Abstract
Defects in the glycosaminoglycan layer (GAG) of the bladder mucosa have been identified as a significant contributor to the pathogenesis and clinical progression of chronic inflammatory diseases of the bladder, such as post-radiation cystitis, bladder pain syndrome and recurrent urinary tract infections. This narrative review aims to explore the contemporary evidence on the role of GAG reconstitution with intravesical installations of hyaluronic acid and chondroitin sulfate in the management of those patients, with a goal to provide valuable insights for clinical practice. The reviewed studies consistently demonstrate that GAG reconstitution can result in varying degrees of clinical improvement in patients with post-radiation cystitis, bladder pain syndrome and recurrent urinary tract infections, and is associated with a very favorable safety profile. While the available evidence is growing, its level is still limited, mainly by relatively low number of randomized controlled trials, with small sample sizes. Further research with larger, well-designed trials is needed to solidify the findings and optimize the clinical application of GAG reconstitution.
Similar content being viewed by others
Why carry out this study? |
Chronic forms of cystitis, including recurrent urinary tract infections, post-radiation cystitis and bladder pain syndrome / interstitial cystitis, are prevalent and morbid diseases with limited options of effective treatment. |
What was learned from this study? |
Intravesical instillations of hyaluronic acid and chondroitin sulfate solution are a promising treatment for patients with chronic forms of cystits, especially when first-line treatment has failed. |
Increasing evidence shows clinical efficacy of this treatment, defined as a reduction in disease symptoms and improvement in urodynamic parameters. |
Intravesical therapy is safe and well-tolerated by patients. |
Introduction
Damage to the protective glycosaminoglycan (GAG) layer covering urothelial epithelial cells in the bladder has been indicated as an important step in the pathogenesis of chronic inflammatory diseases of the bladder, as well as a trigger for clinical manifestation of those conditions [1, 2]. Intravesical GAG restoration is an effective therapy is effective for bladder pain syndrome/interstitial cystitis (BPS/IC) and post-radiation cystitis, as well as it may prevent recurrent urinary tract infections (rUTIs). The goal of this article was to non-systematically review and discuss the contemporary evidence regarding clinical outcomes of intravesical therapy with hyaluronic acid and chondroitin sulfate in patients with various diseases of the bladder. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Structure and Importance of the Glycosaminoglycan Layer in the Bladder
The urothelium lining the bladder is composed of a complex of highly specialized epithelial cells with numerous and multidirectional functions [3]. In addition to participating in the effective collection of urine, the two basic physiological tasks of the urothelial epithelium are:
-
Afferent function, based on the transmission of local impulses to the central nervous system [4];
-
Efferent or paracrine function associated with the secretion of mediators of physiological and pathological bladder function (including P substance, bradykinin, endothelin, and others) [5].
Damage to, and dysfunction of, the urothelial epithelium can lead to several bladder disorders and diseases, including frequent urination, urgency, urinary incontinence, and chronic pain. One of the defense mechanisms ensuring proper functioning of the urothelial epithelium is the presence of a protective GAG layer. The GAG layer is a thin, waterproof polysaccharide coating that plays an isolating role. It separates sensitive urothelial cells from urinary irritants (especially urea, potassium ions, and drug metabolites) and potential infectious agents (especially bacteria). It consists of multiple polysaccharides, including hyaluronic acid, chondroitin sulfate, heparan sulfate, dermatan sulfate, keratan sulfate, and heparin [3]. These polysaccharides combine with urothelial proteins to form proteoglycans [6].
Damage to, and loss of, the GAG layer are considered triggers for chronic inflammatory diseases of the bladder, including BPS/IC, post-radiation cystitis, and rUTIs. Exposure of urothelial cells to the toxic effects of urine leads to their neurogenic damage and inflammation [7]. Activation of nerve fibers in the submucosal layer leads to neuronal hypersensitivity. The cascade of adverse events ends with the presence of bothersome symptoms of disease [3].
Recurrent urinary Tract Infections
One in every two women will experience a UTI during their lifetime [8], and nearly 70% of cases relapse within the following 12 months [9, 10]. The most common definition of rUTIs, adopted, among others, by experts of the European Urological Association, requires two documented episodes of infection within 6 months or three documented episodes within 12 months.
Risk factors for rUTI vary depending on a woman's hormonal status. In women before menopause, sexual activity, use of spermicides, changing sexual partners, maternal history of UTIs, and history of UTIs in childhood are considered the most important. For postmenopausal women, the risk of UTI is higher in women with urinary incontinence, vaginal mucosal atrophy, cystocele, urinary retention after micturition, or urinary catheter, and in those who are under institutional care [11].
Three main pathomechanisms of rUTI have been proposed. The two classic mechanisms include repeated de novo infections caused by ascending bacteria of the anogenital region and activation of persistent infection associated with the presence of a bacterial reservoir [12,13,14]. A third possible mechanism related to the ability of Escherichia coli bacteria to replicate in urothelial cells and form intracellular colonies that periodically cause cystitis is also being considered [15].
Regardless of the pathomechanism of rUTIs, the GAG layer plays an important role in preventing infection. It isolates the urothelial cells from bacteria present in the urine, while also reducing bacterial adhesion [16, 17]. Damage to the GAG layer can lead directly to infection [18]. For this reason, a promising form of UTI prophylaxis is the reconstitution of the GAG layer. As shown in numerous preclinical studies, intravesical GAG infusions lead to reductions in the severity of inflammatory processes, the frequency and amplitude of contractions of the bladder detrusor muscle, urothelial damage, and bacterial growth in urine and cells [19]. In postmenopausal women, intravesical administration of chondroitin sulfate may be particularly important. This polysaccharide has been indicated as extremely defective in rUTI, and is negatively correlated with the development of dysbiosis of the vaginal microbiota [20].
In the prevention of rUTI, a number of interventions, both pharmacological and behavioral, are considered. From a practical point of view, they are often divided into behavioral management, non-antibiotic prophylaxis, and antibiotic prophylaxis (Table 1). In view of the decreasing effectiveness of antibiotics and growing drug resistance, interest in effective non-antibiotic prophylaxis is growing significantly. This is also reflected in clinical management guidelines [21].
Numerous studies have assessed the efficacy and effectiveness of intravesical instillations of hyaluronic acid and chondroitin sulfate in the prevention of rUTIs. They are presented in Table 2. In general, the evidence behind the therapy mainly comes from retrospective observations with consistent outcomes. Intravesical therapy reduces the risk and prolongs time to UTI recurrence, has marginal risk of adverse events and improves the quality of life to a varying extent. The two available randomized trials and two available meta-analyses of studies require separate discussion. Also worth mentioning is a study in which Tasdemir and co-authors showed that, in rats with UTI, intravesical infusions of hyaluronic acid or chondroitin sulfate reduce bacterial multiplication, while infusions of both components also have a positive effect on reducing of pathological morphological lesions of the urothelium [22].
In the study by Damiano and co-authors, 57 women with a history of rUTI were included and randomly assigned to the intervention or control group [23, 24]. The intervention group received prophylaxis based on intravesical instillations of 800 mg of hyaluronic acid and 1 g of chondroitin sulfate, administered every 7 days for 4 weeks and thereafter every 4 weeks for another 5 months. In the control group, placebo was administered. A decrease in the rate of infection episodes by 77% (a decrease of 10% vs 87%) was observed in the group receiving prophylaxis. The mean time to relapse was also prolonged in the intravesical therapy group (53 vs 185 days). In addition, patients from the prophylaxis group rated their quality of life as better on the SF-36 questionnaire and declared less severe disease symptoms in the screening history Pelvic Pain and Urinary Urgency/Frequency (PUF) questionnaire. Three patients in the prophylaxis group had non-infection-related urine collection disorders, one of whom required treatment.
In a study by De Vita and Giordano, 28 women with a history of rUTI were randomly assigned to prophylaxis with intravesical instillations of hyaluronic acid and chondroitin sulfate or prolonged antibiotic prophylaxis with trimethoprim and sulfamethoxazole [25]. In the first group, intravesical instillations containing 800 mg of hyaluronic acid and 1 g of chondroitin sulfate were administered every 7 days for 4 weeks, followed by two infusions at 2 week intervals. In the second group, 200 mg of sulfamethoxazole and 40 mg of trimethoprim were administered every 7 days for 6 weeks. Over 12 months of observation, there were lower numbers of episodes of infection (1.0 vs 2.3), a lower number of micturitions over 3 days (18 vs 24), a greater cystometric capacity of the bladder (380 ml vs 229 ml), lower pain (visual analogue scale scores of 1.6 vs 7.8), lower severity of ailments of overactive bladder syndrome (PUF 11.2 vs 19.6), and better scores for sexual satisfaction and quality of life among those receiving intravesical instillations of hyaluronic acid and chondroitin sulfate.
The first meta-analysis of studies on the effectiveness of intravesical instillations of hyaluronic acid and chondroitin sulfate solution in the prevention of rUTIs was published by De Vita et al. in 2013 [30]. The researchers analyzed four studies, including the two randomized trials discussed above. In total, the meta-analysis included 143 patients. Prophylaxis with intravesical instillations of hyaluronic acid and chondroitin sulfate solution was calculated to avoid, on average, 3.4 episodes of infection per year in each patient. Moreover, the average time to recurrence of infection was prolonged by an average of 187 days in patients receiving prophylaxis. In terms of symptoms reported by patients, prevention led to a reduction in pain, frequency of urination, and urgency as assessed in the PUF questionnaire, but did not significantly affect the average number of micturitions.
A second meta-analysis was published in 2018 by Goddard and Janssen [31]. It included data from eight studies, including the two randomized trials described above. In total, the meta-analysis included 800 patients with rUTI. Prophylaxis with intravesical instillations of hyaluronic acid and chondroitin sulfate was reported to lead to a reduction in the number of infectious episodes in the urinary tract by an average of 2.6 per year and to prolong the time to the next episode by 130 days on average. A beneficial effect of prophylaxis on the PUF questionnaire was also noted. However, no effect was seen on the number of micturitions or quality of life as measured with the SF-36 questionnaire. The authors highlighted that the results of the studies varied from each other, and that the number of studies and number of included patients were small.
Both meta-analyses described above have several limitations. One such limitation is the inclusion of studies in which the intervention involved the administration of a complex formulation of a solution of hyaluronic acid and chondroitin sulfate, as well as studies on hyaluronic acid monotherapy.
The growing role of intravesical therapy with a solution of hyaluronic acid and chondroitin sulfate is illustrated by the presence of this form of treatment in the European Urological Association guidelines. The 2021 guidelines suggested the need for even more studies [32], and in the guidelines from 2022 onwards, the authors additionally refer to the two meta-analyses [33].
Bladder Pain Syndrome/Interstitial Cystitis
BPS/IC can be defined as the presence of persistent or recurrent pain in the bladder region, which is accompanied by worsening with filling of the bladder and/or frequent daily or nocturnal urination [34]. Diagnosis requires the exclusion of UTI and other recognizable local pathologies. The definition of the disease is not universal, which makes it difficult to determine its scale. While the disease is estimated to affect about 3% of the female population and 2% of the male population, a prevalence of up to 30% of the population has also been reported [35, 36].
The pathogenesis of BPS/IC has not yet been clearly determined, and inconsistent data in this area has led to the theory of a multifactorial and multistage process. Urothelial damage is one of the best known pathogenic mechanisms. Loss of the protective GAG layer and urothelial exposure to toxic urine components, including ions of sodium, potassium, and chlorine, drugs and their metabolites, and other toxins, leads to activation of nerve endings, neurogenic inflammation, and disease symptoms [37,38,39]. In preclinical studies, reconstitution of the GAG layer in BPS/IC reduces the inflammatory process and increases the viability and migratory capacity of urothelial cells [40,41,42]. However, regeneration of the urothelium and changes in the severity of symptoms are associated with an improvement in the endoscopic image of the bladder only in some cases [43, 44].
Patients with BPS/IC require complex and holistic care involving doctors of various specialties, a psychologist, and other therapists. A simplified overview of therapeutic options is presented in Table 3. Intravesical treatment has a firmly established position within this indication. The efficacy of intravesical instillations of hyaluronic acid and chondroitin sulfate has been evaluated in eight studies so far (Table 4). To summarize, majority of the studies were prospective and controlled with different comparators, enrolling in total a significant number of individuals. The intravesical instillations of hyaluronic acid and chondroitin sulfate led to a significant subjective and objective improvement as illustrated in dedicated questionnaires (mainly ICSI, ICPI, VAS and PUF) and urodynamic parameters, respectively. When compared to alternative intravesical therapies, hyaluronic acid and chondroitin sulfate combination was at least non-inferior. In addition, five meta-analyses and two randomized controlled trials comparing hyaluronic acid with chondroitin sulfate are discussed below.
Studies on the effectiveness of intravesical therapy in patients with BPS/IC have been the subject of five meta-analyses. A detailed analysis of four of them goes beyond the scope of this study, as they consider all options for pharmacological management in BPS/IC [53] or all possible intravesicular treatment options [54,55,56]. The main limitation of these meta-analyses is the very limited number of studies directly comparing different management strategies in patients with BPS/IC, which in turn leads to the risk of erroneous inference from the comparison of absolute results of different studies or network meta-analyses. As such, these meta-analyses draw quite differing conclusions. Taking intravesical therapies into account, four different meta-analyses indicated four different preparations as the most effective: hyaluronic acid [56], resiniferatoxin [54], dimethylsulfoxide (DMSO) [53], and botulinum toxin [55]. Given this, a personalized combination of several therapeutic options may be a promising form of management in selected patients [57].
In the context of assessment of the effectiveness of hyaluronic acid with chondroitin sulfate in patients with BPS/IC, the meta-analysis published in 2016 by Pyo and Cho, is probably the most valuable [58]. It included 10 observational studies involving a total of 390 patients. Importantly, the meta-analysis considered together studies on the effectiveness of intravesical treatment with hyaluronic acid alone or in combination with chondroitin sulfate. Treatment led to a reduction in the severity of pain (decrease by 3.7 points on the VAS), the severity of symptoms (decrease in the Interstitial Cystitis Symptom Index [ICSI] questionnaire score by 3.2 points), and health problem scores (decrease in the Interstitial Cystitis Problem Index [ICPI] questionnaire score by 2.9 points), and an increase in bladder capacity (by 60 ml) and micturition volume (by 31 ml). However, in view of the results of this meta-analysis, it is impossible to avoid questions about the differences in the effectiveness of treatment with hyaluronic acid alone and in combination with chondroitin sulfate. While the authors of the meta-analyses found no significant differences on the basis of secondary analyses, two randomized controlled trials published in subsequent years compared the efficacy of hyaluronic acid and chondroitin sulfate alone and in combination in patients with BPS/IC.
In the study by Gulpinar and co-authors, 42 patients with BPS/IC were randomized in a 1:1 ratio to treatment with intravesical hyaluronic acid or chondroitin sulfate [59]. They were given 120 mg of hyaluronic acid or 80 mg of chondroitin sulfate every 7 days for the first 4 weeks, then every 2 weeks in the second month, and every 4 weeks in the third and fourth months. The observation lasted 6 months. Study endpoints were: pain severity on the VAS pain intensity scale, functional parameters from the 3-day micturition diary (micturition number, nocturia, micturition volume), symptom severity assessed with the ICSI questionnaire, and health problems assessed with the ICPI questionnaire. In both treatment groups, significant improvements were observed across all endpoints, excluding mean micturition volume in patients treated with hyaluronic acid. Moreover, chondroitin sulfate resulted in greater improvements than hyaluronic acid in terms of the number of micturitions per day, nycturia, and ICPI score. Finally, the authors suggest that chondroitin sulfate has higher efficacy than hyaluronic acid, but point to the need to confirm their observations in larger studies involving longer follow-up.
Ozkidik and co-authors published the results of a randomized controlled trial that compared the efficacy and tolerability of intravesical treatment with hyaluronic acid alone, chondroitin sulfate alone, or their combination [60]. The study enrolled 72 patients with BPS/IC, including 10 men. They were given intravesical instillations of 120 mg of hyaluronic acid or 80 mg of chondroitin sulfate, or a combination of 60 mg of hyaluronic acid with 40 mg of chondroitin sulfate. Intravesical instillations were repeated every 7 days for the first 6 weeks, then every 2 weeks for 6 months, then monthly until the follow-up period of 24 months was completed. Endpoints of the study were functional parameters from micturition diaries and the results of the Patient Perception of Bladder Condition (PPBC), VAS, PUF, ICSI, ICPI and Health-Related Quality of Life (HRQoL) questionnaires. Significant improvements across all endpoints were noted in all groups. The comparison of the groups revealed higher efficacy of the combination therapy in terms of pain, frequency of urination, and urgency assessed with the PUF questionnaire and quality of life assessed with the HRQoL score. There were no other differences between groups. Five patients were diagnosed with UTI during therapy, three had a transient hematuria after catheterization. No other side-effects were reported.
Post-radiation Cystitis
Radiation therapy is an effective treatment for pelvic neoplasms. Most often it is used in patients with prostate cancer, rectal cancer, or endometrial cancer. Despite the increasing precision in delivering the dose of radiation to the target organ, therapy involves the risk of radiation damage to neighboring organs. Possible complications of pelvic radiotherapy include a few affecting the urinary tract, including post-radiation cystitis, lower urinary tract dysfunctions, ureteral stenosis, urethral stricture stenosis, and urinary fistulas. The risk of bladder complications is related to its variable volume, and thus the position in the pelvis changes depending on the degree of urine filling.
Post-radiation cystitis can be divided into early/acute and late/chronic disease [61]. Although the nature of both is similar, they differ in incidence and prognosis [62]. Typically, patients complain of frequent urination, nocturia, and urgency. Additionally, pelvic pain and hematuria may occur. Early inflammation is found in more than half of patients, resulting from inflammatory lesions and urothelial edema after treatment. In urodynamic examination, reduced functional capacity of the bladder can be found, as well as reduced capacity at the first feeling of urgency and reduction in the urine volume backflow after micturition [63]. Late symptoms affect 5–10% of patients [61], at an average of 35 months after radiotherapy, and may occur even 20 years after treatment [64]. Their cause is irreversible bladder fibrosis. Decreased compliance of the bladder and detrusor overactivity are noted on urodynamic examination [65,66,67].
Underlying the development of symptoms of post-radiation cystitis are urothelial edema and chronic inflammation. They result from direct damage to the urothelial epithelium, which occurs as a result of loss of the GAG layer [68]. For this reason, reconstitution of the GAG layer is one of the therapeutic options available to patients with post-radiation cystitis. The scheme of typical treatment is presented in Table 5. Intravesical treatment is considered a second-line treatment. In the case of hemorrhagic inflammation, alum salts, silver nitrate, or formalin can also be considered in addition to hyaluronic acid and chondroitin sulfate. However, their use is burdened with limited availability of efficacy data, and, in the case of formalin, with a high risk of adverse reactions.
It should be emphasized that treatment of patients with post-radiation cystitis should be preceded by a thorough diagnosis, aimed in particular at excluding bladder cancer and upper urinary tract damage. Typical diagnostic tools include: laboratory tests (general examination and urine culture), functional tests (uroflowmetry, micturition diary), imaging (ultrasonography), endoscopic examination (cystoscopy), and questionnaires (International Prostate Symptom Score [IPSS], International Consultation on Incontinence Questionnaire–Urinary Incontinence Short Form [ICIQSF]).
The efficacy of intravesical instillations of hyaluronic acid and chondroitin sulfate solution was confirmed in four prospective observational studies. They are presented in Table 6. In addition, studies confirming the effectiveness of individual components of this combination are available [69,70,71,72,73]. To summarize findings from these studies, intravesical instillations of hyaluronic acid and chondroitin sulfate significantly improve symptoms, quality of life and urodynamic parameters. They also reduce the risk of hematuria and hemorrhagic complications.
Therapeutic efficacy prompted two research groups to evaluate the effectiveness of intravesical instillations in the prevention of post-radiation bladder symptoms. Hazewinkel and co-authors conducted a prospective observational study on a group of 20 women treated with radiotherapy for uterine/endometrial cancer [78]. The intervention included prophylactic instillations of chondroitin sulfate every 7 days for 8 weeks of radiotherapy in 10 women. In this study, good tolerability of treatment (one patient discontinued therapy due to urethral pain) and high efficacy (reduction of symptoms of overactive bladder syndrome, urinary incontinence, micturition disorders, and pain) were noted.
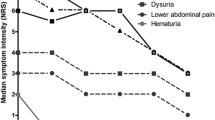
Palou and co-authors conducted a randomized controlled trial that included 49 men treated with radiotherapy for prostate cancer [79]. Patients were randomly assigned to the intervention group or control group. In the intervention group, prophylactic intravesical instillations for post-radiation cystitis were applied. Intravesical chondroitin sulfate and hyaluronic acid were given every 7 days for 6 weeks of radiotherapy and Ialuril oral capsules with chondroitin sulfate, hyaluronic acid, quercetin, and curcumin were administered every 12 h for 12 weeks. In the control group, no additional intervention was implemented. Prophylactic therapy was well tolerated, and no patients discontinued treatment. The endpoints in the study were the severity of symptoms and the quality of life, as measured by the Expanded Prostate Cancer Index Composite (EPIC), International Consultation on Incontinence Questionnaire—Male Lower Urinary Tract Symptoms (ICIQ-MLUTS), and EQ-5D-5L questionnaires. All of these endpoints showed a significant benefit of prevention in annual follow-up. It is worth noting that adverse reactions were reported in 12% of patients; these were mild and self-limiting in nature in all cases (hematuria, nausea, urticaria).
Future Considerations
The confirmed efficacy of GAG replacement therapy in patients with chronic forms of cystitis has led to research into expanded indications and new formulations.
Intravesical instillations of hyaluronic acid and chondroitin sulfate solution are becoming an attractive method for prevention of UTI in patients with neurogenic bladder. Current studies have indicated the high efficacy of this form of management in patients with spinal cord injury [80] and in children with spina bifida using pure intermittent catheterization [81]. The efficacy of this form of prophylaxis for recurrent infections with an etiology of multidrug-resistant bacteria has also been reported, which is of significant clinical importance, especially for patients with neurogenic lower urinary tract dysfunction [82].
Reconstitution of the GAG layer appears to be a valuable form of prevention and treatment of cystitis symptoms after Bacillus Calmette-Guerin (BCG) intravesicular immunotherapy in patients treated for bladder cancer. Two studies on this issue have been published so far. A study by Topazio et al. showed that prophylactic intravesical instillations of hyaluronic acid solution in patients treated with BCG reduces the severity of pain, the severity of lower urinary tract symptoms, and the number of micturitions [83]. A study by Imperatore et al. documented the efficacy of intravesical instillations of hyaluronic acid and chondroitin sulfate solution in the treatment of post-BCG cystitis symptoms refractory to first-line treatment. Treatment led to a reduction in the severity of pain and improvement in basic urodynamic parameters [84].
For the prophylaxis of rUTI, an oral combined preparation containing hyaluronic acid, chondroitin sulfate, quercetin, and curcumin is also available. Two prospective studies published so far have confirmed its effectiveness in both women of reproductive age and in postmenopausal women [85, 86]. In postmenopausal women, the combination of the above-described oral prophylaxis with intravaginal estrogen therapy proved to be particularly effective. Oral use of a combination of hyaluronic acid, chondroitin sulfate, quercetin, and curcumin has also been shown to significantly reduce the severity of chemo-induced cystitis symptoms in patients treated with intravesicular chemotherapy for bladder cancer [87].
Conclusions
Intravesical instillations of hyaluronic acid and chondroitin sulfate solution are a promising treatment for patients with rUTIs and PBS/IC, as well as in patients with post-radiation cystitis in whom first-line treatment has failed. Numerous studies conducted to date have shown the clinical efficacy of this treatment, defined as a reduction in disease symptoms and improvement in urodynamic parameters. At the same time, intravesical therapy is safe and well-tolerated by patients. The biggest limitation remains the small number of randomized trials and the small population of patients included in these studies.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Madersbacher H, van Ophoven A, van Kerrebroeck PE. GAG layer replenishment therapy for chronic forms of cystitis with intravesical glycosaminoglycans—a review. Neurourol Urodyn. 2013;32(1):9–18.
Lazzeri M, Hurle R, Casale P, Buffi N, Lughezzani G, Fiorini G, Peschechera R, Pasini L, Zandegiacomo S, Benetti A, Taverna G, Guazzoni G, Barbagli G. Managing chronic bladder diseases with the administration of exogenous glycosaminoglycans: an update on the evidence. Ther Adv Urol. 2016;8(2):91–9.
Damiano R, Cicione A. The role of sodium hyaluronate and sodium chondroitin sulphate in the management of bladder disease. Ther Adv Urol. 2011;3(5):223–32.
Kanai AJ. Afferent mechanism in the urinary tract. Handb Exp Pharmacol. 2011;202:171–205.
Arms L, Vizzard MA. Neuropeptides in lower urinary tract function. Handb Exp Pharmacol. 2011;202:395–423.
Lilly JD, Parsons CL. Bladder surface glycosaminoglycans is a human epithelial permeability barrier. Surg Gynecol Obstet. 1990;171(6):493–6.
Geppetti P, Nassini R, Materazzi S, Benemei S. The concept of neurogenic inflammation. BJU Int. 2008;101(Suppl 3):2–6.
Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;8(113 Suppl 1A):5S-13S.
Ikäheimo R, Siitonen A, Heiskanen T, Kärkkäinen U, Kuosmanen P, Lipponen P, Mäkelä PH. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis. 1996;22(1):91–9.
Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol. 2010;7(12):653–60.
Vahlensieck W, Perepanova T, Bjerklund Johansen TE, Tenke P, Naber KG, Wagenlehner FE. Management of Uncomplicated Recurrent Urinary Tract Infections. Eur Urol Suppl. 2016;15(4):95–101.
Stamey TA. Recurrent urinary tract infections in female patients: an overview of management and treatment. Rev Infect Dis. 1987;9(2):S195-210.
Schaeffer AJ. Recurrent urinary tract infections in women. Pathogenesis Manag Postgrad Med. 1987;81(3):51–8.
Fowler JE Jr. Urinary tract infections in women. Urol Clin North Am. 1986;13(4):673–83.
Rosen DA, Hooton TM, Stamm WE, Humphrey PA, Hultgren SJ. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 2007;4(12): e329.
Poggi MM, Johnstone PA, Conner RJ. Glycosaminoglycan content of human bladders. A method of analysis using cold-cup biopsies. Urol Oncol. 2000;5(5):234–7.
Morales A, Emerson L, Nickel JC. Intravesical hyaluronic acid in the treatment of refractory interstitial cystitis. Urology. 1997;49(5A Suppl):111–3.
Parsons CL. Epithelial coating techniques in the treatment of interstitial cystitis. Urology. 1997;49(5A Suppl):100–4.
Cicione A, Cantiello F, Ucciero G, Salonia A, Madeo I, Bava I, Aliberti A, Damiano R. Restoring the glycosaminoglycans layer in recurrent cystitis: experimental and clinical foundations. Int J Urol. 2014;21(8):763–8.
Neugent ML, Hulyalkar NV, Kumar A, Xing C, Zimmern PE, Shulaev V, De Nisco NJ. Urinary Glycosaminoglycans Are Associated with Recurrent UTI and Urobiome Ecology in Postmenopausal Women. ACS Infect Dis. 2023;9(4):1022–32.
Naber KG, Bonkat G, Wagenlehner FME. The EAU and AUA/CUA/SUFU Guidelines on Recurrent Urinary Tract Infections: What is the Difference? Eur Urol. 2020;78(5):645–6.
Tasdemir S, Tasdemir C, Vardi N, Yakupogullari Y, Duman Y, Parlakpinar H, Sagir M, Acet A. Intravesical hyaluronic acid and chondroitin sulfate alone and in combination for urinary tract infection: assessment of protective effects in a rat model. Int J Urol. 2012;19(12):1108–12.
Damiano R, Quarto G, Bava I, Ucciero G, De Domenico R, Palumbo MI, Autorino R. Prevention of recurrent urinary tract infections by intravesical administration of hyaluronic acid and chondroitin sulphate: a placebo-controlled randomised trial. Eur Urol. 2011;59(4):645–51.
Damiano R, Quarto G, Bava I, Ucciero G, De Domenico R, Palumbo MI, Autorino R. Corrigendum to "Prevention of Recurrent Urinary Tract Infections by Intravesical Administration of Hyaluronic Acid and Chondroitin Sulphate: A Placebo-Controlled Randomised Trial [Eur Urol 2011;59:645–51]. Eur Urol. 2011;60(1):193.
De Vita D, Giordano S. Effectiveness of intravesical hyaluronic acid/chondroitin sulfate in recurrent bacterial cystitis: a randomized study. Int Urogynecol J. 2012;23(12):1707–13.
Torella M, Schettino MT, Salvatore S, Serati M, De Franciscis P, Colacurci N. Intravesical therapy in recurrent cystitis: a multi-center experience. J Infect Chemother. 2013;19(5):920–5.
Cicione A, Cantiello F, Ucciero G, Salonia A, Torella M, De Sio M, Autorino R, Carbone A, Romancik M, Tomaskin R, Damiano R. Intravesical treatment with highly-concentrated hyaluronic acid and chondroitin sulphate in patients with recurrent urinary tract infections: Results from a multicentre survey. Can Urol Assoc J. 2014;8(9–10):E721–7.
Gugliotta G, Calagna G, Adile G, Polito S, Saitta S, Speciale P, Palomba S, Perino A, Granese R, Adile B. Is intravesical instillation of hyaluronic acid and chondroitin sulfate useful in preventing recurrent bacterial cystitis? A multicenter case control analysis. Taiwan J Obstet Gynecol. 2015;54(5):537–40.
Ciani O, Arendsen E, Romancik M, Lunik R, Costantini E, Di Biase M, Morgia G, Fragalà E, Roman T, Bernat M, Guazzoni G, Tarricone R, Lazzeri M. Intravesical administration of combined hyaluronic acid (HA) and chondroitin sulfate (CS) for the treatment of female recurrent urinary tract infections: a European multicentre nested case-control study. BMJ Open. 2016;6(3): e009669.
De Vita D, Antell H, Giordano S. Effectiveness of intravesical hyaluronic acid with or without chondroitin sulfate for recurrent bacterial cystitis in adult women: a meta-analysis. Int Urogynecol J. 2013;24(4):545–52.
Goddard JC, Janssen DAW. Intravesical hyaluronic acid and chondroitin sulfate for recurrent urinary tract infections: systematic review and meta-analysis. Int Urogynecol J. 2018;29(7):933–42.
EAU Guidelines. Edn. presented at the EAU Annual Congress Milan 2021. ISBN 978-94-92671-13-4.
EAU Guidelines. Edn. presented at the EAU Annual Congress Amsterdam, the Netherlands 2022. ISBN 978-94-92671-16-5
Fall M, Baranowski AP, Elneil S, Engeler D, Hughes J, Messelink EJ, Oberpenning F, de C Williams AC; European Association of Urology. EAU guidelines on chronic pelvic pain. Eur Urol. 2010;57(1):35–48
Konkle KS, Berry SH, Elliott MN, Hilton L, Suttorp MJ, Clauw DJ, Clemens JQ. Comparison of an interstitial cystitis/bladder pain syndrome clinical cohort with symptomatic community women from the RAND Interstitial Cystitis Epidemiology study. J Urol. 2012;187(2):508–12.
Berry SH, Elliott MN, Suttorp M, Bogart LM, Stoto MA, Eggers P, Nyberg L, Clemens JQ. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011;186(2):540–4.
Han XM, Wu XH, Li B, Pan F, Li WC, Liu SL, Zeng FQ, Chen M. The effects of intravesical therapy with hyaluronic acid for painful bladder syndrome: Preliminary Chinese experience and systematic review. Taiwan J Obstet Gynecol. 2015;54(3):240–7.
Parsons CL. The role of a leaky epithelium and potassium in the generation of bladder symptoms in interstitial cystitis/overactive bladder, urethral syndrome, prostatitis and gynaecological chronic pelvic pain. BJU Int. 2011;107(3):370–5.
Hurst RE. Structure, function, and pathology of proteoglycans and glycosaminoglycans in the urinary tract. World J Urol. 1994;12(1):3–10.
Danacioglu YO, Erol B, Ozkanli S, Yildirim A, Atis RG, Silay MS, Caskurlu T. Comparison of Intravesical Hyaluronic Acid, Chondroitin Sulfate, and Combination of Hyaluronic Acid-Chondroitin Sulfate Therapies in Animal Model of Interstitial Cystitis. Int Neurourol J. 2021;25(1):42–50.
Rooney P, Ryan C, McDermott BJ, Dev K, Pandit A, Quinlan LR. Effect of Glycosaminoglycan Replacement on Markers of Interstitial Cystitis In Vitro. Front Pharmacol. 2020;3(11): 575043.
Stellavato A, Pirozzi AVA, Diana P, Reale S, Vassallo V, Fusco A, Donnarumma G, De Rosa M, Schiraldi C. Hyaluronic acid and chondroitin sulfate, alone or in combination, efficiently counteract induced bladder cell damage and inflammation. PLoS ONE. 2019;14(6): e0218475.
Stavropoulos M, Thakare N, Venieris P, Liakouras C, Deliveliotis C, Chrisofos M. The use of intravesical hyaluronic acid in the management of symptomatic premenopausal women with pseudomembranous trigonitis: Are symptoms related to cystoscopy and pathological findings? Low Urin Tract Symptoms. 2022;14(1):57–63.
Lin CJ, Liu CK, Hsieh HY, Chen MJ, Tsai CP. Changes in Cystoscopic Findings after Intravesical Hyaluronic Acid Instillation Therapy in Patients with Interstitial Cystitis. Diagnostics (Basel). 2022;12(8):2009.
Porru D, Leva F, Parmigiani A, Barletta D, Choussos D, Gardella B, Daccò MD, Nappi RE, Allegri M, Tinelli C, Bianchi CM, Spinillo A, Rovereto B. Impact of intravesical hyaluronic acid and chondroitin sulfate on bladder pain syndrome/interstitial cystitis. Int Urogynecol J. 2012;23(9):1193–9.
Cervigni M, Natale F, Nasta L, Mako A. Intravesical hyaluronic acid and chondroitin sulphate for bladder pain syndrome/interstitial cystitis: long-term treatment results. Int Urogynecol J. 2012;23(9):1187–92.
Giberti C, Gallo F, Cortese P, Schenone M. Combined intravesical sodium hyaluronate/chondroitin sulfate therapy for interstitial cystitis/bladder pain syndrome: a prospective study. Ther Adv Urol. 2013;5(4):175–9.
Gülpınar O, Kayış A, Süer E, Gökçe Mİ, Güçlü AG, Arıkan N. Clinical comparision of intravesical hyaluronic acid and hyaluronic acid-chondroitin sulphate therapy for patients with bladder pain syndrome/interstitital cystitis. Can Urol Assoc J. 2014;8(9–10):E610–4.
Cervigni M, Sommariva M, Tenaglia R, Porru D, Ostardo E, Giammò A, Trevisan S, Frangione V, Ciani O, Tarricone R, Pappagallo GL. A randomized, open-label, multicenter study of the efficacy and safety of intravesical hyaluronic acid and chondroitin sulfate versus dimethyl sulfoxide in women with bladder pain syndrome/interstitial cystitis. Neurourol Urodyn. 2017;36(4):1178–86.
Arslan B, Gönültaş S, Gökmen E, Özman O, Avci MA, Özdemir E. Outcomes of intravesical chondroitin-sulfate and combined hyaluronic-acid/chondroitin-sulfate therapy on female sexual function in bladder pain syndrome. Int Urogynecol J. 2019;30(11):1857–62.
Sherif H, Sebay A, Kandeel W, Othman T, Fathi A, Mohey A, Eshazly A. Safety and efficacy of Intravesical hyaluronic acid/chondroitin sulfate in the treatment of refractory painful bladder syndrome. Turk J Urol. 2018;45(4):296–301.
Keane J, Young N, Goh J, Atherton M, Yin J, Moore K, Hall P, Higgs P, Leitch A, Lee J, Rosamilia A. A comparison of two intravesical bladder instillations for interstitial cystitis/bladder pain syndrome. Eur J Obstet Gynecol Reprod Biol. 2021;256:230–4.
Di XP, Luo DY, Jin X, Zhao WY, Li H, Wang KJ. Efficacy and safety comparison of pharmacotherapies for interstitial cystitis and bladder pain syndrome: a systematic review and Bayesian network meta-analysis. Int Urogynecol J. 2021. https://doi.org/10.1007/s00192-020-04659-w. (Epub ahead of print).
Liu S, Zhang C, Peng L, Lu Y, Luo D. Comparative effectiveness and safety of intravesical instillation treatment of interstitial cystitis/bladder pain syndrome: a systematic review and network meta-analysis of randomized controlled trials. Int Urogynecol J. 2020. https://doi.org/10.1007/s00192-020-04490-3. (Epub ahead of print).
Zhang W, Deng X, Liu C, Wang X. Intravesical treatment for interstitial cystitis/painful bladder syndrome: a network meta-analysis. Int Urogynecol J. 2017;28(4):515–25. https://doi.org/10.1007/s00192-016-3079-4.
Barua JM, Arance I, Angulo JC, Riedl CR. A systematic review and meta-analysis on the efficacy of intravesical therapy for bladder pain syndrome/interstitial cystitis. Int Urogynecol J. 2016;27(8):1137–47.
Ghaith AF, Radwan MH, Rashed Taha M, Elbendary MA, Al Damhogy ME, Hagras AM. Evaluation of pain and quality of life after hyaluronic acid instillation in addition to botulinum toxin-A injection in women with refractory Interstitial Cystitis/Painful Bladder Syndrome: A pilot study. Arch Ital Urol Androl. 2022;94(4):447–50.
Pyo JS, Cho WJ. Systematic Review and Meta-Analysis of Intravesical Hyaluronic Acid and Hyaluronic Acid/Chondroitin Sulfate Instillation for Interstitial Cystitis/Painful Bladder Syndrome. Cell Physiol Biochem. 2016;39(4):1618–25.
Gülpınar Ö, Esen B, Kayış A, Gökçe Mİ, Süer E. Clinical comparison of intravesical hyaluronic acid and chondroitin sulfate therapies in the treatment of bladder pain syndrome/interstitial cystitis. Neurourol Urodyn. 2018;37(1):257–62.
Özkıdık M. Assessment of long-term intravesical hyaluronic acid, chondroitin sulfate and combination therapy for patients with bladder pain syndrome. Cent European J Urol. 2019;72(3):270–5.
Smit SG, Heyns CF. Management of radiation cystitis. Nat Rev Urol. 2010;7(4):206–14.
Marks LB, Carroll PR, Dugan TC, Anscher MS. The response of the urinary bladder, urethra, and ureter to radiation and chemotherapy. Int J Radiat Oncol Biol Phys. 1995;31(5):1257–80.
Do V, Choo R, Deboer G, Herschorn S, Danjoux C, Chen CH, Barak I. Urodynamic findings 3 months after radiotherapy in patients treated with conformal external beam radiotherapy for prostate carcinoma. BJU Int. 2002;90(1):62–7.
Levenback C, Eifel PJ, Burke TW, Morris M, Gershenson DM. Hemorrhagic cystitis following radiotherapy for stage Ib cancer of the cervix. Gynecol Oncol. 1994;55(2):206–10.
Blaivas JG, Weiss JP, Jones M. The pathophysiology of lower urinary tract symptoms after brachytherapy for prostate cancer. BJU Int. 2006;98(6):1233–7.
Lin HH, Sheu BC, Lo MC, Huang SC. Abnormal urodynamic findings after radical hysterectomy or pelvic irradiation for cervical cancer. Int J Gynaecol Obstet. 1998;63(2):169–74.
Parkin DE, Davis JA, Symonds RP. Urodynamic findings following radiotherapy for cervical carcinoma. Br J Urol. 1988;61(3):213–7.
Lobo N, Kulkarni M, Hughes S, Nair R, Khan MS, Thurairaja R. Urologic complications following pelvic radiotherapy. Urology. 2018;122:1–9.
Martinez Rodriguez RH, Bayona Arenas S, Ibarz SL. Tratamiento con ácido hialurónico de la hematuria asociada a cistopatía rádica [Hyaluronic acid instillation as treatment of haematuria due to radiation induced cystitis]. Med Clin (Barc). 2014;143(5):230–1.
Sommariva ML, Sandri SD, Ceriani V. Efficacy of sodium hyaluronate in the management of chemical and radiation cystitis. Minerva Urol Nefrol. 2010;62(2):145–50.
Shao Y, Lu GL, Shen ZJ. Comparison of intravesical hyaluronic acid instillation and hyperbaric oxygen in the treatment of radiation-induced hemorrhagic cystitis. BJU Int. 2012;109(5):691–4.
Nordling J, van Ophoven A. Intravesical glycosaminoglycan replenishment with chondroitin sulphate in chronic forms of cystitis. A multi-national, multi-centre, prospective observational clinical trial. Arzneimittelforschung. 2008;58(7):328–35.
Baboudjian M, Fourmarier M, Clement C, Cherasse A, Graziana JP, Bentaleb Y, Rouscoff Y, Ducrocq S, Gondran-Tellier B, Saussine C. Intravesical instillation of high molecular weight sodium hyaluronate in radiation-induced cystitis: a prospective pilot study. World J Urol. 2022;40(1):141–6.
Gacci M, Saleh O, Giannessi C, Detti B, Livi L, Monteleone Pasquetti E, Masoni T, Finazzi Agro E, Li Marzi V, Minervini A, Carini M, Gravas S, Oelke M, Serni S. Sodium hyaluronate and chondroitin sulfate replenishment therapy can improve nocturia in men with post-radiation cystitis: results of a prospective pilot study. BMC Urol. 2015;7(15):65.
Gacci M, Saleh O, Giannessi C, Chini T, Della Camera PA, Detti B, Livi L, Finazzi Agro E, Li Marzi V, Minervini A, Carini M, Oelke M, Gravas S, Serni S. Bladder Instillation Therapy With Hyaluronic Acid and Chondroitin Sulfate Improves Symptoms of Postradiation Cystitis: Prospective Pilot Study. Clin Genitourin Cancer. 2016;14(5):444–9.
Sommariva ML, Lazzeri M, Abrate A, Guazzoni G, Sandri S, Montorsi F. Intravesical Hyaluronic Acid and Chondroitin Sulphate Improve Symptoms and Quality of Life in Patients with Late Radiation Tissue Cystitis: An Investigative Pilot Study. Eur J Inflam. 2014;12:177–85.
Sanguedolce F, Meneghetti I, Bevilacqua G, Montaño B, Martínez C, Territo A, Balaña J, Palou J, Breda A. Intravesical instillation with glycosaminoglycan replacement treatment in patients suffering radiation-induced haemorrhagic cystitis: When and which patients can benefit most from it? Urol Oncol. 2022;40(7):344.e19-344.e25.
Hazewinkel MH, Stalpers LJ, Dijkgraaf MG, Roovers JP. Prophylactic vesical instillations with 0.2% chondroitin sulfate may reduce symptoms of acute radiation cystitis in patients undergoing radiotherapy for gynecological malignancies. Int Urogynecol J. 2011;22(6):725–30.
Palou Redorta J, Sanguedolce F, Sancho Pardo G, Romancik M, Vittori G, Minervini A, Di Maida F, Lunik R, Colombo R, Serretta V, Çetinel B, Bini V, Corradengo D, Lazzeri M. Multicentre International Study for the Prevention with iAluRil of Radio-induced Cystitis (MISTIC): A Randomised Controlled Study. Eur Urol Open Sci. 2021;26:45–54.
King GK, Goodes LM, Hartshorn C, Thavaseelan J, Jonescu S, Watts A, Rawlins M, Woodland P, Synnott EL, Barrett T, Hayne D, Boan P, Dunlop SA. Intravesical hyaluronic acid with chondroitin sulphate to prevent urinary tract infection after spinal cord injury. J Spinal Cord Med. 2022;6:1–7.
Cicek N, Yildiz N, Alpay H. Intravesical hyaluronic acid treatment in recurrent urinary tract infections in children with spina bifida and neurogenic bladder. J Pediatr Urol. 2020;16(3):366.e1-366.e5.
Dinh A, Duran C, Hamami K, Afif M, Bonnet F, Donay JL, Lafaurie M, Chartier-Kastler E. Hyaluronic Acid and Chondroitin Sulphate Treatment for Recurrent Severe Urinary Tract Infections due to Multidrug-Resistant Gram-Negative Bacilli in a Patient With Multiple Sclerosis: Case Report and Literature Review. Open Forum Infect Dis. 2022;9(7):ofac245.
Topazio L, Miano R, Maurelli V, Gaziev G, Gacci M, Iacovelli V, Finazzi-Agrò E. Could hyaluronic acid (HA) reduce Bacillus Calmette-Guérin (BCG) local side effects? Results of a pilot study. BMC Urol. 2014;13(14):64.
Imperatore V, Creta M, Di Meo S, Buonopane R, Longo N, Fusco F, Spirito L, Imbimbo C, Mirone V. Intravesical administration of combined hyaluronic acid and chondroitin sulfate can improve symptoms in patients with refractory bacillus Calmette-Guerin-induced chemical cystitis: Preliminary experience with one-year follow-up. Arch Ital Urol Androl. 2018;90(1):11–4.
Torella M, Del Deo F, Grimaldi A, Iervolino SA, Pezzella M, Tammaro C, Gallo P, Rappa C, De Franciscis P, Colacurci N. Efficacy of an orally administered combination of hyaluronic acid, chondroitin sulfate, curcumin and quercetin for the prevention of recurrent urinary tract infections in postmenopausal women. Eur J Obstet Gynecol Reprod Biol. 2016;207:125–8.
Schiavi MC, Porpora MG, Vena F, Prata G, Sciuga V, D’Oria O, Di Tucci C, Savone D, Aleksa N, Giannini A, Nusiner MP, Zullo MA, Muzii L, Benedetti PP. Orally Administered Combination of Hyaluronic Acid, Chondroitin Sulfate, Curcumin, and Quercetin in the Prevention of Postcoital Recurrent Urinary Tract Infections: Analysis of 98 Women in Reproductive Age After 6 Months of Treatment. Female Pelvic Med Reconstr Surg. 2019;25(4):309–12.
Manfredi C, Spirito L, Calace FP, Balsamo R, Terribile M, Stizzo M, Romano L, Napolitano L, Califano G, Cirillo L, Fusco GM, Rosati C, Quattrone C, Sciorio C, Creta M, Longo N, De Sio M, Arcaniolo D. Oral Preparation of Hyaluronic Acid, Chondroitin Sulfate, Curcumin, and Quercetin (Ialuril® Soft Gels) for the Prevention of LUTS after Intravesical Chemotherapy. Pathophysiology. 2022;29(3):365–437.
Acknowledgements
Medical Writing/Editorial Assistance
The authors thank Proper Medical Writing for support with preparation of the manuscript.
Funding
The publication fees for this article were paid by IBSA.
Author information
Authors and Affiliations
Contributions
Sławomir Poletajew: concept, literature review, manuscript preparation, manuscript submission. Magdalena M. Brzózka: concept, manuscript preparation. Wojciech Krajewski: literature review, manuscript preparation. Hubert Kamecki: literature review, manuscript preparation. Łukasz Nyk: manuscript preparation, critical review. Piotr Kryst: manuscript preparation, critical review.
Corresponding author
Ethics declarations
Conflict of interest
Sławomir Poletajew is consultant to IBSA. Magdalena M. Brzózka is employee of IBSA Poland. Wojciech Krajewski has nothing to disclose. Hubert Kamecki has nothing to disclose. Łukasz Nyk has nothing to disclose. Piotr Kryst has nothing to disclose.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Poletajew, S., Brzózka, M.M., Krajewski, W. et al. Glycosaminoglycan Replacement Therapy with Intravesical Instillations of Combined Hyaluronic Acid and Chondroitin Sulfate in Patients with Recurrent Cystitis, Post-radiation Cystitis and Bladder Pain Syndrome: A Narrative Review. Pain Ther 13, 1–22 (2024). https://doi.org/10.1007/s40122-023-00559-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-023-00559-1