Abstract
Introduction
Helping Opioid Prescription Elimination (HOPE) is a project designed to provide surgeons with practical, real-world solutions to effectively manage postoperative pain and eliminate the need for opioids using HTX-011 (extended-release bupivacaine/low-dose meloxicam). In phase 3 herniorrhaphy and bunionectomy studies, HTX-011 without multimodal analgesia (MMA) was superior to bupivacaine hydrochloride in reducing pain and opioid consumption. Here, we examine the HOPE Hernia-1 study, which was designed to compare alternating ibuprofen/acetaminophen with concurrent use as part of an HTX-011-based non-opioid MMA regimen in patients undergoing herniorrhaphy and to evaluate the effectiveness of a personalized opioid prescription algorithm.
Methods
Patients undergoing outpatient open inguinal herniorrhaphy with intraoperative administration of HTX-011 (300 mg bupivacaine/9 mg meloxicam) were randomly assigned to receive a scheduled oral regimen of ibuprofen plus acetaminophen, either taken together every 6 hours or alternating every 3 hours, for 5 days following surgery, while awake. Based on the opioid prescription algorithm evaluated here, patients could receive an oxycodone prescription upon discharge only if they had a numeric rating scale pain score of ≥ 6 at discharge and/or had received a postoperative rescue opioid.
Results
The majority of patients did not require an opioid prescription through 2 weeks following surgery, and this was similar between cohorts (alternating MMA, 89.1%; concurrent MMA, 93.6%). Patient satisfaction was high for both regimens, and 95% of patients had an opioid-free recovery. No patient discharged without a prescription called back to request one. Treatment was well tolerated, without evidence of nonsteroidal anti-inflammatory drug-related toxicity.
Conclusions
HTX-011, used with over-the-counter products ibuprofen/acetaminophen and personalized opioid prescription algorithm in a real-world environment, has the potential to reduce opioid use and opioid prescriptions after herniorrhaphy without compromising patient satisfaction.
Trial Registration
ClinicalTrials.gov, NCT03237481.
Similar content being viewed by others
Effective, easy-to-use analgesia regimens are needed to effectively manage postoperative pain while minimizing the need for opioids. |
HTX-011 combines bupivacaine and meloxicam in an extended-release polymer, allowing for sustained analgesia up to 72 hours. |
This study, HOPE Hernia-1, compared two HTX-011-containing, non-opioid multimodal analgesia regimens—concurrent versus alternating ibuprofen and acetaminophen—following herniorrhaphy. |
Both regimens resulted in similarly high patient satisfaction and minimized the need for opioids, with 95% of patients experiencing an opioid-free recovery. |
Introduction
Worldwide, herniorrhaphy is a common surgery, with more than 20 million procedures performed each year [1]; 800,000 inguinal hernias are repaired annually in the United States [2, 3]. Despite the increased use of non-opioid multimodal analgesia (MMA) and successful efforts to decrease opioid prescribing, current evidence indicates that opioid medications are still overprescribed after common general surgical procedures, including herniorrhaphy, with approximately 70% of tablets going unused by patients [2,3,4,5,6]. Opioids and their overprescribing in the postoperative setting are associated with adverse events (AEs) and the potential for long-term dependence and diversion [7]. The goals of effective postoperative pain management are to adequately manage pain, minimize opioid consumption, and appropriately identify patients who require opioid prescriptions at discharge. Unfortunately, effective non-opioid therapies for postoperative pain are limited, and there is a lack of personalized treatment algorithms to identify patients who can be appropriately discharged without an opioid prescription.
HTX-011 (ZYNRELEF™) combines bupivacaine and low-dose meloxicam in a proprietary Biochronomer® polymer allowing for controlled release of the active ingredients over 72 hours, providing enhanced and sustained analgesia [8,9,10]. HTX-011 is administered via needle-free application into the surgical site prior to wound closure. Bupivacaine is the active ingredient in HTX-011; low-dose meloxicam, a nonsteroidal anti-inflammatory drug (NSAID), increases the ability of bupivacaine to penetrate nerves and enhances its analgesic effect by decreasing local inflammation, which normalizes the pH surrounding the surgical site. The synergistic mechanism of action overcomes the acidic environment that can limit the duration of effects of other local anesthetics after surgery.
The efficacy and safety of HTX-011 have been established throughout the HTX-011 clinical program, in which more than 1000 patients received HTX-011 across a variety of surgical procedures. In a double-blind, active- and placebo-controlled, randomized phase 3 study (EPOCH-2, NCT03237481) HTX-011 alone (i.e., without MMA) demonstrated superior pain relief and reduced opioid consumption after herniorrhaphy, with a similar safety profile compared with both bupivacaine hydrochloride and placebo [9]. In the EPOCH-2 single-arm follow-on study (NCT03695367), the use of HTX-011 as the foundation of a scheduled, non-opioid, over-the-counter (OTC) MMA regimen of alternating acetaminophen and ibuprofen resulted in 83% of patients having an opioid-free recovery through 28 days after surgery [11]. A retrospective analysis identified that all patients who required opioids had a numeric rating scale (NRS) of pain intensity score of ≥ 6 at 2 hours after surgery and/or received opioid rescue medication within the first 2 hours after surgery. These characteristics were used to develop a personalized opioid prescription algorithm that would be assessed prospectively.
This study, Helping Opioid Prescription Elimination Hernia-1 (HOPE Hernia-1), is part of the HOPE project, which is designed to provide surgeons with practical real-world solutions to effectively manage postoperative pain and eliminate the need for opioid prescriptions using an HTX-011-based non-opioid MMA regimen. Oral ibuprofen and acetaminophen are commonly used after surgery [11,12,13,14,15]; however, there are limited data regarding the optimal schedule for their concomitant use. This study was designed to identify which of two postoperative MMA regimens—concurrent or alternating ibuprofen/acetaminophen—following intraoperative administration of HTX-011 in patients undergoing open inguinal herniorrhaphy, with prospective verification of an opioid prescribing algorithm, would result in a higher proportion of patients not requiring a prescription for postoperative opioids in a real-world setting. Secondary objectives were to assess post-discharge opioid consumption and patient satisfaction.
Methods
Study Design and Treatments
Patients were scheduled to undergo an open inguinal herniorrhaphy with mesh at one of seven centers across the United States. This trial was conducted in accordance with the principles of the Declaration of Helsinki. The study protocol, amendments, and informed consent forms were obtained and approved by Aspire IRB (Santee, CA, USA; reference number 520190073). Aspire IRB approval was obtained for all seven study sites before patients were screened, and patients provided written informed consent prior to any study-related procedures. The study was registered with ClinicalTrials.gov (NCT03907176).
All patients were shown a 5-minute educational video prior to surgery that provided an overview of the study, set expectations for postsurgical pain, gave instructions on the assigned post-discharge MMA regimen, and reviewed potential side effects of ibuprofen, acetaminophen, and oxycodone. All patients received oral ibuprofen 400 mg and acetaminophen 1 g approximately 2 hours before surgery. At the end of surgery, after final irrigation and suction of fascial layers and prior to suturing, all patients received intraoperative HTX-011 (300 mg bupivacaine/9 mg meloxicam) administered via needle-free application into the surgical site.
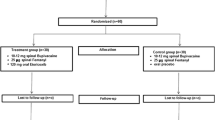
Patients were randomly assigned to one of two parallel cohorts with different postoperative non-opioid MMA regimen schedules (Fig. 1) [13]. In cohort 1 (alternating), patients received postoperative oral ibuprofen 600 mg and 3 hours later received oral acetaminophen 1 g. These two medications were alternated such that patients received an analgesic approximately every 3 hours while awake. In cohort 2 (concurrent), patients received the same doses of ibuprofen/acetaminophen taken together every 6 hours while awake. In both cohorts, the first dose of assigned medication was administered once the patient was able to tolerate oral intake. Opioid rescue medication was administered as per institutional standard of care, and patients were discharged as per normal site practice. Postoperative pain was assessed at the time of discharge using an 11-point NRS; 0 represented “no pain” and 10 represented “worst pain imaginable.”
Patients were eligible to receive a discharge prescription for oxycodone (ten 5 mg tablets, no substitutions) only if they reported an NRS score ≥ 6 and/or if they had received a postoperative opioid prior to discharge. Upon discharge, patients were given a medication guide with approximate times for their scheduled MMA doses and were instructed to adhere to them while awake for 5 days following surgery. Patients could continue the MMA medications as needed thereafter. Follow-up visits were scheduled for postoperative day 15 (efficacy, treatment satisfaction, and safety) and day 29 (safety). AEs and serious AEs were monitored through day 29, and clinical safety laboratory tests (hematology and serum chemistry) were conducted at baseline and on day 15.
Patient Population
Adult patients scheduled to undergo a unilateral open inguinal herniorrhaphy with mesh under deep sedation or general anesthesia and who had an American Society of Anaesthesiologists Physical Status classification of I, II, or III were enrolled. Patients were excluded if they had a planned concurrent surgical procedure, had a preexisting painful physical/restrictive condition expected to require analgesic treatment, had known or suspected daily use of opioids for ≥ 7 days within the previous 6 months, or had used any opioids within 24 hours prior to surgery.
Endpoints
The primary endpoint was the proportion of patients who did not receive a postoperative opioid prescription through 2 weeks following surgery. Additional endpoints included the proportion of patients requiring no opioids (opioid-free) through the 2-week follow-up period, proportion of patients not receiving an opioid prescription at discharge, proportion of patients calling back for pain and receiving an opioid prescription, pain intensity at discharge, number of opioid tablets taken between discharge and 2 weeks after surgery (via patient recall), incidence of AEs, and mean nine-item Treatment Satisfaction Questionnaire for Medication (TSQM-9) scores. The TSQM-9 survey is a validated nine-question instrument used to assess patient satisfaction with medication, providing scores for three domains: effectiveness (satisfaction regarding symptom management), convenience (feasibility of regimen), and global satisfaction (benefits outweighing risks) [16]. Each survey question uses either a seven- or a five-point Likert scale. Scale scores are transformed into scores ranging from 0 to 100, with higher scores indicating higher satisfaction.
Statistical Analysis
Patients were randomly assigned to one of two parallel cohorts. Randomization was based on a centralized, computer-generated, blocked randomization algorithm and was stratified by site. The sample size of 45 patients per cohort was selected to provide greater than 80% power to detect a 20% absolute difference between cohorts 1 and 2, assuming the proportions of patients who did not receive a postoperative opioid prescription through the day 15 visit were 10% and 30% in the two groups, respectively (Fisher’s exact test at α = 0.2, two-sided). All data were summarized by each cohort regimen and in totality using descriptive statistics. All patients who received HTX-011 were included in efficacy and safety analyses, and results were summarized using observed cases with no imputation.
Results
Ninety-three patients were randomly assigned 1:1 to the two cohorts (alternating MMA, n = 46; concurrent MMA, n = 47) and were treated with HTX-011. All patients completed the study through the day 15 visit and contributed to the primary endpoint. Nearly all patients (92/93, 98.9%) completed the study (Fig. 2). Baseline characteristics were similar between cohorts; most patients were male (98.9%) and white (84.9%), with a mean (standard deviation [SD]) age of 49.5 (11.7) years and body mass index of 28.2 (3.8) kg/m2 (Table 1).
Opioid Prescriptions
The majority of patients (85/93; 91.4%) did not receive an opioid prescription at discharge or at any time through 2 weeks after surgery, and the results were similar between cohorts (alternating MMA, 89.1%; concurrent MMA, 93.6%). Not all patients who received an opioid prescription at discharge took opioids. Overall, 94.6% of patients were opioid-free through the 2-week follow-up period: 91.3% and 97.9% for the alternating and concurrent regimens, respectively (Fig. 3). There were no callbacks from patients who were discharged without an opioid prescription, and all of those patients remained opioid-free throughout the 2-week follow-up period.
Pain Intensity and Discharge Time
The mean (SD) discharge time following surgery was 2.6 (2.0) hours in the alternating MMA cohort and 2.2 (1.6) hours in the concurrent MMA cohort. At discharge, the mean (SD) NRS pain scores were 2.3 (1.5) and 2.9 (1.7) in the alternating and concurrent MMA cohorts, respectively (Table 2). The majority of patients (76.3%) reported mild pain (NRS score of < 4), 22.6% of patients reported moderate pain (NRS score of 4–6), and one patient reported severe pain (NRS score of 7). Results were similar between cohorts.
Treatment Satisfaction
Of the 98% of patients who completed the TSQM-9, most patients were “very” or “extremely” satisfied with their MMA regimen. Results between the two cohorts were similar. On a scale of 0 to 100, in which higher numbers indicate higher satisfaction, mean TSMQ-9 scores for alternating versus concurrent MMA schedules, respectively, were 84.5 versus 79.3 for effectiveness, 89.3 versus 86.3 for convenience, and 85.6 versus 82.1 for global satisfaction (Table 2).
Personalized Opioid Prescription Algorithm
Of the eight patients who received an opioid prescription at discharge, seven patients met the algorithm-specified criteria: five patients received postoperative opioids prior to discharge and two patients had an NRS score of ≥ 6 at discharge. One patient who did not meet the algorithm-specified criteria was prescribed an opioid due to “normal site practice” (surgical site was closing for a long weekend); this patient did not take any opioids.
Of the seven patients who were prescribed opioids per the algorithm at discharge, four reported not taking any opioids. The three patients (alternating regimen, 2; concurrent regimen, 1) who required opioids took 10, 10, and 13 oxycodone 5 mg tablets, respectively, through 2 weeks after surgery.
Safety
HTX-011 plus acetaminophen and ibuprofen was well tolerated; safety results were similar between cohorts. The most common AEs were nausea (10.9% vs. 14.9%, for alternating vs. concurrent MMA, respectively), constipation (2.2% vs. 8.5%), and vomiting (6.5% vs. 4.3%) (Table 3). A total of three (6.4%) patients in the concurrent MMA cohort experienced an AE considered by the investigator to be possibly related to HTX-011: one (2.1%) tinnitus, one (2.1%) constipation, and one (2.1%) nausea. No serious AEs or severe AEs were reported, and no AEs led to study withdrawal. There was no evidence of local anesthetic systemic toxicity or NSAID-related toxicity. There were no clinically meaningful changes from baseline in laboratory test results, including no clinically meaningful trends in serum creatinine.
Discussion
Acetaminophen and ibuprofen are commonly used components of MMA regimens [13, 17]; however, there are limited data comparing dosing schedules. To our knowledge, this is the first study to evaluate the effectiveness of, and patient satisfaction with, the use of alternating versus concurrent ibuprofen/acetaminophen in an adult postoperative setting. In this study involving patients undergoing open inguinal herniorrhaphy with HTX-011, the alternating and the concurrent MMA regimens were similarly effective in managing postoperative pain with minimal use of opioids.
HTX-011, used with a scheduled non-opioid MMA regimen plus an opioid prescription algorithm for identifying individual patients who might require a discharge opioid prescription, enabled more than 90% of patients to be discharged without an opioid prescription, with no callbacks for pain management, and 95% of patients to recover opioid-free. The results of this study are similar to those observed in the EPOCH-2 single-arm follow-on study, in which 87% of patients who received HTX-011 and scheduled (alternating) acetaminophen/ibuprofen were managed opioid-free through postoperative day 10 [11]. The results of the HOPE Hernia-1 study demonstrate the reproducibility of HTX-011 clinical trial data and prospectively confirm the clinical utility of a simple personalized algorithm to guide opioid prescribing in a real-world setting. Unlike EPOCH-2 and the EPOCH-2 single-arm follow-on study, in which patients were required per protocol to stay in the hospital for 72 hours, patients in the current study were discharged at a time determined by the investigator, which was an average of 2–3 hours after surgery.
Opioids are commonly used for postoperative pain but can result in opioid-related AEs and potential long-term dependence. As such, opioids should only be prescribed when necessary and at the lowest possible effective dose [18]. Substantial progress has been made over the past 5 years regarding opioid-sparing postoperative protocols and decreased discharge prescription opioid quantities. Current literature recommends a discharge prescription range of 0 to 15 oxycodone (5 mg) pills for inguinal herniorrhaphy [2, 6, 18,19,20], with growing evidence that many patients may require at most only three pills [2, 6, 14, 21, 22]. In addition, limits on duration and/or doses of opioids have been enacted by state programs, large payers, and pharmacy chains and benefit managers [23].
Although this progress is notable, recent evidence suggests that clinical practice is lagging and opioids are still overprescribed after inguinal herniorrhaphy, possibly because of the inability to accurately predict which patients will need opioid-level analgesia. A retrospective study of administrative health claims from 60,000 patients undergoing inguinal hernia repair identified a median of approximately 210 oral morphine equivalents that were prescribed preoperatively (for use postoperatively), which equates to roughly 28 oxycodone pills [3]. A recent meta-analysis of 44 studies in adult surgical populations estimated a total of 2,909,744 prescribed morphine milligram equivalents for 13,068 patients, with 61% of prescribed pills left over. This translates to an average of approximately 30 prescribed oxycodone (5 mg) pills per patient, with 18 unused [4]. In addition, the initial results of opioid duration and/or dose limits by states and organizations have indicated that these programs have not resulted in decreased opioid prescribing for acute pain [23]. Procedure-specific opioid prescribing recommendations and identification of risk factors for increased opioid consumption could help to avoid opioid overprescribing; however, additional guidance is needed for the individual patient [14, 19].
The use of scheduled non-opioid MMA alone for inguinal hernia repair (open and laparoscopic), based on current literature, results in approximately 15–64% of patients recovering opioid-free [2, 3, 6, 12, 14, 22]. Though the primary objective of the current study was not to assess the impact of HTX-011 on the rate of opioid-free patients, the use of HTX-011 in combination with scheduled non-opioid MMA resulted in 95% of patients overall with an opioid-free recovery through the 15-day follow-up period. Use of HTX-011 as the foundation of non-opioid MMA, in conjunction with an algorithm that distinguishes between patients who need or do not need a discharge opioid prescription, could further increase the proportion of opioid-free patients, decrease the amount of opioids required per patient, and decrease the total number of opioid pills prescribed. In combination, this could potentially result in several million fewer unused opioid tablets available for misuse or diversion.
Both MMA regimens resulted in high levels of patient satisfaction in all three domains (effectiveness, convenience, and global satisfaction) of the validated TSQM-9 survey (Table 2). Because similar efficacy, tolerability, and high patient satisfaction were demonstrated in the two cohorts, applying these findings to clinical practice could allow the patient and provider to determine the preferred MMA schedule, which could improve convenience and medication adherence. Increasing patient autonomy in medical decision-making has been shown to increase patient satisfaction [24]. In the second part of the HOPE Hernia study (HOPE Hernia-2), currently ongoing, the alternating or concurrent MMA schedule is selected for each patient by the investigator, and may reflect patient preference [25].
We formulated an algorithm to guide discharge opioid prescriptions for individual patients based on an analysis of the EPOCH-2 single-arm follow-on study, which revealed that patients who required postoperative opioids had an NRS pain score of ≥ 6 at 2 hours after surgery and/or received an opioid rescue medication within the first 2 hours after surgery. In the current study, we prospectively evaluated the effectiveness of the algorithm in a real-world setting. Application of the algorithm successfully differentiated patients who needed an opioid prescription from those who did not, enabling more than 90% of patients to be discharged without an opioid prescription. The algorithm was not overly restrictive; of the seven patients who met algorithm-specified criteria and received a discharge opioid prescription, four did not take any opioids after discharge. More importantly, the algorithm did not wrongly withhold an opioid prescription; there were no callbacks for pain management from anyone discharged without a prescription, and no patients discharged without a prescription reported taking opioids from any other source.
Though decreasing opioid use can decrease opioid-related AEs, the scheduled use of acetaminophen and NSAIDs, especially long-term use, is also not without risk. In single-arm follow-on studies involving HTX-011 as the foundation of non-opioid MMA, coadministration of HTX-011 with scheduled NSAIDs and acetaminophen for a minimum of 3 days did not impact safety; there was no evidence of NSAID- or acetaminophen-related toxicity based on treatment-emergent AEs or clinical laboratory test results [11, 15]. The current study confirmed these findings for a 5-day MMA regimen. Although these data provide confidence in the safety of real-world use of HTX-011 with scheduled acetaminophen and ibuprofen, clinicians should not dismiss the common drug-class–related AEs for these OTC medications.
The 2019 American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on Perioperative Opioid Minimization in Opioid-Naive Patients recommends that clinicians provide patients with individually tailored education, including information about postoperative pain management [26]. In this study, before surgery, all patients were shown a short educational video describing their assigned MMA protocol. We believe that patient education is a key component to setting appropriate expectations regarding postoperative pain and ensuring adherence to a scheduled non-opioid MMA regimen after discharge.
This study is not without limitations. In the HOPE Hernia-1 study, the alternating and concurrently administered acetaminophen and ibuprofen regimens were similarly effective; as such, the study may have been underpowered to show a statistically significant difference between the two regimens. However, these results indicate that alternating or concurrent administration of ibuprofen and acetaminophen appear to offer similar clinical benefit.
This study did not contain a non-HTX-011 control arm, for two reasons: first, the superiority of HTX-011 compared with bupivacaine hydrochloride in pain reduction and reduced opioid consumption was previously established in the phase 3 EPOCH-2 study [9], and second, the primary objective was to determine which of two postoperative non-opioid (MMA) regimens, with intraoperative administration of HTX-011 as the foundation, would result in a higher proportion of patients not requiring a prescription for opioid medication following unilateral open inguinal herniorrhaphy.
A wide variety of non-opioid MMA regimens are commonly used in clinical practice, so it is not known whether use of a different non-opioid MMA regimen could have affected the results presented herein. However, because MMA regimens comprising the two most commonly used OTC analgesics were evaluated, the results should be applicable to a large number of patients [13].
Lastly, although this study provided evidence of the feasibility of a personalized opioid prescription algorithm to identify which patients required or did not require an opioid prescription, the algorithm was used only with HTX-011 as the foundation of non-opioid MMA. Because of the sustained pain control provided by HTX-011 over 72 hours, applicability of the algorithm to regimens not containing HTX-011 is not known. Future research into HTX-011 with additional non-opioid MMA in other surgical models and confirmation of the generalizability of the algorithm, as well as exploration of different algorithms, are desirable.
Conclusions
Efficacy, safety, and patient satisfaction were similar between alternating and concurrent acetaminophen/ibuprofen non-opioid MMA regimens following intraoperative administration of HTX-011 in patients undergoing open inguinal herniorrhaphy. Treatment with HTX-011 and either of the scheduled non-opioid OTC MMA regimens, coupled with a personalized opioid prescription algorithm, minimized and even eliminated the use of opioids. In this study, 90% of patients were discharged without an opioid prescription and did not call back for pain management, and 95% of patients reported an opioid-free recovery. Applied to clinical practice, the simple opioid prescription algorithm would allow the surgeon to confidently identify patients unlikely to require postoperative opioids, and has the potential to reduce the number of unnecessary opioid prescriptions while ensuring that patients have appropriate pain control. The results of this study suggest that use of HTX-011 as the foundation of a non-opioid MMA regimen, combined with a personalized opioid prescription algorithm, could transform opioid prescribing habits, avoiding unnecessary prescriptions and unused pills, to help stem the current opioid crisis.
References
Simons MP, Smietanski M, Bonjer HJ, et al. International guidelines for groin hernia management. Hernia. 2018;22(1):1–165.
Knight AW, Habermann EB, Ubl DS, Zielinski MD, Thiels CA. Opioid utilization in minimally invasive versus open inguinal hernia repair. Surgery. 2019;166(5):752–7.
Howard R, Gunaseelan V, Brummett C, Waljee J, Englesbe M, Telem D. New persistent opioid use after inguinal hernia repair. Ann Surg. 2020. https://doi.org/10.1097/sla.0000000000004560 (ePub ahead of print).
Schirle L, Stone AL, Morris MC, et al. Leftover opioids following adult surgical procedures: a systematic review and meta-analysis. Syst Rev. 2020;9(1):139.
Hill MV, McMahon ML, Stucke RS, Barth RJ Jr. Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Ann Surg. 2017;265(4):709–14.
Mylonas KS, Reinhorn M, Ott LR, Westfal ML, Masiakos PT. Patient-reported opioid analgesic requirements after elective inguinal hernia repair: a call for procedure-specific opioid-administration strategies. Surgery. 2017;162(5):1095–100.
Brummett CM, Waljee JF, Goesling J, Moser S, Lin P, Englesbe MJ, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6):e170504.
Viscusi E, Gimbel JS, Pollack RA, Hu J, Lee GC. HTX-011 reduced pain intensity and opioid consumption versus bupivacaine HCl in bunionectomy: phase III results from the randomized EPOCH 1 study. Reg Anesth Pain Med. 2019;44:700–6.
Viscusi E, Minkowitz H, Winkle P, Ramamoorthy S, Hu J, Singla N. HTX-011 reduced pain intensity and opioid consumption versus bupivacaine HCl in herniorrhaphy: results from the phase 3 EPOCH 2 study. Hernia. 2019;23:1071–80.
Ottoboni T, Quart B, Pawasauskas J, Dasta JF, Pollak RA, Viscusi ER. Mechanism of action of HTX-011: a novel, extended-release, dual-acting local anesthetic formulation for postoperative pain. Reg Anesth Pain Med. 2020;45:117–23.
Singla N, Winkle P, Bertoch T, Hu J, Beaton A, Redan J. Opioid-free recovery after herniorrhaphy with HTX-011 as the foundation of a multimodal analgesic regimen. Surgery. 2020;168(5):915–20.
Hallway A, Vu J, Lee J, Palazzolo W, Waljee J, Brummett C, et al. Patient satisfaction and pain control using an opioid-sparing postoperative pathway. J Am Coll Surg. 2019;229(3):316–22.
Ong CK, Seymour RA, Lirk P, Merry AF. Combining paracetamol (acetaminophen) with nonsteroidal antiinflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg. 2010;110(4):1170–9.
Millard JL, Moraney R, Childs JC, Ewing JA, Carbonell AM, Cobb WS, et al. Opioid use after inguinal and ventral hernia repair. Am Surg. 2020;86(8):965–70.
Pollak R, Cai D, Gan TJ. Opioid-free recovery from bunionectomy with HTX-011, a dual-acting local anesthetic combining bupivacaine and meloxicam, as the foundation of non-opioid multimodal analgesia. J Am Podiatr Med Assoc. 2021. https://doi.org/10.7547/20-204 (ePub ahead of print).
Bharmal M, Payne K, Atkinson MJ, Desrosiers MP, Morisky DE, Gemmen E. Validation of an abbreviated Treatment Satisfaction Questionnaire for Medication (TSQM-9) among patients on antihypertensive medications. Health Qual Life Outcomes. 2009;7:36.
Beverly A, Kaye AD, Ljungqvist O, Urman RD. Essential elements of multimodal analgesia in Enhanced Recovery After Surgery (ERAS) guidelines. Anesthesiol Clin. 2017;35(2):e115–43. https://doi.org/10.1016/j.anclin.2017.01.018.
Overton HN, Hanna MN, Bruhn WE, Hutfless S, Bicket MC, Makary MA. Opioid-prescribing guidelines for common surgical procedures: an expert panel consensus. J Am Coll Surg. 2018;227(4):411–8.
Michigan Opioid Prescribing Engagement Network. Prescribing recommendations. 2021. https://michigan-open.org/prescribing-recommendations/. Accessed 3 May 2021.
Thiels CA, Ubl DS, Yost KJ, Dowdy SC, Mabry TM, Gazelka HM, et al. Results of a prospective, multicenter initiative aimed at developing opioid-prescribing guidelines after surgery. Ann Surg. 2018;268(3):457–68.
Linnaus ME, Neville MR, Habermann EB, Gray RJ. Postoperative opioid utilization and patient satisfaction in general surgery procedures: A prospective observational study. Am Surg. 2021. https://doi.org/10.1177/0003134821989040 (ePub ahead of print).
Anderson M, Hallway A, Brummett C, Waljee J, Englesbe M, Howard R. Patient-reported outcomes after opioid-sparing surgery compared with standard of care. JAMA Surg. 2021;156(3):286–7.
Chua K-P, Kimmel L, Brummett CM. Disappointing early results from opioid prescribing limits for acute pain. JAMA Surg. 2020;155(5):375–6.
Hill AE, Smith CV, Hadden BW. Autonomy in the obstetrician/gynecologist-patient relationship as a predictor of patient satisfaction. Yale J Biol Med. 2013;86(2):179–88.
US National Library of Medicine: Herniorrhaphy Study for Opioid Elimination. 2021. https://clinicaltrials.gov/ct2/show/NCT03907176 Accessed 3 May 2021.
Wu CL, King AB, Geiger TM, et al. American society for enhanced recovery and perioperative quality initiative joint consensus statement on perioperative opioid minimization in opioid-naïve patients. Anesth Analg. 2019;129(2):567–77.
Acknowledgements
We thank the patients, investigators, and study site staff for their valuable contributions to this work.
Funding
This study was funded by Heron Therapeutics (San Diego, CA, USA). Sponsorship for this study and the journal’s Rapid Service Fee were funded by Heron Therapeutics.
Editorial and Other Assistance
Editorial and submission assistance in the preparation of this article was provided by Katie Luepke, PharmD, BCPS, of Heron Therapeutics, and ApotheCom. Support for this assistance was funded by Heron Therapeutics.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Jia Hu. The first draft of the manuscript was written by Katie Luepke, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
List of Investigators
Harold Minkowitz, HD Research Group, 2001 Hermann Dr, Houston, TX 77,004, USA.
Prior Presentation
These findings were presented in part at annual or semi-annual meetings of the American Society of Health-System Pharmacists (Las Vegas, NV; December 8–12, 2019) and Americas Hernia Society (Virtual meeting; September 25–26, 2020).
Disclosures
Harold Minkowitz reports grants and personal fees from Acacia Pharmaceuticals, AcelRx, Durect, Innocoll, Heron Therapeutics, and Pacira BioSciences; and grants from Concentric Analgesics, Recro, and Trevena during the conduct of the study. Roy Soto reports consulting fees from Heron Therapeutics outside the submitted work. John Fanikos reports nonfinancial editorial support from Heron Therapeutics and other fees from Alexion, Allergan, AstraZeneca, Boehringer Ingelheim, Pacira BioSciences, Pfizer, and Portola outside the submitted work. Gregory B. Hammer has nothing to disclose. Neel Mehta reports personal fees from Heron Therapeutics during the conduct of the study. Jia Hu reports personal fees from Heron Therapeutics during the conduct of the study and outside the submitted work and is an employee of Heron Therapeutics. Jay Redan reports consulting fees from Heron Therapeutics outside the submitted work.
Compliance with Ethics Guidelines
This trial was conducted in accordance with the principles of the Declaration of Helsinki of 1964 and its later amendments. The study protocol, amendments, and informed consent form were approved by Aspire IRB (Santee, CA, USA; reference number 520190073) for all seven study sites. Informed consent forms were obtained from investigational sites before patients were screened, and patients provided written informed consent prior to any study-related procedures.
Data Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Minkowitz, H., Soto, R., Fanikos, J. et al. Opioid-Free Recovery After Hernia Repair with HTX-011 as the Foundation of a Non-Opioid, Multimodal Analgesia Regimen in a Real-World Setting: A Randomized, Open-Label Study. Pain Ther 10, 1295–1308 (2021). https://doi.org/10.1007/s40122-021-00289-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-021-00289-2