reports from clinicians, patients reported reading and driving were substantially impacted by PDR.
Emotional impacts were aggravation, concern for future/worsening symptoms, decreased self-perception, loss of independence, other negative emotions, sadness, and fear and anxiety.
“There’s anxiety caused by knowing that my diabetes has caused me to have some eye issues that could become progressively worse.” Male, 37 years old.
Problems recognizing people was a reported social impact. Patients reported three impacts not included in the preliminary conceptual model: walking, writing, and loss of independence.
Treatment Experience
During Wave 1 interviews, aspects of disease management reported by patients who received PRP and/or aflibercept included side effects (e.g., nausea, temporary vision problems), treatment effects (e.g., better able to do daily activities, prevents further decline), treatment concerns and fears, and challenges related to treatment access (Figs. 2 and 3c). Headaches, nausea, temporary eye problems, and temporary vision problems were side effects from treatment not represented in the literature search. Similarly, all reported treatment effects (e.g., better able to do daily activities, improves headaches) were not identified in the literature search. Office wait times were not reported in the literature as a challenge to treatment access.
Most treatment experiences were reported by patients irrespective of PDR treatment. However, three experiences (improves headaches, worsens eye problems, office wait times) were reported by patients who received PRP only and two experiences (stress of treatment and traveling long distances for appointments) were reported by patients who received aflibercept only. These experiences were generally comparable to those reported by clinicians given that disadvantages of PRP and aflibercept included exacerbation of blurry vision and burden of returning for multiple visits, respectively.
During Wave 2 interviews, seven patients who received both PRP and aflibercept were asked to describe differences in treatment experience during and after treatment, impact of treatment, and reasons for switching treatments. No strong treatment preference between PRP and aflibercept was identified (preference for PRP: n = 3; preference for aflibercept: n = 4).
“You know, they’re both helpful in their own particular way. I don’t know that I like one better than the other. To me, I guess there’s a lot more discomfort initially with the laser, but the injection stays sore for a little while longer than what the lasers do. I can usually recover from the lasers pretty fast.” Male, 62 years old.
Patients reported differences in side effects between treatments, such as associations of headaches with PRP and temporary floaters with aflibercept, and differences in opinions on the more painful or uncomfortable treatment and the treatment associated with a prolonged recovery period. Although patients considered the aflibercept injection procedure to be faster than the PRP procedure, they were more fearful of aflibercept. All three patients who provided reasons for switching treatments reported that treatment switches were based on doctor recommendations. Taken together, patients with PDR compared difficulties during and after treatment separately and stated that eye problems (e.g., redness and swelling), complications, and recovery delays influenced treatment preferences.
Discussion
The aim of this qualitative study was to explore the full spectrum of patient experiences with PDR and its treatment by examining symptoms and impacts of PDR on the daily lives of patients and treatment experiences with PRP and aflibercept. A targeted literature search and patient and clinician interviews served as the basis for an extensive evaluation of DR symptoms, impacts, and treatment experiences in patients with PDR. Findings from this study showed that patients with PDR experience a wide range of symptoms and impacts on physical, emotional, and social functioning that negatively affect HRQoL. Moreover, this study adds to knowledge of the treatment experience of PDR by providing valuable insights into comparative patient experience with PRP and aflibercept. Clinicians and patients reported benefits and disadvantages of both treatments, which were shown to influence treatment preferences.
A conceptual model was developed in which the patient experience of PDR was described in three key domains: symptoms, impacts, and managing the disease. Data saturation was reached, which supported development of a robust conceptual model illustrating a wide range of symptoms, impacts on daily life, and treatment experiences in PDR. Concepts identified in the literature were supported by clinicians and patients, confirming the relevance of the conceptual model to the patient experience of PDR. This conceptual model represents the wide variety of patient experiences with PDR and its associated treatments and emphasizes that many unmet needs remain. The conceptual model may therefore be useful for informing assessments of HRQoL in clinical trials and real-world settings.
Symptoms described by patients varied but were consistent with previous reports [7, 8]. Symptoms including blurred vision, floaters, and difficulty with distance vision were reported previously [7, 8] and were also reported by patients and clinicians in the current study. Additional symptoms such as red vision, eye burning and itching, eye fatigue, and watery eyes were uncovered in this study and emphasized the symptom burden experienced by patients with DR.
Substantial impacts of DR symptoms on daily activities, emotions, and social activities were described in this study. Patients reported substantial difficulty with daily activities such as driving and reading, as was observed in previous studies [7, 12]. Consistent with results from other qualitative studies of patients with DR [7, 14], loss of independence and mobility were reported by patients in this study along with difficulty recognizing faces and decreased participation in social activities. Taken together, these findings underscore the negative impact of DR on HRQoL and the importance of treatments that effectively minimize or prevent vision loss.
Negative experiences were reported by patients irrespective of treatment with PRP or aflibercept. Eye problems, treatment complications, and recovery time were important considerations in patients’ choice of treatment. Patients also cited barriers to treatment, such as long wait times and long traveling distances for appointments, that may negatively affect treatment compliance. Preferences for PRP, aflibercept, corticosteroids, and focal laser treatment were previously evaluated in a study of patients with DR in the presence or absence of DME; however, any possible effect of treatment accessibility was not assessed [32]. This study highlights unmet needs of patients with DR that may not have previously been considered but must be addressed to increase treatment satisfaction and compliance.
There were several limitations to this study. This study did not include patients with NPDR. Although patients with mild or moderate NPDR may be asymptomatic [6, 33], future studies are warranted to examine potential differences in the experiences of patients with symptomatic NPDR and those with PDR. Patients were recruited from a single region and did not represent all races or ethnicities. A more diverse patient population may have further enriched findings from qualitative patient interviews. It is also possible that most patients in this sample had a high level of visual functioning, potentially limiting the analysis of patients with severe PDR. Another limitation is that patient experiences may have been confounded by comorbid conditions despite study exclusion criteria. Given that interviews were conducted via telephone, patients may have withheld details of their experiences with PDR if a house member was present, potentially leading to underreporting of the impact of PDR in their lives. Finally, the effect of PRP or aflibercept on post-treatment vision issues and impact on daily activities was not reported by patients given that PDR progresses slowly.
Findings of this study have several implications for the management of patients with PDR. First, a broad range of symptoms was reported by patients with PDR, many of which may not be recognized in clinical practice. It is therefore important that healthcare providers be aware of the heterogeneity of clinical presentations in PDR. Second, this study highlighted significant burdens on patients with PDR, including those from treatment, indicating unmet needs remain in disease management. In addition to side effects, patients reported fear, anxiety, and stress with treatment. Efforts should be directed to improve the treatment experience of patients given that negative emotions associated with treatment may exacerbate the emotional burden of PDR. In this regard, the development of long-lasting and effective treatment options should be prioritized to improve visual outcomes and reduce the frequency of visits. Finally, future studies are warranted to further characterize differences in patient experiences with PRP and aflibercept and optimize treatment regimens based on patient needs.
Conclusions
In summary, this qualitative study provided a comprehensive and insightful analysis of the patient experience in PDR. Findings from this study will be integral for increasing understanding of PDR symptomology, evaluating treatment approaches and response, and supporting efforts to optimize patient care.
References
Teo ZL, Tham YC, Yu M, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology. 2021;128:1580–91. https://doi.org/10.1016/j.ophtha.2021.04.027.
Lechner J, O’Leary OE, Stitt AW. The pathology associated with diabetic retinopathy. Vision Res. 2017;139:7–14. https://doi.org/10.1016/j.visres.2017.04.003.
Duh EJ, Sun JK, Stitt AW. Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI Insight. 2017;2: e93751. https://doi.org/10.1172/jci.insight.93751.
Jampol LM, Glassman AR, Sun J. Evaluation and care of patients with diabetic retinopathy. N Engl J Med. 2020;382:1629–37. https://doi.org/10.1056/NEJMra1909637.
Stitt AW, Curtis TM, Chen M, et al. The progress in understanding and treatment of diabetic retinopathy. Prog Retin Eye Res. 2016;51:156–86. https://doi.org/10.1016/j.preteyeres.2015.08.001.
Fenwick EK, Pesudovs K, Rees G, et al. The impact of diabetic retinopathy: understanding the patient’s perspective. Br J Ophthalmol. 2011;95:774–82. https://doi.org/10.1136/bjo.2010.191312.
Coyne KS, Margolis MK, Kennedy-Martin T, et al. The impact of diabetic retinopathy: perspectives from patient focus groups. Fam Pract. 2004;21:447–53. https://doi.org/10.1093/fampra/cmh417.
American Optometric Association. Diabetic retinopathy. 2021. Available at: https://www.aoa.org/healthy-eyes/eye-and-vision-conditions/diabetic-retinopathy?sso=y. Accessed September 15, 2022.
Mazhar K, Varma R, Choudhury F, et al. Severity of diabetic retinopathy and health-related quality of life: the Los Angeles Latino Eye Study. Ophthalmology. 2011;118:649–55. https://doi.org/10.1016/j.ophtha.2010.08.003.
Cooper OAE, Taylor DJ, Crabb DP, Sim DA, McBain H. Psychological, social and everyday visual impact of diabetic macular oedema and diabetic retinopathy: a systematic review. Diabet Med. 2020;37:924–33. https://doi.org/10.1111/dme.14125.
Gupta P, Liang Gan AT, Kidd Man RE, et al. Impact of incidence and progression of diabetic retinopathy on vision-specific functioning. Ophthalmology. 2018;125:1401–9. https://doi.org/10.1016/j.ophtha.2018.02.011.
Willis JR, Doan QV, Gleeson M, et al. Vision-related functional burden of diabetic retinopathy across severity levels in the United States. JAMA Ophthalmol. 2017;135:926–32. https://doi.org/10.1001/jamaophthalmol.2017.2553.
Khoo K, Man REK, Rees G, Gupta P, Lamoureux EL, Fenwick EK. The relationship between diabetic retinopathy and psychosocial functioning: a systematic review. Qual Life Res. 2019;28:2017–39. https://doi.org/10.1007/s11136-019-02165-1.
Devenney R, O’Neill S. The experience of diabetic retinopathy: a qualitative study. Br J Health Psychol. 2011;16:707–21. https://doi.org/10.1111/j.2044-8287.2010.02008.x.
Flaxel CJ, Adelman RA, Bailey ST, et al. Diabetic retinopathy Preferred Practice Pattern®. Ophthalmology. 2020;127(1):P66–145. https://doi.org/10.1016/j.ophtha.2019.09.025.
Diabetic Retinopathy Research Group. Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS report number 8. Ophthalmology. 1981;88:583–600. https://doi.org/10.1016/S0161-6420(81)34978-1.
Reddy SV, Husain D. Panretinal photocoagulation: a review of complications. Semin Ophthalmol. 2018;33:83–8. https://doi.org/10.1080/08820538.2017.1353820.
Writing Committee for the Diabetic Retinopathy Clinical Research Network, Gross JG, Glassman AR, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2015;314:2137–46. https://doi.org/10.1001/jama.2015.15217.
Regeneron Pharmaceuticals, Inc. EYLEA® (aflibercept) Injection, for intravitreal use [US prescribing information]. 2022. Available at: https://www.regeneron.com/sites/default/files/EYLEA_FPI.pdf. Accessed September 15, 2022.
Genentech, Inc. LUCENTIS® (ranibizumab injection) for intravitreal injection [US prescribing information]. 2018. Available at: https://www.gene.com/download/pdf/lucentis_prescribing.pdf. Accessed September 15, 2022.
Halim S, Nugawela M, Chakravarthy U, et al. Topographical response of retinal neovascularization to aflibercept or panretinal photocoagulation in proliferative diabetic retinopathy: post hoc analysis of the CLARITY randomized clinical trial. JAMA Ophthalmol. 2021;139:501–7. https://doi.org/10.1001/jamaophthalmol.2021.0108.
Sivaprasad S, Prevost AT, Vasconcelos JC, et al. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet. 2017;389:2193–203. https://doi.org/10.1016/S0140-6736(17)31193-5.
Brown DM, Wykoff CC, Boyer D, et al. Evaluation of intravitreal aflibercept for the treatment of severe nonproliferative diabetic retinopathy: results from the PANORAMA randomized clinical trial. JAMA Ophthalmol. 2021;139:946–55. https://doi.org/10.1001/jamaophthalmol.2021.2809.
Hutton DW, Stein JD, Bressler NM, et al. Cost-effectiveness of intravitreous ranibizumab compared with panretinal photocoagulation for proliferative diabetic retinopathy: secondary analysis from a Diabetic Retinopathy Clinical Research Network randomized clinical trial. JAMA Ophthalmol. 2017;135:576–84. https://doi.org/10.1001/jamaophthalmol.2017.0837.
Diabetic Retinopathy Study Research Group. Preliminary report on effects of photocoagulation therapy. Am J Ophthalmol. 1976;81:383–96. https://doi.org/10.1016/0002-9394(76)90292-0.
Rothman M, Burke L, Erickson P, Leidy NK, Patrick DL, Petrie CD. Use of existing patient-reported outcome (PRO) instruments and their modification: the ISPOR Good Research Practices for Evaluating and Documenting Content Validity for the Use of Existing Instruments and Their Modification PRO Task Force Report. Value Health. 2009;12:1075–83. https://doi.org/10.1111/j.1524-4733.2009.00603.x.
Bryman A, Burgess B. Analyzing qualitative data. New York: Routledge; 2002.
Thomas DR. A general inductive approach for analyzing qualitative evaluation data. Am J Eval. 2006;27:237–46.
Bowling A. Research methods in health: investigating health and health services. Maidenhead: Open University Press; 2009.
Fabbrocini G, Cacciapuoti S, Monfrecola G. A qualitative investigation of the impact of acne on health-related quality of life (HRQL): development of a conceptual model. Dermatol Ther (Heidelb). 2018;8:85–99. https://doi.org/10.1007/s13555-018-0224-7.
Pope C, Ziebland S, Mays N. Qualitative research in health care. Analysing qualitative data. BMJ. 2000;320:114–6. https://doi.org/10.1136/bmj.320.7227.114.
Wirostko B, Beusterien K, Grinspan J, et al. Patient preferences in the treatment of diabetic retinopathy. Patient Prefer Adherence. 2011;5:229–37. https://doi.org/10.2147/PPA.S11972.
National Eye Institute. Diabetic retinopathy. 2021. Available at: https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/diabetic-retinopathy. Accessed September 15, 2022.
Acknowledgements
The authors thank the participants involved in this study.
Funding
This study and the journal’s Rapid Service Fee were funded by Regeneron Pharmaceuticals, Inc., Tarrytown, NY, USA. The sponsor conceived the need for the study, designed the study, and oversaw all operational aspects. Modus Outcomes conducted the study and analyzed the data. Regeneron Pharmaceuticals, Inc. and Modus Outcomes participated in the preparation of this manuscript.
Medical Writing, Editorial, and Other Assistance
Medical writing and assistance were provided by Stephanie Agbu, PhD, of Regeneron Pharmaceuticals, Inc. Editorial assistance was provided by Katie Groschwitz, PhD, of Core Medica and was funded by Regeneron Pharmaceuticals, Inc.
Author Contributions
Steven A. Sherman and Diana Rofail conceived the need for the project, designed the study, and oversaw all aspects. Adele Levine, Jessica Baldasaro, and Patrick Marquis participated in data acquisition and analysis. Steven A. Sherman, Diana Rofail, Adele Levine, Christopher R. Hartford, Jessica Baldasaro, Patrick Marquis, Rohini Rao, and Diana V. Do contributed to the interpretation of data and approved the final manuscript for submission.
Disclosures
Steven A. Sherman, Diana Rofail, Christopher R. Hartford, and Rohini Rao are employees and stockholders of Regeneron Pharmaceuticals, Inc. Adele Levine was an employee of Modus Outcomes at the time of the study (current affiliation: Center for the Evaluation of Value and Risk in Health, Boston, MA, USA). Jessica Baldasaro and Patrick Marquis are employees of Modus Outcomes. Diana V. Do is a consultant to Allergan, AsclepiX, Boehringer Ingelheim, Clearside, Genentech, Kodiak Sciences, and Regeneron Pharmaceuticals, Inc.; and has received research funding from AsclepiX, Boehringer Ingelheim, Genentech, and Regeneron Pharmaceuticals, Inc.
Compliance with Ethics Guidelines
This study was conducted in accordance with the principles of the Declaration of Helsinki and all applicable regulatory requirements. All study documents—including the protocol, interview guides, demographic and health information form, screener form, and informed consent form—were approved by the WCG Independent Review Board (reference number: 20202896) before study initiation. All patients provided written informed consent before participation in this study, and all researchers were trained in qualitative methods and Good Clinical Practice.
Data Availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Sherman, S.A., Rofail, D., Levine, A. et al. The Patient Experience with Diabetic Retinopathy: Qualitative Analysis of Patients with Proliferative Diabetic Retinopathy. Ophthalmol Ther 12, 431–446 (2023). https://doi.org/10.1007/s40123-022-00614-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-022-00614-8






