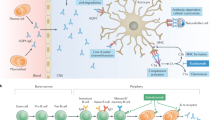
Abstract
Introduction
Anti-aquaporin-4 antibody-positive (AQP4-Ab+) neuromyelitis optica spectrum disorder (NMOSD) is a complement-mediated autoimmune disease in which unpredictable and relapsing attacks on the central nervous system cause irreversible and accumulating damage. Comparative efficacy of new NMOSD therapies, such as ravulizumab, with established therapies is critical in making informed treatment decisions.
Methods
Efficacy of ravulizumab relative to established AQP4-Ab+ NMOSD treatments, such as eculizumab, inebilizumab, and satralizumab, was evaluated in a Bayesian network meta-analysis (NMA). Data were extracted from trials identified by a systematic literature review. The final evidence base consisted of 17 publications representing five unique and global studies (PREVENT, N-MOmentum, SAkuraSky, SAkuraStar, and CHAMPION-NMOSD). The primary endpoint was time-to-first relapse; other outcomes included annualized relapse rates (ARRs).
Results
For patients receiving monotherapy (monoclonal antibody only), ravulizumab was associated with a lower risk of relapse than inebilizumab (hazard ratio [HR] 0.09, 95% credible interval [CrI] 0.02, 0.57) or satralizumab (HR 0.08, 95% CrI 0.01, 0.55) and was comparable to eculizumab (HR 0.86, 95% Crl 0.16, 4.52). Ravulizumab + immunosuppressive therapy (IST) was associated with a lower risk of relapse than satralizumab + IST (HR 0.15, 95% CrI 0.03, 0.78); the comparison with eculizumab + IST suggested no difference. No patients treated with inebilizumab received background IST and were thus excluded from analysis. The ARR with ravulizumab monotherapy was 98% lower compared with inebilizumab (rate ratio [RR] 0.02, 95% Crl 0.00, 0.38) and satralizumab (RR 0.02, 95% Crl 0.00, 0.42) monotherapies. The ARR with ravulizumab ± IST showed the strongest treatment-effect estimates compared with other interventions.
Conclusion
In the absence of head-to-head randomized controlled trials, NMA results suggest ravulizumab, a C5 inhibitor, is likely to be more effective in preventing NMOSD relapse in patients with AQP4-Ab+ NMOSD when compared with other treatments having different methods of action.
Plain Language Summary
Anti-aquaporin-4 antibody-positive neuromyelitis optica spectrum disorder, also called AQP4-Ab+ NMOSD, is a rare autoimmune disease that causes repeated episodes of symptoms such as blindness, arm/leg weakness, painful spasms, vomiting, and hiccups, among other symptoms. Each episode can cause nervous system damage to worsen, making it more difficult to recover back to regular abilities. Repeated episodes are likely to cause permanent damage, such as blindness and paralysis. Medical treatments that reduce episodes also reduce the damage and the chances symptoms will become permanent. One treatment, ravulizumab, is being studied to treat adults with AQP4-Ab+ NMOSD. This analysis looked at information from published clinical studies to compare ravulizumab with three other treatments (eculizumab, inebilizumab, and satralizumab) to determine how well each treatment reduced NMOSD episodes. There are no studies that have tested all four treatments in one study. Here, the treatments were compared by a method used to estimate the likelihood of a treatment being better than the others. While all four treatments successfully reduced episodes in their own studies, this analysis predicts that ravulizumab would likely be best in preventing episodes compared with inebilizumab or satralizumab when used alone or in combination with other immunosuppressive treatments. These findings, in consideration along with other relevant factors such as cost, safety, dosing delivery method, and frequency of treatment, may help doctors and patients decide what is the best treatment option for each individual patient to prevent attacks in adults with AQP4-Ab+ NMOSD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Ravulizumab was under investigation for the treatment of adults with anti-aquaporin-4 antibody-positive neuromyelitis optica spectrum disorder (AQP4-Ab+ NMOSD). |
A systematic literature review was conducted to identify data from controlled clinical trials for ravulizumab, as well as the three established AQP4-Ab+ NMOSD treatments, eculizumab, inebilizumab, and satralizumab. A fixed-effects Bayesian network meta-analysis under the proportional hazards assumption was used to perform an indirect treatment comparison to estimate relative efficacies in preventing relapses. |
A total of 17 publications were included in the evidence base, representing five unique, global clinical trials (CHAMPION-NMOSD, N-MOmentum, PREVENT, SakuraSky, and SakuraStar). Hazard ratios suggest that patients on ravulizumab monotherapy had a reduced risk of relapse of 91% and 92% when compared with patients on inebilizumab or satralizumab monotherapy, respectively. Ravulizumab and eculizumab treatment effects in the monotherapy setting were comparable. |
Ravulizumab had the greatest likelihood of being the best treatment option for delaying time-to-first relapse in all three treatment-setting scenarios tested (monotherapy, combination therapy, and combined mono- and combination therapy), and reducing the rate of relapses. Results suggest C5 inhibition may prevent AQP4-Ab+ NMOSD relapses more effectively than treatments targeting other mechanisms of action. |
Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is a complement-mediated autoimmune disease affecting the central nervous system [1, 2]. This rare, severely disabling disease primarily affects the optic nerves and spinal cord and causes unpredictable and sudden attacks of partial or complete vision loss, body numbness, leg or arm weakness or paralysis, as well as persistent nausea, uncontrolled vomiting, and unrelenting hiccups among other symptoms [1, 2]. The inflammatory attacks can cause immediate and irreversible neurological damage that accumulates with each relapse, and that can lead to permanent disability, including blindness and paralysis [3,4,5,6,7,8,9]. As 80–90% of those with NMOSD have relapsing disease, effective immunotherapies to prevent relapse are vital [1, 10].
One of the primary drivers of the NMOSD pathogenesis is the activation of the complement cascade by aquaporin-4 (AQP4) immunoglobulin G [11,12,13]. Several interventions have been developed for the treatment of adults with anti-AQP4 antibody-positive (AQP4-Ab+) NMOSD [14,15,16]: eculizumab (C5 protein inhibitor) [3, 17], satralizumab (monoclonal antibody [mAb] targeting interleukin-6 receptor [IL-6R]) [18, 19], and inebilizumab (mAb that binds to the B cell surface antigen cluster of differentiation 19 [CD19] inducing antibody-dependent cellular cytolysis) [20]. A fourth treatment, ravulizumab, a second-generation C5 inhibitor engineered from eculizumab, was recently approved as a treatment for NMOSD by the European Union (EU) and Japan [21]. Ravulizumab was evaluated in a phase 3, externally placebo-controlled, open-label, multicenter study (CHAMPION-NMOSD; NCT04201262) of AQP4-Ab+ NMOSD [22]. The external control arm consisted of the eculizumab PREVENT trial placebo group [3].
Comparative efficacy analyses can aid healthcare providers in making informed treatment decisions when creating a care plan with a patient and is also considered by payers and fundholders in determining coverage and reimbursement. The most credible sources of comparative evidence are direct comparisons of randomized controlled trials (RCTs); however, such sufficiently powered studies evaluating all relevant treatment options are not always feasible, especially with rare diseases such as AQP4-Ab+ NMOSD, which has low prevalence and incidence rates. Statistical methods, such as network meta-analysis (NMA), can be useful tools to simultaneously compare trial-specific treatment effects of several therapies—provided that trials were designed similarly, and enrolled patients consisted of the same target population of interest—to evaluate relative treatment effects as if a head-to-head RCT was conducted [23, 24]. A previous indirect treatment comparison was published; however, new therapies are under investigation and subsequent NMAs are needed to aid in decision-making. The aim of this study is to compare the performance of ravulizumab relative to approved treatments for adults with AQP4-Ab+ NMOSD by conducting an NMA of clinical trial data identified from our review.
Methods
Selection of Relevant Trials
A systematic literature review (SLR) search was initiated on March 8, 2023 across Embase, MEDLINE, and the Cochrane Central Register of Controlled Trials databases to identify relevant data. The pre-defined Population, Intervention, Comparator, Outcome, and Study design (PICOS) eligibility criteria guided identification and selection of studies (Table 1). Additional searching of specific congress proceedings from 2021 to 2022 included a review of European Committee for Treatment and Research for Multiple Sclerosis (ECTRIMS), American Academy of Neurology (AAN), Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS), and European Academy of Neurology (EAN) meetings as well as a www.clinicaltrials.gov screening for relevant trials with registered outcomes that had not yet been formally published. Information outside of traditional publishing and distribution channels (e.g., government documents, corporate dossiers, company-provided datasets, dissertations/theses) were also searched to identify relevant and supporting data.
Two reviewers worked independently to perform the screening, data extraction, and risk of bias assessments of the studies in duplicate. Risk of bias was assessed on the basis of randomization process, deviations from the intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. The reviewers updated the literature search that was previously executed by the same organization on January 27, 2020, up until March 8, 2023. Any discrepancies between the two reviewers were resolved through discussion and by involving a third reviewer, if necessary.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals.
Feasibility Assessment for NMA
The final included studies from the SLR were evaluated in a feasibility assessment to gauge the appropriateness of proceeding with an NMA. The assessment included (1) a determination of whether the evidence for the interventions of interest forms one evidence network for each population group and outcome of interest; (2) an assessment of the similarity of common comparator treatments; (3) an exploration of the distribution of baseline patient characteristics both within and between comparisons to identify factors that may bias indirect estimates (i.e., identify effect modifiers); (4) an assessment of outcome availability, definitions, and the time points at which outcomes are reported; and (5) an exploration of the observed treatment effects to assess variability in outcome reporting and proportional hazards assumption for time-to-event outcomes. Important treatment-effect modifiers considered were AQP4 status and the use of background immunosuppressive therapy (IST).
The feasibility assessment process was conducted in accordance with recommendations by the International Society for Pharmacoeconomics and Outcomes Research (ISPOR), the National Institute for Health and Care Excellence (NICE), and PRISMA guidelines [25,26,27].
Network Meta-analysis
In order to simultaneously compare multiple treatments within a single analysis, ISPOR recommends an NMA approach that combines available data from a network of clinical trials [26, 28].
Analyses used a Bayesian NMA, assuming fixed-effects and proportional hazards to estimate relative treatment effects. Data extracted from the clinical trials identified during the SLR were used. The analysis followed the recommended guidelines from ISPOR, NICE, and PRISMA guidelines [25,26,27]. In the case of zero relapses observed under ravulizumab and eculizumab, we used the Firth correction method applied to the Cox proportional hazards model to estimate a non-zero hazard ratio (HR) and credible intervals (CrIs) [29,30,31].
Time-to-first relapse and annualized relapse rates (ARRs) were the only endpoints available across all selected comparators deemed feasible for NMA. The primary endpoint in all included trials was adjudicated time-to-first relapse. This endpoint was defined as the presence or worsening of NMOSD-related neurologic symptoms. For time-to-event outcomes (e.g., time-to-first relapse), the NMA was performed using a regression model with a contrast-based normal likelihood for the logHR and corresponding standard error of each trial or comparison in the network, under the assumption of proportional hazards. Relative treatment effects were expressed as HRs and 95% Crls. For ARR, the NMA was performed on the basis of the number of events over the total exposure time, or patient-years at risk, using a regression model with a Poisson likelihood and log link. Relative treatment effects were expressed as rate ratios (RRs) and 95% Crls.
NMAs on health-related quality of life (QoL) outcomes (i.e., Expanded Disability Status Scale [EDSS], European Quality of Life-5D Visual Analog Scale [EQ-5D VAS], and Short Form Survey [SF-36]) were considered; however, difference in the QoL measures used across studies and the duration of the reported data limited the ability to form any valid comparisons or generalizable conclusions as a result, thus they have been omitted from this publication.
CHAMPION-NMOSD and PREVENT were combined in the NMA and treated effectively as a single comparative study. To address any biases arising from slight differences in study designs and unforeseen enrollment differences resulting in disparity in patient characteristics between CHAMPION-NMOSD and PREVENT, sensitivity analyses for the efficacy endpoints using propensity score weights were performed to balance baseline covariates between groups and to further reduce potential bias introduced through an external control. The variables included in the propensity score calculation were region, gender, age at first dose, background IST use, baseline EDSS, and historical ARR 24 months prior to screening. Two sets of analyses were performed, one in an average treatment effect (ATE) framework and the other using an average treatment effect on treated population (ATT) framework (Table 2).
Software
The Just Another Gibbs Sampler (JAGS) software package, implementing a Markov chain Monte Carlo (MCMC) method, was used to estimate the parameters of the different models. All analyses were performed using R version 4.2.1 (http://www.r-project.org/) and JAGS version 4.3.1.
Results
Evidence Base
The SLR search identified a total of 442 citations. Of 375 citations identified via databases, 278 were screened at the title and abstract stage after removing automated duplicates via Endnote and trial registries. The full text from 53 citations was reviewed, with 15 records excluded because of irrelevant study design, 20 because of irrelevant population, 1 because of irrelevant intervention, and 4 because of irrelevant outcomes. Four of 67 additional citations were included from gray literature searches, including hand-retrieved materials that served as secondary data sources pertaining to N-MOmentum (inebilizumab), SakuraStar (satralizumab), and SakuraSky (satralizumab) trials, released by the Gemeinsamer Bundesausschuss (G-BA). Inebilizumab and satralizumab dossiers from the G-BA were supplied by Horizon Therapeutics and Roche, respectively, for reimbursement purposes (https://www.g-ba.de/english/). Additionally, PREVENT (eculizumab) and CHAMPION-NMOSD (ravulizumab) clinical study reports were provided by Alexion Pharmaceuticals. A total of 17 citations associated with the following five unique clinical trials (CHAMPION-NMOSD, PREVENT, N-MOmentum, SakuraSky, and SakuraStar) were selected for our evidence base for NMA (Fig. 1).
PRISMA diagram: Study identification and selection. AAN American Academy of Neurology Annual Meeting, ACTRIMS Americas Committee for Treatment and Research in Multiple Sclerosis, CENTRAL Cochrane Central Register of Controlled Trials, EAN European Academy of Neurology, ECTRIMS European Committee for Treatment and Research in Multiple Sclerosis
Feasibility Assessment
All patients from CHAMPION-NMOSD and PREVENT had confirmed AQP4-Ab+ NMOSD at enrollment with demonstrated benefit in the active treatment group. The inebilizumab trial (N-MOmentum) and satralizumab trials (SakuraSky and SakuraStar) additionally enrolled AQP4-Ab− patients; however, these trials reported insufficient evidence to support a claim of risk reduction in this population compared to placebo as was observed in AQP4-Ab+ patients. Given the different association of AQP4 status and treatment outcome, only AQP4-Ab+ patient cohorts from N-MOmentum, SakuraSky, and SakuraStar were considered relevant for NMA; patients who were AQP4-Ab− were excluded from analyses.
Notably, CHAMPION-NMOSD, PREVENT, and SakuraSky allowed the use of background IST on-trial, which was not allowed in SakuraStar or N-MOmentum (Table S1 in the electronic supplementary material). As the association of additive IST with treatment outcome is unclear, analyses were performed for three clinically relevant population scenarios (Fig. 2): monotherapy only (patients receiving mAb only; Table S2), combination therapy (i.e., patients receiving mAb with background IST; Table S3), and combined monotherapy or combination therapy (i.e., patients receiving mAb with or without background IST; Table S4).
Network of evidence for indirect treatment comparison of time-to-first relapse in adults with AQP4-Ab+ NMOSD. Parentheses indicate trial subgroup populations relevant for analyses. Dashed lines indicate externally controlled three-arm trial with combined PREVENT & CHAMPION patient-level data. ISTs are defined as azathioprine, mycophenolate mofetil, glucocorticoids, and/or others. AQP4-Ab+ anti-aquaporin-4 antibody-positive, IST immunosuppressive therapy, NMOSD neuromyelitis optica spectrum disorder
For the findings to be relevant, there should not be substantial differences in treatment effect-modifiers between the studies that form the evidence base and the target population or setting of interest. AQP4 status and background IST were considered important effect modifiers that were accounted for in NMA. All other between-study differences, such as age or baseline EDSS, assessed across included studies in an evidence network related to trial, treatment, or patient characteristics were minimal and deemed unlikely to substantially bias NMA results. While differences in these trial and patient characteristics could impact the absolute number of events (e.g., relapses) observed, as a result of the randomization they would do so in both arms equally. The relative difference between the active and the control arm, the measure used in an NMA, is likely to remain unaffected.
Estimated Treatment Effects
Monotherapy
Time-to-first relapse and ARR for a population treated in a monotherapy setting were reported in four trials, CHAMPION-NMOSD, PREVENT, N-MOmentum, and SakuraStar, evaluating ravulizumab, eculizumab, inebilizumab, and satralizumab, respectively (Fig. 2). At any time, patients treated with ravulizumab monotherapy were 91% less likely to experience a first relapse than patients treated with inebilizumab monotherapy (HR 0.09; 95% CrI 0.02, 0.57), and were 92% less likely to experience a first relapse than patients treated with satralizumab monotherapy (HR 0.08; 95% CrI 0.01, 0.55) (Fig. 3). Treatment effects with eculizumab and ravulizumab as monotherapy were comparable.
Forest plot of NMA results for time-to-first relapse with ravulizumab versus alternate interventions in adults with AQP4-Ab+ NMOSD. In SAkuraSky and PREVENT, background IST such as azathioprine, mycophenolate mofetil, and glucocorticoids were allowed, whereas IST was explicitly excluded from the N-MOmentum and SAkuraStar population. AQP4-Ab+ anti-aquaporin-4 antibody-positive, CrI credible interval, IST immunosuppressive therapy, NMOSD neuromyelitis optica spectrum disorder
The ARR of ravulizumab monotherapy was 98% lower compared with inebilizumab (RR 0.02, 95% CrI 0.00, 0.38) and satralizumab (RR 0.02, 95% CrI 0.00, 0.42) monotherapy, and was comparable between ravulizumab and eculizumab. Similar to time-to-first relapse, ravulizumab and eculizumab monotherapy were the most efficacious in improving ARR among the interventions being compared (Fig. 4).
Forest plot of NMA results for annualized relapse rate with ravulizumab versus alternate interventions in adults with AQP4-Ab+ NMOSD. In SAkuraSky and PREVENT, background IST such as azathioprine, mycophenolate mofetil, and glucocorticoids were allowed, whereas IST was explicitly excluded from the N-MOmentum and SAkuraStar population. AQP4-Ab+ anti-aquaporin-4 antibody-positive, CrI credible interval, IST immunosuppressive therapy, NMOSD neuromyelitis optica spectrum disorder
Based on rank order probabilities, ravulizumab monotherapy had the highest likelihood of being the best treatment for improving time-to-first relapse and ARR, followed by eculizumab monotherapy (Table 3).
Combination Therapy
Time-to-first relapse and ARR in patients treated in a combination therapy setting were reported in three trials, CHAMPION-NMOSD, PREVENT, and SakuraSky, evaluating ravulizumab + IST, eculizumab + IST, and satralizumab + IST, respectively (Fig. 2). Patients treated with ravulizumab + IST were 85% less likely to experience a first relapse than those treated with satralizumab + IST (HR 0.15; 95% CrI 0.03, 0.78) (Fig. 3). It could not be concluded that ravulizumab + IST was more efficacious than eculizumab + IST regarding improving time-to-first relapse as a result of the uncertainty in the relative treatment-effect estimate.
Ravulizumab + IST showed the strongest treatment effect in improving ARR; however, the uncertainty in the relative treatment-effect estimates between alternative interventions did not allow for claims of one combination treatment being better over another (Fig. 4).
On the basis of rank order probabilities, ravulizumab + IST had the highest likelihood of being the best treatment for improving time-to-first relapse and improving ARR of the compared interventions, followed by eculizumab + IST (Table 3).
Combined Mono- and Combination Therapy
Time-to-first relapse for a mixed population of patients on monotherapy or combination therapy was reported in four trials: CHAMPION-NMOSD, PREVENT, SakuraStar, and SakuraSky, evaluating ravulizumab ± IST, eculizumab ± IST, and satralizumab ± IST, respectively (Fig. 2). The relative treatment effect of satralizumab ± IST was obtained by pooling the results of SakuraStar (monotherapy) and SakuraSky (combination therapy).
At any particular time during the reported study periods, patients on ravulizumab ± IST were 94% less likely to experience a first relapse than patients on satralizumab ± IST (HR 0.06; 95% CrI 0.02, 0.18; Fig. 3). Similarly, patients on ravulizumab ± IST were 76% less likely to experience a first relapse than those on eculizumab ± IST (HR 0.24, 95% Crl 0.08, 0.71).
Ravulizumab ± IST was more efficacious than satralizumab ± IST in improving ARR. The ARR was 98% (RR 0.02, 95% Crl 0.00, 0.32) lower with ravulizumab ± IST than with satralizumab ± IST (Fig. 4). Results of ravulizumab ± IST versus eculizumab ± IST were comparable.
On the basis of rank order probabilities, ravulizumab ± IST had the highest likelihood of being the best treatment for improving time-to-first relapse and ARR, followed by eculizumab ± IST (Table 3).
Discussion
Comparative efficacy analysis is often used by healthcare providers to aid in treatment decision-making. In the absence of head-to-head RCTs comparing the treatment modalities of interest, an NMA offers an alternative method to compare treatment effects. Previous studies using the NMA comparison approach did not include ravulizumab. This is the first study to implement an NMA to evaluate the relative treatment effects between ravulizumab and established therapies, eculizumab, inebilizumab, and satralizumab, for treating patients with AQP4-Ab+ NMOSD.
The results of this comparative efficacy analysis suggest that ravulizumab was more efficacious than inebilizumab and satralizumab in improving time-to-first relapse in a monotherapy setting. In the combination therapy and combined mono- and combination therapy setting, ravulizumab was also more efficacious against satralizumab in improving time-to-first relapse. Similarly, analysis of the ARR also demonstrated the benefit of ravulizumab in reducing the rate of relapses relative to the alternate interventions in a monotherapy setting. Results suggest no difference between ravulizumab and eculizumab in either monotherapy or combination therapy settings but when combined, a significant difference in time to first relapse was detected in favor of ravulizumab; this counterintuitive result is likely an artifact of a small number of events and the nature of the HR (i.e., its non-collapsibility) used as the basis of this comparison.
Although not approved in the USA or EU for the treatment of NMOSD, historically rituximab has been used off-label for NMOSD relapse prevention [32]. There is only one known RCT comparing rituximab and placebo in NMOSD (RIN-1), and it showed the efficacy of rituximab in preventing relapses in all patients with AQP4-Ab+ NMOSD treated during the study period [32]. The RIN-1 study was not included in this NMA because of fundamental trial differences such as patients were not required to have had a history of prior relapses, patients had less severe baseline relapse risk (e.g., monophasic patients, lower baseline ARR), and the trial had an extremely small sample size [32]. Thus, including RIN-1 in this indirect treatment comparison was unreasonable considering it contrasts with the other studies in this NMA.
While RCTs are the most credible source of evidence to obtain insight into relative treatment effects for indirect treatment comparisons, the single-arm, external placebo-controlled, unblinded design of CHAMPION-NMOSD is different from the other studies included. The rationale and justification for the design has been previously published [3, 22]. The question is whether and to what extent the findings could have been influenced by the study design and, as a result, impacted the findings of this NMA.
The prospective design of CHAMPION-NMOSD aimed to mimic those of PREVENT with regard to its enrollment criteria, permittance of concomitant medications, adjudication of procedures, and prespecified endpoints [3, 22]. The inclusion criteria between both studies were largely similar but had differences. CHAMPION-NMOSD required fewer prior relapses as inclusion criteria and used updated diagnostic criteria consistent with those used in the more recent NMOSD trials. Other differences were observed in baseline EDSS, historical ARR, and the proportion of prior rituximab treatment. Given the overall similarity of placebo response across different NMOSD trials where a small difference in the included patient population was observed [3, 22], it is unlikely a placebo arm as part of CHAMPION-NMOSD would have resulted in a substantially different number of on-trial relapses.
Given the external placebo arm used for the CHAMPION-NMOSD trial, we performed two sets of sensitivity analysis in the NMA to identify the potential impact on the relative effectiveness estimation and found marginal impact on the observed treatment differences. We cannot rule out that differences in the patient population between both studies introduced a bias in the efficacy of ravulizumab included in the NMA. Nevertheless, the study found that the overwhelming evidence of relapse benefit observed in patients treated with ravulizumab far outweighed any measure of uncertainty arising from its nonrandomized trial design.
A limitation of this study was that each treatment group was informed by only a single trial (i.e., ravulizumab, eculizumab, inebilizumab) or two trials (i.e., satralizumab) with limited sample size. The largest patient sample size analyzed involved 161 patients treated with inebilizumab; all other treatment groups had fewer than 100 patients. As such, there was limited ability to account for heterogeneity in the NMA estimates and prevent any cross-trial adjustment of patient characteristics by means of a meta-regression analysis. An NMA with safety endpoints was not conducted because of substantial heterogeneity in the reporting of adverse events across included trials.
Long-term follow-up data from the identified RCTs (i.e., extension periods) are not incorporated. The main reason is that extension periods no longer have a control arm, which makes it impossible to reliably estimate HR. All outcomes of interest across the included studies were similarly defined; however, there were discrepancies in enrollment criteria such as NMOSD relapse activity inclusion criteria and relapse definition criteria across N-MOmentum, SAkuraSky, SAkuraStar, and PREVENT. Differences in NMOSD relapse inclusion and definition criteria are unlikely to impact our results. All included trials reported adjudicated relapse outcomes. Differences in attack criteria across included trials have been previously described as a limitation due to no current consensus on the impact of differing attack definitions and adjudication methods [33].
Duration of follow-up was not deemed as a treatment-effect modifier regarding the primary endpoint of time-to-first relapse under the proportional hazards assumption. AQP4 status as a treatment-effect modifier was considered by assessing only AQP4-Ab+ patients. The presence of background IST use as a treatment-effect modifier was also considered by conducting an NMA based on three population scenarios (combined monotherapy or combination therapy, monotherapy only, and combination therapy), each representing different treatment settings. An additional strength of this NMA is that these stratified analyses are likely to be more relevant for clinical decision-making.
Conclusion
This analysis provides estimates of relative treatment efficacy of ravulizumab compared with other established interventions for patients with AQP4-Ab+ NMOSD in the absence of head-to-head RCTs. NMA results based on currently available evidence suggest ravulizumab monotherapy is more efficacious than satralizumab and inebilizumab monotherapies in preventing relapse, while ravulizumab + IST is more efficacious than satralizumab + IST for this endpoint in the combination therapy setting. Findings between eculizumab and ravulizumab were largely comparable, suggesting that C5 inhibition may likely prevent relapse more effectively than other therapeutic strategies such as IL-6R inhibition or CD19-mediated B-cell depletion. Although efficacy is only one consideration when making treatment decisions, knowledge of the comparative efficacy effects between treatments is a vital component in the shared decision-making process.
Data Availability
Data sharing is not applicable to this article as no datasets were generated during the current study. All data for this manuscript were publicly available and can be accessed using the criteria described in the Methods and Supplementary Material.
References
Jarius S, Ruprecht K, Wildemann B, et al. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: a multicentre study of 175 patients. J Neuroinflamm. 2012;9:14.
Hyun JW, Kim Y, Kim SY, Lee MY, Kim SH, Kim HJ. Investigating the presence of interattack astrocyte damage in neuromyelitis optica spectrum disorder: longitudinal analysis of serum glial fibrillary acidic protein. Neurol Neuroimmunol Neuroinflamm. 2021;8(3):e965.
Pittock SJ, Berthele A, Fujihara K, et al. Eculizumab in aquaporin-4-positive neuromyelitis optica spectrum disorder. N Engl J Med. 2019;381(7):614–25.
Hamid SHM, Whittam D, Mutch K, et al. What proportion of AQP4-IgG-negative NMO spectrum disorder patients are MOG-IgG positive? A cross sectional study of 132 patients. J Neurol. 2017;264(10):2088–94.
Wingerchuk DM. Diagnosis and treatment of neuromyelitis optica. Neurologist. 2007;13(1):2–11.
Hinson SR, Romero MF, Popescu BF, et al. Molecular outcomes of neuromyelitis optica (NMO)-IgG binding to aquaporin-4 in astrocytes. Proc Natl Acad Sci U S A. 2012;109(4):1245–50.
Morrow MJ, Wingerchuk D. Neuromyelitis optica. J Neuroophthalmol. 2012;32(2):154–66.
Levy M, Fujihara K, Palace J. New therapies for neuromyelitis optica spectrum disorder. Lancet Neurol. 2021;20(1):60–7.
Kim SH, Jang H, Park NY, et al. Discontinuation of immunosuppressive therapy in patients with neuromyelitis optica spectrum disorder with aquaporin-4 antibodies. Neurol Neuroimmunol Neuroinflamm. 2021;8(2):e947.
Akaishi T, Misu T, Takahashi T, et al. Progression pattern of neurological disability with respect to clinical attacks in anti-MOG antibody-associated disorders. J Neuroimmunol. 2021;351:577467.
Huda S, Whittam D, Bhojak M, Chamberlain J, Noonan C, Jacob A. Neuromyelitis optica spectrum disorders. Clin Med (Lond). 2019;19(2):169–76.
Duan T, Smith AJ, Verkman AS. Complement-dependent bystander injury to neurons in AQP4-IgG seropositive neuromyelitis optica. J Neuroinflamm. 2018;15(1):294.
Jarius S, Paul F, Weinshenker BG, Levy M, Kim HJ, Wildemann B. Neuromyelitis optica. Nat Rev Dis Primers. 2020;6(1):85.
SOLIRIS® (eculizumab). Prescribing Information. Alexion Pharmaceuticals, Inc., Boston, MA; 2020 November.
ENSPRYNG® (satralizumab-mwge). Prescribing Information. Genentech, Inc., San Francisco; 2022 March.
Uplizna® (inebilizumab-cdon). Prescribing Information. Horizon Therapeutics, Dublin, 2021 July.
Soliris: product information. European Medicines Agency, London, 2021.
Yamamura T, Kleiter I, Fujihara K, et al. Trial of satralizumab in neuromyelitis optica spectrum disorder. N Engl J Med. 2019;381(22):2114–24.
Traboulsee A, Greenberg BM, Bennett JL, et al. Safety and efficacy of satralizumab monotherapy in neuromyelitis optica spectrum disorder: a randomised, double-blind, multicentre, placebo-controlled phase 3 trial. Lancet Neurol. 2020;19(5):402–12.
Cree BAC, Bennett JL, Kim HJ, et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial. Lancet. 2019;394(10206):1352–63.
Update on US regulatory review of ULTOMIRIS® in NMOSD [press release]. AstraZeneca, 6 September 2023.
Pittock SJ, Barnett M, Bennett JL, et al. Ravulizumab in aquaporin-4-positive neuromyelitis optica spectrum disorder. Ann Neurol. 2023;93(6):1053–68.
Dias S, Ades AE, Welton NJ, Jansen JP, Sutton AJ. Generalised linear models. In: Dias S, Welton NJ, Jansen JP, Sutton AJ, editors. Network meta-analysis for decision making. Wiley; 2018. p. 93–153.
Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR task force on indirect treatment comparisons good research practices: part 1. Value Health. 2011;14(4):417–28.
Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Mak. 2013;33(5):607–17.
Hoaglin DC, Hawkins N, Jansen JP, et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR task force on indirect treatment comparisons good research practices: part 2. Value Health. 2011;14(4):429–37.
Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84.
Rouse B, Chaimani A, Li T. Network meta-analysis: an introduction for clinicians. Intern Emerg Med. 2017;12(1):103–11.
Evrenoglou T, White IR, Afach S, Mavridis D, Chaimani A. Network meta-analysis of rare events using penalized likelihood regression. Stat Med. 2022;41(26):5203–19.
Heinze G, Schemper M. A solution to the problem of monotone likelihood in Cox regression. Biometrics. 2001;57(1):114–9.
Lin DY, Dai L, Cheng G, Sailer MO. On confidence intervals for the hazard ratio in randomized clinical trials. Biometrics. 2016;72(4):1098–102.
Tahara M, Oeda T, Okada K, et al. Safety and efficacy of rituximab in neuromyelitis optica spectrum disorders (RIN-1 study): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2020;19(4):298–306.
Cree BA, Bennett JL, Kim HJ, et al. Sensitivity analysis of the primary endpoint from the N-MOmentum study of inebilizumab in NMOSD. Mult Scler. 2021;27(13):2052–61.
Acknowledgements
Medical Writing
Medical writing support was provided by Danielle E. Dalechek, MS, Yashodhara Dasgupta, PhD, and Leanne M. Low, PhD, of Oxford PharmaGenesis Inc., Newtown, PA, USA, and was funded by Alexion Pharmaceuticals, Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
The authors disclosed financial support for the research, authorship, and/or publication of this article. Research funding and publication fees, including the journal’s Rapid Service Fee, were provided by Alexion, AstraZeneca Rare Disease.
Author information
Authors and Affiliations
Contributions
Conception and design (all authors), acquisition of data (Ina Zhang, Sami Fam, Adrian Kielhorn, Jeroen Jansen), analysis or interpretation of data (all authors); and preparation of manuscript (all authors), critical review of manuscript (all authors). The study sponsor reviewed the analysis plan and study manuscript; all aspects of the SLR (e.g., searches, screening, extraction), data management, processing, and analyses were conducted by PRECISIONheor, and all final analytic decisions were made by study investigators. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Stacey L. Clardy is an employee of the University of Utah and the Salt Lake City VA. She and her institution have received research support from NIH/NINDS (U01, The ExTINGUISH Trial), the Western Institute for Veteran Research, the Siegel Rare Neuroimmune Association, the Immune Deficiency Foundation, Viela Bio/Horizon, Alexion/AstraZeneca, the Barbara Gural Steinmetz Foundation, and the Sumaira Foundation for NMO, and consulting/advisory board fees from Alexion, VielaBio/Horizon, and Genentech/Roche. Sean J. Pittock has received personal compensation for serving on scientific advisory boards for F. Hoffmann-La Roche (which manufactures satralizumab, an approved targeted therapy for NMOSD) and his institution has received grants; his institution has received grants, personal fees, nonfinancial support, research support, and compensation for serving as a consultant for Alexion, AstraZeneca Rare Disease (which holds the patent rights to ravulizumab and eculizumab). He holds Patent # 9,891,219B2, Application # 12–573,942, Methods for Treating Neuromyelitis Optica (NMO) by Administration of Eculizumab to an Individual That Is Aquaporin-4 (AQP4)-IgG Autoantibody Positive, which has been issued and for which he has received royalties. He also reports grants, personal fees, nonfinancial support, and other support from Horizon, MedImmune (which produces inebilizumab). Orhan Aktas reports grants from the German Ministry of Education and Research (BMBF) and the German Research Foundation (DFG); grants and personal fees from Biogen, Genzyme, Novartis, and Teva, and personal fees from Alexion, Almirall, Bristol Myers Squibb, Horizon Therapeutics, Merck Serono, and Roche, and is a member of the European Reference Network for Rare Eye Diseases (ERN-EYE), co-funded by the Health Program of the European Union under the Framework Partnership Agreement No 739434 ‘ERN-EYE’. Jin Nakahara reports personal fees from AbbVie, Alexion Pharma GK, Asahi Kasei Medical, Biogen, Bristol Myers Squibb, Chugai, CSL Behring, Daiichi Sankyo, Eisai, Kyorin, Mitsubishi Tanabe Pharma, Novartis, Otsuka, Roche, Takeda, and Teijin Pharma; research scholarships from AbbVie, Boehringer Ingelheim, Chugai, Daiichi Sankyo, EA Pharma, Eisai, JB, Mitsubishi Tanabe Pharma, Otsuka, Shionogi, Sumitomo Pharma, Teijin Pharma, and Tsumura; and grants from the Ministry of Education, Science and Technology of Japan, the MHLW, and Biogen. Noriko Isobe reports speaker honorarium from Alexion Pharma GK, Biogen Japan, Chugai, CSL Behring, Daiichi Sankyo, Eisai, Mitsubishi Tanabe Pharma, Novartis, Takeda Pharma, Teijin Pharma; research grants from Teijin Pharma, Chugai; and collaborative research with Biogen Japan. Diego Centonze is an employee of the University of Rome Tor Vergata. Sami Fam is an employee and stockholder of Alexion Pharmaceuticals. Adrian Kielhorn is an employee and stockholder of Alexion Pharmaceuticals. Jeffrey Yu is an employee and stockholder of Alexion Pharmaceuticals. Jeroen Jansen is an employee of PRECISIONheor, a consultancy firm, which received funding from Alexion Pharmaceuticals to support this work. Ina Zhang is an employee of PRECISIONheor, a consultancy firm, which received funding from Alexion Pharmaceuticals to support this work.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Clardy, S.L., Pittock, S.J., Aktas, O. et al. Network Meta-analysis of Ravulizumab and Alternative Interventions for the Treatment of Neuromyelitis Optica Spectrum Disorder. Neurol Ther (2024). https://doi.org/10.1007/s40120-024-00597-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40120-024-00597-7