Abstract
Introduction
Acquired amyloid neuropathy is an iatrogenic disease that appears years after a domino liver transplant. The objectives of our study are to analyze the efficacy and tolerability of tafamidis for the treatment of acquired amyloid neuropathy in domino liver transplant recipients. This post-authorization, prospective, longitudinal study included seven domino liver transplant recipients with acquired amyloid neuropathy who received treatment with tafamidis for 18 months.
Methods
The primary endpoints were the response rate, defined as those patients with an increase of < 2 points on the Neurological Impairment Score (NIS) from baseline, and the change in the NIS score from baseline. Secondary endpoints included the Quantitative Sensory Test, 10-m walk test, quality of life (Norfolk), and disability (Rasch-built Overall Disability Scale). As safety parameters, the evidence of graft rejection, changes in immunosuppressive trough levels and changes in antiviral and allogeneic cellular immunity before and 12 months after tafamidis treatment were also assessed.
Results
Six patients (85.7%) had responded at 18-months. Compared to baseline, we observed non-statistically significant improvement in mean NIS score at 6 months (− 2.54 points, CI − 5.92 to 0.84), 12 months (− 3.25 points; CI − 6.63 to 0.13), and 18 months (− 2.35 points; CI − 5.74 to 1.02). Changes in the Quantitative Sensory Test, 10-m walk tests and the quality of life and disability questionnaires were not statistically significant. The use of tafamidis did not induce relevant side effects or drug interactions. Also, no acute rejections events nor changes in functional adaptive immunity were observed.
Conclusion
Our study supports the safety and tolerability of tafamidis for the treatment of acquired amyloid neuropathy in domino liver transplant recipients. Tafamidis shows promise as a useful treatment in the clinical management of these patients. Future randomized placebo-controlled clinical trials with longer follow-up durations are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
There is no medical treatment for acquired amyloid neuropathy in domino liver transplant recipients. |
Medical treatments for hereditary transthyretin amyloidosis may be useful for acquired amyloid neuropathy in domino liver transplant recipients. |
Tafamidis treatment was safe and well-tolerated in our patients with domino liver transplant and acquired amyloid neuropathy. |
Tafamidis shows promise as a useful treatment in the clinical management of domino liver transplant with acquired amyloid neuropathy. |
Introduction
Hereditary transthyretin amyloidosis (hATTR) is caused by mutations in the transthyretin (TTR) gene, encoding an unstable protein that triggers the multisystemic deposition of amyloid aggregates. The extracellular deposition of amyloid in the somatic and autonomic peripheral nervous systems produces familial amyloid neuropathy (FAP), which is the main cause of disability [1].
Given that 95% of TTR production occurs in the liver, the main therapeutic strategy for these patients is orthotopic liver transplantation [2]. The livers in patients with hATTR are otherwise functionally and morphologically healthy, making them suitable for domino liver transplantation (DLT) in patients with hepatic failure [3]. Once in the DLT recipient, the liver graft continues to synthesize mutated TTR and over time can lead to acquired amyloid polyneuropathy (AAN) [4,5,6,7,8,9,10,11,12]. When the manifestations of the disease appear, liver retransplantation can be considered as a strategy to halt disease progression [4, 5], but this is often not viable due to technical reasons or patient comorbidity. However, medical treatments have emerged over the last decade as an alternative to orthotopic liver transplantation in patients with hATTR and FAP. These treatments aim to stabilize the TTR monomers (e.g., diflunisal and tafamidis) or to inhibit TTR synthesis (e.g., inotersen, patisiran, vutrisiran, eplontersen) [13,14,15,16]. Despite patients with AAN due to DLT having the same pathophysiology as patients with FAP due to hATTR, these treatments are not indicated for the treatment of AAN due to DLT.
In the largest series of DLT recipients with AAN, 5 of 7 patients who received diflunisal for 1 year reported neurological worsening of ≥ 2 points on the Neurological Impairment Score (NIS), with side effects contributing to low retention rates [17]. Only isolated case reports exist that describe DLT recipients with AAN undergoing treatment with tafamidis [18,19,20] and patisiran [21]. In 2020, the expert advisory committee for highly complex treatments in Catalonia decided in favor of treating patients with tafamidis or patisiran for AAN after receiving a DLT from donors with hATTR [22]. However, the impact of amyloid neuropathy treatments on adaptive immunity and interaction with immunosupressant treatments remain unclear.
This study describes our experience of the safety and tolerability of tafamidis in a series of seven DLT recipients with AAN treated for 18 months.
Methods
Study Design
We conducted a longitudinal, prospective, post-authorization study of tafamidis used to treat AAN in DLT recipients in a Familial Amyloidosis Multidisciplinary Unit. The study covers an 18-month follow-up period from February 2021 to December 2022. It included DLT recipients without pre-existing polyneuropathy, in whom other causes of polyneuropathy different of amyloid deposits were excluded or corrected. All patients underwent sural nerve biopsy to investigate the presence of amyloid deposits in nerve tissue. The full inclusion and exclusion criteria are detailed in Supplementary Table 1.
Dose Administration
All patients received treatment with tafamidis [2-(3,5-dichlorophenyl)-1,3-benzoxazole-6-carboxylic acid] at an oral dosage of 20 mg once daily. In patients who had previously received diflunisal, we waited 72 h after the last dose of diflunisal before starting tafamidis.
Evaluation of Efficacy and Safety
Efficacy Parameters
Primary endpoints were change in the NIS from baseline to 18 months of treatment (range, 0–113 points) and responder rate. Responders were defined as those with an increase of < 2 points (the minimum detectable change), whereas non-responders were defined as those who experienced an increase of ≥ 2 points.
Secondary objectives for efficacy included changes in the following parameters by 18 months: the NIS-Lower Limbs Scale (NIS-LL); the Quantitative Sensory Testing Scale (QST); electromyographic study (sural and ulnar nerve sensory neurography, plus ulnar, peroneal, and anterior tibial nerve motor neurography, unilaterally); the 10-m walking test (10MWT); the Norfolk Quality of Life Scale (range − 4 to 136, with higher scores indicating poorer quality of life), the Rasch-built Overall Disability Scale (range 0–48, with lower scores reflecting greater disability); and the Composite Autonomic Symptom Score (COMPASS 31 Scale; range 0–100, with higher scores indicating more autonomic symptoms). In the neurophysiological tests, reductions of ≥ 50% in the amplitude of motor or sensory potentials in the explored nerves were considered clinically significant.
All study assessments were performed by the same investigator.
Safety Parameters
Before starting tafamidis, all patients underwent cardiology assessment with physical examination, staging on the New York Heart Association (NYHA) Scale, electrocardiography (ECG), and the determination of troponin T and natriuretic peptide (NT-proBNP) levels. Echocardiography and 24-h Holter-ECG were also performed in the first 6 months of treatment. Follow-up assessment included the NYHA Scale and determination of troponin T and NT-proBNP at 6, 12, and 18 months, with echocardiography repeated after a year of treatment.
Abdominal ultrasound was performed to confirm the morphology and function of each liver graft at baseline. Follow-up included liver function parameters and immunosuppressant levels at the beginning of treatment and at 6, 12, and 18 months, plus annual abdominal ultrasound in each year of treatment. An additional ultrasound study was performed in the event of deteriorating liver function.
Periodic monitoring of renal function was also performed, with deterioration defined as a glomerular filtration rate < 60 mL/min or a decrease > 10 mL/min from baseline.
Adverse effects were recorded during treatment with tafamidis.
Assessment of Cell-Mediated Immunity
Cell-mediated immunity (CMI) specific to viral and allogeneic antigens was assessed before initiation of treatment with tafamidis and at 12 months of follow-up.
An IFN-Y ELISPOT assay was used to measure CMI specific to three different viruses; cytomegalovirus (CMV), Epstein–Barr virus (EBV), and influenza, as well as total allogeneic cellular stimuli [23, 24]. Briefly, 3·105 peripheral blood mononuclear cells (PBMCs) in a 100-µL volume were stimulated in duplicates with six different allogeneic fully HLA-mismatched cellular stimuli (CD3 + depleted PBMCs) and overlapping peptide pools of two main immunogenic CMV antigens (IE-1 and pp65 (Oxford Immunotec), EBV (Oxford Immunotec) and influenza (Autoimmun Diagnostik®) for 22 h. We detected IFN-γ spots using a biotinylated anti-human IFN-γ antibody developed by the addition of alkaline phosphatase conjugate substrate. The resulting spots were counted semiautomatically with an ELISPOT reader (7th generation Autoimmun Diagnostik®, Strasburg, Germany). Medium alone was used as a negative control and Pokeweed Mitogen (Autoimmun Diagnostik®, Strasberg, Germany) as a positive control.
Statistical Analysis
Counts and percentages were presented for categorical variables and the mean with the standard deviation or the median with the first and last quantile for numerical variables.
To compare the values of the Scale scores in successive visits to the values in the initial visit, a mixed linear regression was used taking in account the random effect of the patient. The conditions of application of the models have been validated and confidence intervals at 95% of the estimators have been calculated whenever possible. P values < 0.05 were considered statistically significant. All analyses were carried out with the statistic package R version 4.2.2 (2022-10-31) for Windows.
For cell-mediated immunity, statistical analysis were performed using SPSS version 26 software and graphs were generated using GraphPad Prism version 8.0 software (Graphpad Software, San Diego, CA, USA). Continuous variables were expressed as median (IQR) A comparison between groups was performed using Kruskall–Wallis and Mann–Whitney U for non-normally distributed data. P values < 0.05 were considered statistically significant.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Ethical approval was obtained by the Ethics Committee for Drug Research of Bellvitge University Hospital and the Spanish Agency of Medicines and Medical Devices (reference numbers: EPA 020/20; CCP-TAF-2020-01). All methods were performed in accordance with the relevant guidelines and regulations. Written informed consent was obtained from all participants in the study for data collection.
Results
Clinical and Demographic Characteristics
As shown in Table 1, seven DLT recipients who developed AAN received tafamidis (mean age at baseline 74.3 years; range 71–80 years). All had received grafts from patients with hATTR who had the Val30Met mutation. The median time from DLT to the onset of neurological symptoms was 8.71 years (range 5–15 years), with all patients reporting painful dysesthesias, impaired thermal sensitivity, and distal hypoesthesia in the lower limbs. Six patients had previously received treatment with diflunisal, but this had been suspended due to lack of efficacy or side effects. Five patients had diabetes (71.43%), but all had good glycemic control before starting tafamidis. One patient had a history of harmful alcohol use, but he had been abstinent years before receiving the liver graft. We identified no other causes of polyneuropathy after taking detailed medical histories and searching for other causes in laboratory tests (e.g., glycated hemoglobin, vitamin B12, folic acid, and protein electrophoresis).
Sural nerve biopsy revealed amyloid deposits in five patients. Although the biopsy was negative in the two remaining patients, neither presented with results suggestive of polyneuropathy by anamnesis, neurological examination, or electromyography before receiving the transplant. Moreover, other causes of polyneuropathy were excluded or controlled before starting treatment with tafamidis, and the post-DLT clinical progression could only be explained by amyloid deposition. At the beginning of the study, the NIS Scale score (mean ± SD) was 18.43 ± 9.85 points (range 5–33 points). Concerning the FAP Scale, six patients were stage I and one patient was stage II. Four patients were stage I on the polyneuropathy disability (PND) score (range, I–IIIA). All patients had electromyographic findings of length-dependent axonal polyneuropathy at study initiation that had not been present before receipt of the DLT.
Efficacy of Treatment (Table 2)
Six patients were responders (< 2 point increase in the NIS Scale) and one patient showed neurological deterioration (3.5 points worsening on the NIS Scale) when comparing the scores at baseline and 18 months of treatment with tafamidis. Six patients also showed a slight improvement on the NIS Scale at 6 months, but only two maintained this improvement to 18 months. A non-statistically significant improved NIS Scale was shown from baseline to 6 months (− 2.54 points; range − 7 to 2.25; CI − 5.92 to 0.84; p = 0.134), 12 months (− 3.25 points; range − 11 to 4.25; CI − 6.63 to 0.13; p = 0.059) and 18 months (− 2.35 points; range − 13 to 3.5; CI − 5.74 to 1.02; p = 0.162) of treatment with tafamidis (Figs. 1, 2).
A non-statistically significant improvement was also observed on the NIS-LL Scale, with mean changes of − 1.39 points (range − 5 to 4.25, CI − 4.35 to 1.57; p = 0.340) at 6 months, − 2.11 points (range − 6 and 6.25; CI − 5.07 to 0.85; p = 0.154) at 12 months, and − 1.07 points (range − 9 to 5; CI − 4.03 to 1.89; p = 0.461) at 18 months of treatment. One patient presented with progression on the PND and FAP Scales, but, at the functional level, the worsening was related to post-COVID pulmonary fibrosis and was not accompanied by a worsening on the NIS Scale. The electromyographic studies at 6, 12, and 18 months revealed no significant changes compared to the start of treatment.
Compared to baseline, no statistically significant differences were observed after 18 months of treatment with tafamidis on the Quantitative Sensory Testing Scale, Norfolk Quality of Life Scale, Rasch-built Overall Disability Scale, COMPASS-31, and 10-m walking test (Table 2; Fig. 2).
Tolerability and Side Effects of Treatment
Only one patient presented a side effect related to tafamidis, which involved only self-limited gastrointestinal discomfort during the first weeks of treatment. However, one patient presented with a deterioration in the glomerular filtration rate that was attributed to COVID-19 complicated by pulmonary fibrosis and diuretic treatment rather than tafamidis.
No patient showed echocardiographic evidence of cardiac amyloidosis, worsening of cardiac symptoms based on the NYHA Scale, during follow-up (Supplementary Table 2). Troponin T values did not change statistically significantly from baseline (mean 23.71 ± 14.71 ng/L) to 18 months (mean 29.14 ± 19.12 ng/L; p = 0.031). NT-proBNP also showed no statistical significance from baseline (mean 661.86 ± 726.36 ng/L) to 18 months (mean 517.14 ± 436.68 ng/L; p = 0.312).
Immunosuppression levels remained unchanged during tafamidis treatment and no patient presented with liver graft rejection. The study of both allogeneic and anti-viral adaptive immunity through the functional assessment of circulating alloreactive and virus-specific T-cell frequences showed no interference in these responses between pre- and post-treatment assessment (Fig. 3).
Assessment of circulating alloreactive T-cells using a panel of 6 complete HLA mismatch B-cell lines and activation on antiviral circulating T-cell. Allogeneic responses: 22 (0.75–247.75) vs. 20 (0–197.75), p = 0.345; 74.50 (0–213.25) vs. 63 (0–228.25), p = 0.593; 15.50 (11.50–304.25) vs. 42.50 (4.50–294.50), p = 0.753; 28.50 (0–51.25) vs. 32 (0–61), p = 0.715; 13 (7.50–41.50) vs. 21.50 (0–35), p = 0.674; 54 (3–340.50) vs. 32 (0–395.50), p = 0.893. Statistics for CMV responses: 80 (1.50–1161) vs. 241 (15.50–1204.50), p = 0.345 for IE-1 and 46 (974–1324) vs. 657 (143.50–1718.50), p = 0.345 for pp65. Statistics for EBV responses: 16 (0.50–40) vs. 6 (0.50–57), p = 1.000. Statistics Influenza responses: 12 (5.50–24) vs. 14 (6–20.50), p = 1.000
Discussion
According to the Domino Liver Transplant Registry, 1288 DLTs had been performed by the end of 2019, of which 1264 came from donors with hATTR [25]. Most of these recipients can be expected to develop AAN from the amyloid deposits of not only the mutated but also non-mutated form of TTR (wild type) [11]. Although this complication was initially expected to arise decades after transplantation, experience has shown that it can appear much earlier [4,5,6,7,8,9,10,11,12]. Thus, although a DLT may improve the vital prognosis of patients waiting for a conventional liver transplant, it can cause a new pathology and have a great impact on patient prognosis, with few therapeutic alternatives available (e.g., liver retransplantation is not viable in most cases). However, medical treatments have been developed over the last decade only for patients with hATTR, who show a pathophysiology very similar to those of patients with AAN following a DLT. On the basis of these rationales, it seems reasonable to believe that these treatments, such as a TTR stabilizer like tafamidis, could benefit patients with AAN following a DLT.
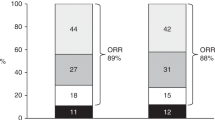
The current study demonstrated a response rate of 85.71% at 18 months, using the same criteria as the seminal clinical trial of tafamidis in patients with hATTR and FAP [14]. However, in this clinical trial, a response rate of 60% was reported after 18 months of treatment with tafamidis versus 38.1% for placebo, excluding the patients who had received an orthotopic liver transplantation. The response in that clinical trial was defined as an increase in the score of the NIS-LL Scale of < 2 points [14]. The higher proportion of responders in our series (85, 71%) may reflect the earlier treatment initiation and a lower baseline NIS score in this study compared to the clinical trial, underlining the importance of periodic follow-up of DLT recipients in a similar way to that of asymptomatic hATTR carriers.
In the present study, we defined responders as patients who showed an increase of < 2 points on the NIS Scale from baseline to 18 months. However, as shown in Fig. 1, some patients who presented an improvement in the NIS Scale at 6 months subsequently experienced a decrease of ≥ 2 points by 12 and/or 18 months. We considered this group of patients as partial responders. Thus, our sample comprised 14.28% of non-responders, 28.57% of partial responders, and 57.14% of responders. These results are similar to those of Monteiro et al. [26], who reported that 29.5% of their sample were non-responders, 36.2% were partial responders, and 34.3% were responders. The lower response rate in their series likely reflects a much longer follow-up period (range, 18–66 months).
The patients included in our study had a much higher mean age than those included in the trials and observational studies of hATTR [14, 26, 27]. This makes comorbidities more frequent, which can be difficult to distinguish from polyneuropathy, thus affecting the outcomes of the assessments of quality of life and disability. An example of this can be seen in the patient who experienced deteriorations in the PND and FAP Scales without showing an accompanying deterioration in the NIS Scale from baseline. Despite the high levels of comorbidity in our series, tafamidis did not lead to a higher incidence of side effects, compared with other studies [14, 26,27,28].
Since thrombocytopenia and glomerulonephritis of a probable autoimmune origin have previously been described for inotersen [15, 29, 30], and as immune-mediated graft damage through the development of allograft rejection via anti-donor alloimmune responses could occur, we assessed the interaction of this therapy with allogeneic as well as protective anti-viral cellular immune responses. Changes in immunosuppressive trough levels were also assessed. Notably, no relevant changes in liver function, immunosuppressive trough levels, or other signs of liver rejection were observed. Likewise, thorough characterization at the single-cell level confirmed the absence of any immunological interference of this therapy with adaptive immunity.
As limitations of the study, we must highlight firstly the small number of the sample and, secondly, that five of the seven patients had diabetes mellitus, despite the optimal control of the disease.
Conclusions
This is the largest series in the literature describing the use of tafamidis for AAN in DLT recipients where the donor had hATTR. Our results support the safety and tolerability of tafamidis for the neurological management of AAN in DLT recipients, without any significative impact on adaptive immunity that could eventually challenge liver transplant outcomes. However, despite being a rare condition, the sample size of seven patients is still too small. With this limitation in mind, our study supports tafamidis potential utility in the neurological management of patients with AAN following DLT. Future randomized placebo-controlled clinical trials with longer follow-up durations are needed to assess the long-term efficacy of this treatment option.
Data Availability
All data generated or analyzed during this study are included in this published article as supplementary information files. Any anonymized data not published within the article will be shared at reasonable request by any qualified investigator.
References
Adams D, Koike H, Slama CT. Hereditary transthyretin amyloidosis: a model of medical progress for a fatal disease. Nat Rev Neurol. 2019;15:387–404. https://doi.org/10.1038/s41582-019-0210-4.
Holmgren G, Ericzon BG, Groth CG, et al. Clinical improvement and amyloid regression after liver transplantation in hereditary transthyretin amyloidosis. Lancet. 1993;341:1113–6. https://doi.org/10.1016/0140-6736(93)93127-M.
Kitchens W. Domino liver transplantation: indications, techniques, and outcomes. Transplant Rev. 2011;25(4):167–77. https://doi.org/10.1016/j.trre.2011.04.002.
Mnatsakanova D, Živković S. Iatrogenic amyloid polyneuropathy after domino liver transplantation. World J Hepatol. 2017;9(3):126–30. https://doi.org/10.4254/wjh.v9.i3.126.
Abdelfatah MM, Hayman SR, Gertz MA. Domino liver transplantation as a cause of acquired familial amyloid polyneuropathy. Amyloid. 2014;21:136–7. https://doi.org/10.3109/13506129.2014.885894.
Ericzon B-G. Domino transplantation using livers from patients with familial amyloidotic polyneuropathy: should we halt? Liver Transpl. 2007;13(2):185–7. https://doi.org/10.1002/lt.21073.
Takei Y, Gono T, Yazaki M, et al. Transthyretin-derived amyloid deposition on the gastric mucosa in domino recipients of familial amyloid polyneuropathy liver. Liver Transpl. 2007;13(2):215–8.
Yamamoto S, Wilczek HE, Iwata T, et al. Long-term consequences of domino liver transplantation using familial amyloidotic polyneuropathy grafts. Transpl Int. 2007;20:926–33. https://doi.org/10.1111/j.1432-2277.2007.00516.x.
Adams D, Lacroix C, Antonini T, et al. Risk of developing de novo amyloid deposits and induced polyneuropathy in FAP domino liver recipients [abstract]. Eur J Neurol. 2009;16(suppl 13):16–54.
Barreiros AP, Geber C, Birklein F, Galle PR, Otto G. Clinical symptomatic de novo systemic transthyretin amyloidosis 9 years after domino liver transplantation. Liver Transpl. 2010;16:109. https://doi.org/10.1002/lt.21928.
Lladó L, Baliellas C, Casasnovas C, et al. Risk of transmission of systemic transthyretin amyloidosis after domino liver transplantation. Liver Transpl. 2010;16:1386–92. https://doi.org/10.1002/lt.22174.
Marques H, Barros I, Li J, et al. Current update in domino liver transplantation. Int J Surg. 2020;82S:163–8. https://doi.org/10.1016/j.ijsu.2020.03.017.
Berk JL, Suhr OB, Obici L, et al. Repurposing diflunisal for familial amyloid polyneuropathy: a randomized clinical trial. JAMA. 2013;310(24):2658–67. https://doi.org/10.1001/jama.2013.283815.
Coelho T, Maia LF, da Silva AM, et al. Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology. 2012;79(8):785–92. https://doi.org/10.1212/WNL.0b013e3182661eb1.
Benson MD, Waddington-Cruz M, Berk JL, Polydefkis M, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):22–31. https://doi.org/10.1056/NEJMoa1716793.
Adams D, Gonzalez-Duarte A, O’Riordan WD, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):11–21. https://doi.org/10.1056/NEJMoa1716153.
Nedkova-Hristova V, Baliellas C, González-Castello J, et al. Treatment with diflunisal in domino liver transplant recipients with acquired amyloid neuropathy. Transpl Int. 2022. https://doi.org/10.3389/ti.2022.10454.
Misumi Y, Ueda M, Masuda T, et al. Characteristics of acquired transthyretin amyloidosis. Neurology. 2019;93(17):e1587–96. https://doi.org/10.1212/WNL.000000000000836.
Yoshinaga T, Yazaki M, Sekijima Y, et al. The pathological and biochemical identification of possible seed-lesions of transmitted transthyretin amyloidosis after domino liver transplantation. J Pathol Clin Res. 2016;2:72–9. https://doi.org/10.1002/cjp2.36.
Matsushima M, Yabe I, Tsuda M, Sakakibara M, Shimamura T, Sasaki H. Amyloid polyneuropathy and myocardial amyloidosis 10 years after domino liver transplantation from a patient with a transthyretin Ser50Arg mutation. Intern Med. 2017;56:3231–5. https://doi.org/10.2169/internalmedicine.8434-16.
Tsamis K, Mytilinaios D, Heneghan M, Gillmore J, et al. Treatment of acquired transthyretin amyloidosis in domino liver transplantation. Clin Transplant. 2022. https://doi.org/10.1111/ctr.14822.
Agreement of the Pharmacotherapeutic Committee for the Comprehensive Public Use Health System of Catalonia (CFT-SISCAT) of CatSalut on the use of tafamidis, inotersen and patisiran for the treatment of polyneuropathy due to hereditary transthyretin amyloidosis. https://catsalut.gencat.cat/web/.content/minisite/catsalut/proveidors_professionals/medicaments_farmacia/harmonitzacio/informes/_compartits/amiloidosi-polineuropatia/acord-CFT-SISCAT-tafamidis-inotersen-patisiran-ATTRh.pdf. Accessed Mar 2024.
Bestard O, Crespo E, Stein M, et al. Cross-validation of IFN-γ Elispot assay for measuring alloreactive memory/effector T cell responses in renal transplant recipients. Am J Transplant. 2013;13(7):1880–90. https://doi.org/10.1111/ajt.12285.
Lúcia M, Crespo E, Melilli E, et al. Preformed frequencies of cytomegalovirus (CMV)-specific memory T and B cells identify protected CMV-sensitized individuals among seronegative kidney transplant recipients. Clin Infect Dis. 2014;59(11):1537–45. https://doi.org/10.1093/cid/ciu589.
Familial Amyloidotic Polyneuropathy World Transplant Registry and Domino Liver Transplant Registry. www.fapwtr.org. Accessed Mar 2024.
Monteiro C, Mesgazardeh J, Anselmo J, et al. Predictive model of response to tafamidis in hereditary ATTR polyneuropathy. JCI Insight. 2019;4(12): e126526. https://doi.org/10.1172/jci.insight.126526.
Planté-Bordeneuve GF, Salhi H, Nordine T, Ayache S, Corvoisier P, et al. Long-term treatment of transthyretin familial amyloid polyneuropathy with tafamidis: a clinical and neurophysiological study. Neurology. 2017;264(2):268–76. https://doi.org/10.1007/s00415-016-8337-3.
Damy T, Garcia-Pavia P, Hanna M, Judge D, Merlini G, Gundapaneni B. Efficacy and safety of tafamidis doses in the tafamidis in transthyretin cardiomyopathy clinical trial (ATTR-ACT) and long-term extension study. Eur J Heart Fail. 2021;23(2):277–85. https://doi.org/10.1002/ejhf.2027.
Narayanan P, Curtis B, Shen L, et al. Underlying immune disorder may predispose some transthyretin amyloidosis subjects to inotersen-mediated thrombocytopenia. Nucleic Acid Ther. 2020;30(2):94–103. https://doi.org/10.1089/nat.2019.0829.
Law S, Arnold J, Rauf M, et al. Focal segmental glomerulosclerosis complicating therapy with inotersen, an antisense oligonucleotide inhibitor: a case report. Am J Kidney Dis. 2022. https://doi.org/10.1053/j.ajkd.2022.08.018.
Acknowledgements
We express our gratitude to the patient for their collaboration. We thank CERCA Programme/Generalitat de Catalunya for institutional support. Four of the authors of this publication (Velina Nedkova, Valentina Vélez, Miosés Morales de la Prida and Carlos Casasnovas) are members of the European Reference Network for Neuromuscular Diseases.
Funding
This study was funded through an independent research grant (ID#63238211) by Pfizer. The funder had no influence on the study design, data processing, statistical analysis, decision to publish this article and did not fund the journal's open access and rapid service fees. IDIBELL (Instituto de Investigación Biomédica de Bellvitge) will fund the journal's open access and rapid service fees.
Author information
Authors and Affiliations
Contributions
Velina Nedkova and Carlos Casasnovas designed and conceptualized study, had major role in acquisition of data, analyzed the data and drafted the manuscript for intellectual content. Laura Donadeu and Oriol Bestard contributed to the acquisition of CMI data, analyzed the CMI data; revised the manuscript for intellectual content. Valentina Vélez-Santamaría, Miosés Morales de la Prida, Carmen Baliellas, José González-Costello, Laura Lladó and Emma González-Vilatarsana contributed to the acquisition of data and revised the manuscript for intellectual content. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Laura Doneu’s funding: Predoctoral Contract for Health Research Training (PFIS) funded by Instituto de Salud Carlos III. Oriol Bestard´s funding: Competitive grant PI19/01710 funded by Instituto de Salud Carlos III and co-fundede by European Union (ERDF/ESF)—A way to build Europe. RICORS (Redes de Investigación Cooperativa Orientadas a Resultados en Salud) consortia (Intituto de Salud Carlos III).Competitive grant PI22/01019 funded by Instituto de Salud Carlos III and co-fundede by European Union (ERDF/ESF)—A way to build Europe. Carmen Baliellas, José González-Costello, Laura Lladó and Emma González-Vilatarsana has nothing to disclose.
Ethical approval
This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Ethical approval was obtained by Ethics Committee for Drug Research of Bellvitge University Hospital and the Spanish Agency of Medicines and Medical Devices (reference number: EPA 020/20; CCP-TAF-2020-01). All methods were performed in accordance with the relevant guidelines and regulations. Written informed consent was obtained from all participants in the study for data collection.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Nedkova-Hristova, V., Donadeu, L., Baliellas, C. et al. Safety, Tolerability, and Outcomes of Tafamidis for the Treatment of Acquired Amyloid Neuropathy in Domino Liver Transplant Recipients. Neurol Ther (2024). https://doi.org/10.1007/s40120-024-00621-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40120-024-00621-w