Abstract
Introduction
In STRIVE, natalizumab treatment demonstrated effectiveness in clinical, magnetic resonance imaging (MRI), and patient-reported outcomes (PROs) in patients with early relapsing–remitting multiple sclerosis (RRMS). This post hoc analysis examined the effectiveness and safety of natalizumab in patients who self-identified as either Black/African American (AA) or Hispanic/Latino.
Methods
Clinical, MRI, and PROs were assessed for the Black/AA subgroup (n = 40) and compared with the non-Hispanic White subgroup (n = 158). As a result of the very small sample size, outcomes for the Hispanic/Latino subgroup (n = 18) were assessed separately, including a sensitivity analysis with Hispanic/Latino patients who completed the 4-year study on natalizumab.
Results
Clinical, MRI, and PROs were comparable between the Black/AA and non-Hispanic White subgroups except for MRI outcomes at year 1. A higher proportion of non-Hispanic White than Black/AA patients achieved MRI no evidence of disease activity (NEDA; 75.4% vs. 50.0%, p = 0.0121) and no new or newly enlarging T2 lesions (77.6% vs. 50.0%, p = 0.0031) at year 1; these differences were not observed in years 2–4 of the study. For the Hispanic/Latino subgroup in the intent-to-treat population, 46.2% and 55.6% achieved NEDA at years 1 and 2; 66.7% and 90.0% achieved clinical NEDA at years 3 and 4. Annualized relapse rate was reduced by 93.0% at year 1 versus the year before natalizumab initiation; this reduction was maintained throughout the study. Over 4 years, 37.5–50.0% of patients had a clinically meaningful improvement in their Symbol Digit Modalities Test score, and 81.8–100.0% and 90.9–100.0% had stable/improved Multiple Sclerosis Impact Scale-29 physical and psychological scores, respectively. Similar results were observed in the sensitivity analysis with Hispanic/Latino subgroup of the 4-year natalizumab completers.
Conclusion
These results highlight the effectiveness and safety of natalizumab in patients with early RRMS who self-identified as Black/AA or Hispanic/Latino.
ClinicalTrials.gov
NCT01485003.
Similar content being viewed by others
Data on the effectiveness and safety of multiple sclerosis (MS) disease-modifying therapies in minority underrepresented populations are limited. |
STRIVE, a 4-year observational study, demonstrated safety and effectiveness of natalizumab treatment in patients with early relapsing–remitting MS (RRMS). |
This post hoc analysis of STRIVE examined these outcomes among patients who self-identified as either Black/African American (AA) or Hispanic/Latino with early RRMS. |
Natalizumab treatment demonstrated effectiveness on clinical, MRI, and patient-reported outcomes in Black/AA and Hispanic/Latino patients with early RRMS. |
These data may help clinicians make treatment decisions for patients with early RRMS in these minority underrepresented populations. |
Introduction
Data on the efficacy and safety of disease-modifying therapies (DMTs) in Black/African American (AA) and Hispanic/Latino patients are limited because of the lack of information and underrepresentation of these minority patient populations in multiple sclerosis (MS) clinical studies [1,2,3,4]. Of the 45 MS phase III DMT trials, only 14 (31.1%) reported the patients’ racial breakdown, while the remaining 31 trials (68.9%) did not report race/ethnicity or categorized White patients only [4]. Studies examining the long-term effectiveness and safety of MS DMTs in real-world settings are also limited for these minority groups, regardless of the specific DMT [5,6,7]. Such sparsity of evidence-based data on DMTs in minority populations makes it challenging for clinicians when their patients ask for information on the benefit/risk profile of specific treatments based on racial and/or ethnic backgrounds.
Although there has been an effort to increase focus on studying MS DMTs in minority populations, it will take time to correct this underrepresentation. This is evident from a recent survey that found Black/AA and Hispanic patients with MS were more concerned about receiving poor-quality medical care as well as being taken advantage of by the research team than White and non-Hispanic patients [8]. In the interim, any effectiveness or safety data on the use of specific MS DMTs in these minority populations, regardless of the sample size, is urgently needed, given the current paucity of information.
Natalizumab (TYSABRI®) is an infusible DMT that is approved for the treatment of adult patients with relapsing forms of MS [9] on the basis of results from the phase III AFFIRM (NCT00027300) [10] and SENTINEL (NCT00030966) [11] trials. ln AFFIRM, natalizumab significantly reduced clinical and magnetic resonance imaging (MRI) disease activity in patients with relapsing–remitting MS (RRMS) compared with placebo over 2 years [10]. As only 10 patients in AFFIRM self-identified as Black/AA, the post hoc analysis evaluating the efficacy of natalizumab in patients of African descent included an additional 39 Black/AA patients from SENTINEL. The results of this post hoc analysis showed that natalizumab significantly reduced the annualized relapse rate (ARR) over 2 years by 60% (0.21 vs. 0.53, p = 0.02), and the mean number of gadolinium-enhancing (Gd+) and new/newly enlarging T2 lesions by 79% (0.19 vs. 0.91, p = 0.03) and 90% (0.88 vs. 8.52, p = 0.008), respectively, versus the comparator group [2]. Similarly, as only 13 patients in AFFIRM self-identified as Hispanic, the post hoc analysis evaluating the efficacy of natalizumab in Hispanic patients included an additional 22 Hispanic patients from SENTINEL. The results showed that natalizumab significantly reduced ARR over 2 years by 85% (0.21 vs. 1.41, p < 0.001) and the mean number of Gd+ and new/newly enlarging T2 lesions at 2 years by 100% (0 vs. 1.55, p = 0.02) and 95% (0.57 vs. 11.64, p = 0.01), respectively, versus the comparator arm [3].
Prospective long-term real-world use of natalizumab in patients with RRMS who self-identify as belonging to minority racial or ethnic groups is limited to the 4-year STRIVE study (NCT01485003), which looked at the effectiveness and safety of natalizumab in patients with RRMS who had a disease duration of 3 years or less [12]. In this post hoc analysis of STRIVE, we examined the effectiveness and safety of natalizumab over 4 years of the study in patients with RRMS who self-identified as either Black/AA or Hispanic/Latino.
Methods
Study Design and Patients
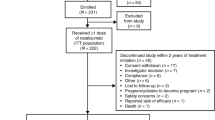
STRIVE was a prospective, 4-year, multicenter, observational, open-label, single-arm, phase IV study, conducted in the USA between February 2012 and November 2018. Patients received 300 mg of natalizumab intravenously every 4 weeks. This post hoc subgroup analysis included patients with RRMS enrolled in STRIVE who self-identified as either Black/AA or Hispanic/Latino. Patients who self-identified as non-Hispanic White were included as a comparator group. Complete details of the STRIVE study design were published previously [12]. Briefly, eligible patients were aged 18 to 65 years, tested negative for anti-JC virus (JCV) antibodies within 6 months prior to screening or at the baseline visit, and had a disease duration of 3 years or less at the time of informed consent. Patients were either naïve to DMTs or had been treated with a DMT for 36 months or less prior to the date of informed consent. Patients were excluded, however, if they had prior natalizumab treatment [12].
In STRIVE, the intent-to-treat (ITT) population included all enrolled patients who completed informed consent and received at least one dose of natalizumab. If a patient permanently discontinued natalizumab treatment but chose to remain in the study, data were collected on the reasons for discontinuation. In such cases, the investigative site continued to follow up with the patient as per the protocol schedule of assessments through month 48. If a patient withdrew from the study, the participating neurologist documented the reason for early withdrawal on the study exit form and conducted the final assessments, after which no further data were collected.
All patients provided written informed consent prior to enrollment. Approval was granted by the Copernicus Group IRB #1 (reference number IRB00001313) and, at the rest of the study sites, by an independent ethics committee. The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Outcomes
Effectiveness analyses in the racial/ethnic subgroups were consistent with those conducted in the overall ITT population and included no evidence of disease activity (NEDA), ARR, 24-week confirmed disability worsening (CDW) and confirmed disability improvement (CDI), as well as MRI, Symbol Digit Modalities Test (SDMT), and Multiple Sclerosis Impact Scale (MSIS-29) as described previously [12, 13]. NEDA encompassed both clinical and MRI NEDA. The primary endpoints of STRIVE were the proportion of patients who achieved NEDA in years 1 and 2 and clinical NEDA in years 3 and 4. Clinical NEDA was defined as no relapses or CDW. CDW was defined as an Expanded Disability Status Scale (EDSS) score increase of at least 0.5 from a baseline of 6.0 or higher, at least 1.0 from a baseline of 1.0 to less than 6.0, or at least 1.5 from a baseline of 0.0, confirmed after 24 weeks. MRI NEDA was defined as no Gd+ lesions and no new or newly enlarging T2 lesions. MRI was conducted at baseline and yearly thereafter and analyzed by a central reader, NeuroRx Research (Montreal, Quebec, Canada). Details of the MRI acquisition and analysis were published previously [12]. CDI was defined as a decrease of at least 1.0 point from a baseline EDSS score of 2.0 or higher, confirmed after 24 weeks. SDMT was assessed at baseline and yearly thereafter as a measure of cognitive processing speed [13]. The SDMT score reflects the number of correct matches, with a higher score indicating better cognitive processing speed. A clinically meaningful improvement in SDMT score was defined as an increase of at least 4 points [14]. Patient quality of life was assessed using the MSIS-29, a brief, self-reported measurement that assesses the physical (20 questions) and psychological (9 questions) impact of MS [15]. Physical and psychological MSIS-29 scores can be divided into five categories: “no problems” (0–19), “few problems” (20–39), “moderate problems” (40–59), “quite a few problems” (60–79), and “extreme problems” (80–100). A change in the MSIS-29 physical or psychological score resulting in a downward move of at least one category was considered an improvement, as described previously [16]. No change or an improvement in the MSIS-29 category was regarded as a positive outcome for the patient. Patients completed the MSIS-29 at screening and yearly thereafter.
Safety was evaluated on the basis of any serious adverse event (SAE) experienced between the time of informed consent and the end of study and reported within 24 h by the neurologist or designee becoming aware of the event.
Statistical Analyses
The analysis population included Black/AA and Hispanic/Latino subgroups of the ITT population. Comparative effectiveness analyses against the non-Hispanic White comparator subgroup were only conducted for the Black/AA subgroup, given the very small sample size of the Hispanic/Latino cohort. Baseline characteristics were compared between the Black/AA and non-Hispanic White subgroups. In general, continuous variables were summarized using mean and standard deviation and were tested using the two-sample t test or the Mann–Whitney U test. Categorical variables were summarized using count and percentage and tested using chi-squared test or Fisher’s exact test. Statistical significance was defined as p < 0.05.
NEDA was analyzed over 4 years and used observed data only; missing data were not imputed. Patients who had missing data but evidence of disease activity on at least one NEDA subcomponent were included in the analysis. Patients were excluded, however, if they had missing data and did not exhibit disease activity on available NEDA subcomponents. To account for potential disease activity shortly after natalizumab initiation, an exploratory analysis assessed NEDA over years 2 to 4 following MRI re-baselining at year 1. NEDA, clinical NEDA, MRI NEDA, SDMT, and MSIS-29 outcomes were compared between the Black/AA and non-Hispanic White subgroups using multiple logistic regression adjusting for baseline confounders. Adjusted ARRs were compared using a negative binomial model with repeated measures, and cumulative probabilities of CDW were assessed with Kaplan–Meier methods.
To provide a better appreciation of the long-term effectiveness of natalizumab in patients who self-identified as Hispanic/Latino, sensitivity analyses were conducted for NEDA, MRI, SDMT, and MSIS-29 using data from Hispanic/Latino patients who completed the 4-year study on natalizumab (i.e., 4-year natalizumab completers).
Results
Black/AA Patients
Patient Enrollment and Baseline Characteristics
Of 222 patients in the STRIVE ITT population, 40 patients (18.0%) self-identified as Black/AA (Table S1 in the supplementary material). During the study, 15 patients (37.5%) in the Black/AA subgroup discontinued natalizumab; the most common reason was seroconversion to anti-JCV antibody-positive status/elevated index/progressive multifocal leukoencephalopathy risk (n = 5). Reported lack of efficacy was not listed as a reason for discontinuation in any of the Black/AA patients. Of the 15 Black/AA patients who discontinued natalizumab, 7 withdrew from the study.
Patients in the Black/AA subgroup had active disease at baseline, as indicated by a Gd+ lesion count ranging from 0 to 54 (median = 0) and a median (range) EDSS score of 2.0 (0–4), as well as a median (range) of 1 (0–4) relapse in the year before natalizumab treatment initiation. Almost half of the Black/AA patients had a history of prior MS treatment. Baseline characteristics of the non-Hispanic White patients were similar to those of the Black/AA patients, with the exception that the Black/AA subgroup had a higher T2 lesion volume compared to the non-Hispanic White subgroup (Table S1 in the supplementary material).
No Evidence of Disease Activity
In the Black/AA subgroup, 41.2% and 68.6% achieved NEDA at years 1 and 2, respectively (Fig. 1a); 80.0% and 96.8% achieved clinical NEDA at years 3 and 4, respectively (Fig. 1b). Over the study, between 50.0% and 87.9% of Black/AA patients achieved MRI NEDA (Fig. 1c). In the exploratory analysis with MRI re-baselining at year 1, 62.5%, 72.7%, and 83.9% of the Black/AA patients achieved NEDA, clinical NEDA, and MRI NEDA, respectively, in years 2 to 4 (Fig. 1).
Similar results were observed in the non-Hispanic White subgroup, with the exception that significantly more non-Hispanic White patients achieved MRI NEDA at year 1 than Black/AA patients [75.4% vs. 50.0%, p = 0.0121] (Fig. 1c).
Clinical Outcomes: ARR, CDW, and CDI
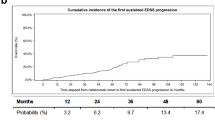
In the Black/AA subgroup, the adjusted ARR decreased by 90.2% from 1.12 (95% confidence interval [CI] 0.81–1.56) in the year prior to natalizumab initiation to 0.11 (95% CI 0.03–0.36) at year 4 (Fig. 2a). The cumulative probability of CDW (Fig. 2b) and CDI (Fig. 2c) at year 4 was 28.1% and 31.0%, respectively, for the Black/AA subgroup. Similar results were observed in the non-Hispanic White subgroup, with no statistically significant differences found between the two groups (Fig. 2).
Summary of clinical outcome measures a adjusted ARR,a b CDW, c CDI, and d SDMTb over 4 years in STRIVE Black/AA and non-Hispanic White subgroups of the ITT population. AA African American, ARR annualized relapse rate, CDI confirmed disability improvement, CDW confirmed disability worsening, CI confidence interval, GEE generalized estimating equation, ITT intent-to-treat, SDMT Symbol Digit Modalities Test. Dashed lines indicate 95% confidence interval. aType 3 GEE analysis showed that there is a significant change over time (p < 0.0001), but no significant difference detected between the Black/AA and non-Hispanic White ITT subgroups (p = 0.6297). bBaseline SDMT score was added as an additional covariate for the multiple logistic regression model
Clinical Outcomes: Symbol Digit Modalities Test
The percentage of Black/AA patients experiencing clinically meaningful improvement in their SDMT score (i.e., at least 4 points) from baseline ranged from 41.7% to 48.6% over the 4-year study (Fig. 2d). Similar results were observed in the non-Hispanic White subgroup with 42.3–57.7% experiencing clinically meaningful improvement in their SDMT score (Fig. 2d; p > 0.05 at all 4 years).
MRI Outcomes
In the Black/AA subgroup, 50.0% of patients had no new or newly enlarging T2 lesions at year 1 with over 80.0% of the patients having no new lesions at years 2–4 (Fig. 3a). Throughout the study, over 90.0% of the Black/AA patients had no Gd+ lesion at each annual assessment (Fig. 3b). Similar results were seen in the non-Hispanic White group with the exception that there were more non-Hispanic White patients who had no new or newly enlarging T2 lesions at year 1 compared to Black/AA patients (77.6% vs 50.0%, p = 0.0031) (Fig. 3a).
Patient-Reported Outcomes: MSIS-29
The percentage of Black/AA patients experiencing stability or improvement in their annual MSIS-29 physical score from screening ranged from 77.8% to 87.9% over the study (Fig. 4a); 80.6–90.9% of Black/AA patients experienced stability or improvement in their annual MSIS-29 psychological score from screening (Fig. 4b). Similar results were seen in the non-Hispanic White subgroup with 86.3–90.8% and 90.2–93.1% experiencing stability or improvement from screening in their annual MSIS-29 physical and psychological scores, respectively (Fig. 4).
Safety
SAEs were reported in 6 of 40 (15%) Black/AA patients (Table S2 in the supplementary material). No cases of progressive multifocal leukoencephalopathy were reported, and no single SAE was reported in two or more Black/AA patients. One patient who self-identified as Black/AA had a treatment-related SAE of anaphylactic reaction but was not hospitalized for evaluation.
Hispanic/Latino Patients
Patient Enrollment and Baseline Characteristics
Of 222 patients in the STRIVE ITT population, 18 patients (8.1%) self-identified as Hispanic/Latino (Table S3 in the supplementary material). A total of 9 patients (50%) in the Hispanic/Latino subgroup discontinued natalizumab during the study. The most common reasons for natalizumab discontinuation were consent withdrawal and reported lack of efficacy (n = 2 for both). Of the 9 Hispanic/Latino patients who discontinued natalizumab, 7 withdrew from the study. One patient, who did not discontinue natalizumab but was lost to follow-up, also withdrew from the study.
In the ITT population, patients who self-identified as Hispanic/Latino had active disease at baseline, as indicated by a Gd+ lesion count ranging from 0 to 21 (median = 1) and a median (range) EDSS score of 2.0 (0–4), as well as a median (range) of 2 (1–4) relapses in the year before natalizumab treatment initiation. Most Hispanic/Latino patients had a history of MS treatment. Baseline characteristics for the Hispanic/Latino 4-year natalizumab completer subgroup were generally similar to those of the Hispanic/Latino ITT subgroup, with the exceptions that the 4-year natalizumab completer subgroup appeared to have higher MRI disease activity as well as a shorter prior MS treatment duration than the Hispanic/Latino ITT subgroup (Table S3 in the supplementary material).
No Evidence of Disease Activity
In the Hispanic/Latino ITT subgroup, 46.2% and 55.6% achieved NEDA at years 1 and 2, respectively (Fig. 5a); 66.7% and 90.0% achieved clinical NEDA at years 3 and 4, respectively (Fig. 5b). Over the study, between 66.7% and 90.0% of Hispanic/Latino ITT patients achieved MRI NEDA (Fig. 5c). The exploratory analysis with MRI re-baselining at year 1 found that 27.3%, 36.4%, and 90.0% of the Hispanic/Latino ITT patients achieved NEDA, clinical NEDA, and MRI NEDA, respectively, in years 2 to 4 (Fig. 5). In general, similar results were observed in the Hispanic/Latino 4-year natalizumab completer subgroup (Fig. 5).
Clinical Outcomes: ARR, EDSS, SDMT
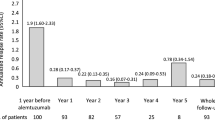
In the Hispanic/Latino ITT subgroup, the ARR decreased by 91.3% from 1.72 (95% CI 1.38–2.15) in the year prior to natalizumab initiation to 0.15 (0.05–0.44) at year 4 (Fig. 6a).
Summary of clinical outcome measures a ARR, b EDSS, and c SDMT over 4 years in STRIVE Hispanic/Latino subgroups of the ITT population and the 4-year natalizumab completers.a ARR annualized relapse rate, CI confidence interval, EDSS Expanded Disability Status Scale, ITT intent-to-treat, SDMT Symbol Digit Modalities Test. aAs a result of the extremely low sample size (n = 6) it was not possible to calculate the ARR for the 4-year natalizumab completers
In both Hispanic/Latino subgroups the median EDSS score remained stable at 1.5 at all annual assessments except for year 2, where it was 1.0 (Fig. 6b).
The percentage of Hispanic/Latino ITT patients experiencing clinically meaningful improvement in their SDMT score (i.e., at least 4 points) from baseline ranged from 37.5% to 50% over the 4-year study (Fig. 6c). Similar results were observed in the Hispanic/Latino 4-year natalizumab completer subgroup with the exception of year 1, where more patients experienced clinically meaningful improvement in their SDMT score than in the ITT subgroup (83.3% vs. 50.0%) (Fig. 6c).
MRI Outcomes
In the Hispanic/Latino ITT subgroup, the median number of new or newly enlarging T2 lesions was 0 at all annual assessments (Table S4 in the supplementary material). Throughout the study, the median number of Gd+ lesions at each annual assessment was also 0 in the Hispanic/Latino ITT subgroup. Similar results were seen in the Hispanic/Latino 4-year natalizumab completer subgroup with the median number of new or newly enlarging T2 lesions and Gd+ lesions equal to 0 at all annual assessments (Table S4 in the supplementary material).
Patient-Reported Outcome: MSIS-29
The percentage of Hispanic/Latino ITT patients experiencing stability or improvement in their annual MSIS-29 physical score from screening ranged from 81.8% to 100% over the study (Fig. 7a); 90.9–100% of Hispanic/Latino ITT patients experienced stability or improvement in their annual MSIS-29 psychological score from screening (Fig. 7b). Similar results were seen in the Hispanic/Latino 4-year natalizumab completer subgroup with 83.3–100% experiencing stability or improvement from screening in both their annual MSIS-29 physical and psychological scores, respectively (Fig. 7).
Safety
SAEs were reported in 5 of 18 (27.8%) Hispanic/Latino patients (Table S2 in the supplementary material). No cases of progressive multifocal leukoencephalopathy were reported, and no single SAE was reported in two or more Hispanic/Latino patients.
Discussion
Only two high-efficacy DMTs, natalizumab and ocrelizumab, have published post hoc analyses of their phase III pivotal trials examining efficacy in patients of African descent [1, 2]. Both analyses found that the treatment reduced clinical and MRI activity versus placebo/interferon beta-1a in patients of African descent [1, 2]. Although these results are promising, authors of both publications stated further research was needed. This post hoc analysis of STRIVE represents the first prospective real-world effectiveness and safety data published for a high-efficacy monoclonal antibody DMT in patients with early RRMS who self-identify as Black/AA. As research has suggested a more aggressive disease course in Black/AA than in non-Hispanic White patients [17,18,19,20,21], patients who self-identified as non-Hispanic White were included as a comparator group in this post hoc analysis of STRIVE to determine whether the effectiveness of natalizumab in Black/AA and non-Hispanic White patients is comparable.
The only difference found between the two subgroups regarding baseline characteristics was T2 lesion volume, with the Black/AA subgroup having a higher T2 lesion volume compared to the non-Hispanic White subgroup. This finding is consistent with that observed in the OPERA trials, where patients of African descent had higher T2 lesion volumes than those of non-African descent [1]. However, unlike STRIVE, the post hoc analysis of the OPERA trials also found that compared with patients of non-African descent, those of African descent tended to have a shorter disease duration and were less likely to have had DMT treatment within the 2 years prior to baseline. This apparent difference in disease duration may be in part due to the inclusion criteria for STRIVE, which required a disease duration of at most 3 years at the time of enrollment [12]. In comparison, OPERA I and II did not specify disease duration in their inclusion criteria and only excluded patients who had a disease duration of more than 10 years, with an EDSS of 2.0 or less at screening [22].
The proportion of patients achieving NEDA, clinical NEDA, and MRI NEDA was generally similar in the Black/AA and non-Hispanic White subgroups, apart from MRI NEDA at year 1, where a higher proportion of non-Hispanic White than Black/AA patients achieved MRI NEDA (75.4% vs. 50.0%, p = 0.0121). Consistent with this finding, a higher proportion of non-Hispanic White patients had no new/newly enlarging T2 lesions at year 1 compared to Black/AA patients (77.6% vs. 50.0%, p = 0.0031). Although all statistical analyses were adjusted for differences in baseline T2 lesion volume, there is still a possibility of new MRI disease activity occurring after the baseline scan before the onset of effectiveness. As none of the Black/AA patients had Gd+ lesions at year 1, the presence of new/newly enlarging T2 lesions was solely responsible for only 50% of the Black/AA patients achieving MRI NEDA in year 1. Based on these findings, one plausible hypothesis could be that the onset of radiological effectiveness occurs earlier in the non-Hispanic White than Black/AA patients since T2 lesions reflect overall burden of disease. This could potentially explain why the differences were only evident at year 1; however, further studies with more frequent imaging and larger sample sizes adequately powered to detect a difference would be needed to test this hypothesis.
The high annual rates of clinical NEDA in both the Black/AA and non-Hispanic White subgroups were consistent with the dramatically reduced adjusted ARRs as well as the low 24-week CDW seen in both subgroups while on natalizumab treatment.
The proportion of patients in the Black/AA and non-Hispanic White subgroups experiencing clinically meaningful improvement in their SDMT score from baseline was similar over the course of the study. Similarly, the percentage of patients experiencing stability or improvement in their annual MSIS-29 physical and psychological score from screening was comparable between the two subgroups. Over 4 years, safety in Black/AA patients with RRMS was consistent with that observed in the overall STRIVE population and with natalizumab’s well-established safety profile [9, 10, 12, 23, 24].
This post hoc analysis of STRIVE also represents the first prospective real-world effectiveness and safety data published for a high-efficacy monoclonal antibody DMT in patients with early RRMS who self-identify as Hispanic/Latino. As a result of the very small sample size of the Hispanic/Latino subgroup in the ITT population (n = 18), it was not feasible to conduct a comparative analysis with the non-Hispanic White subgroup. However, given the importance of this information in the absence of any effectiveness and safety data for high-efficacy monoclonal DMTs in Hispanic/Latino patients, results for the STRIVE Hispanic/Latino subgroup analysis were included separately as a case series. As patients in the ITT population only had to have one dose of natalizumab, data from the Hispanic/Latino 4-year natalizumab completer subgroup was also presented to provide some appreciation of the long-term effectiveness of natalizumab in this ethnic group. The patients in the Hispanic/Latino 4-year natalizumab completer subgroup had a higher MRI disease activity at baseline than the overall Hispanic/Latino ITT subgroup. This high level of MRI disease activity may have played a role in the decision to keep the patients on therapy.
NEDA, clinical NEDA, and MRI NEDA at each annual assessment were generally similar between the Hispanic/Latino ITT and 4-year natalizumab completer subgroups. There were numerical differences between the groups in the proportion of patients achieving NEDA at year 1 (46.2% ITT vs. 66.7% 4-year completers) and year 2 (55.6% ITT vs. 80.0% 4-year completers). A combination of missing data and very small sample sizes likely played a role in these differences. As a result of the potential for disease activity prior to the onset of action, a sensitivity analysis was done looking at NEDA, clinical NEDA, and MRI NEDA in both subgroups following MRI re-baselining at year 1. A smaller proportion of the Hispanic/Latino subgroup achieved NEDA and clinical NEDA in years 2 to 4 after MRI re-baselining in both the ITT population and 4-year natalizumab completers. As the proportion of patients achieving MRI NEDA in years 2 to 4 after MRI re-baselining was greater than 80% in both Hispanic/Latino subgroups, it suggests that the clinical disease activity may have played a large role in the lower proportion of patients achieving NEDA. However, this is not supported by the other clinical data. Specifically, over the course of the study the ARR was low, ranging from 0.12 to 0.15, and the median EDSS was stable between 1 and 1.5. Hence, the low proportions of patients achieving NEDA and clinical NEDA in two groups may again be a consequence of missing data and very small sample sizes. In support of this, the ARR and MRI results in the Hispanic/Latino ITT subgroup are consistent with those of the AFFIRM/SENTINEL Hispanic post hoc analysis. Further research is needed, however, given the very small sample sizes. The proportion of patients in the Hispanic/Latino ITT and 4-year natalizumab completer subgroups experiencing clinically meaningful improvement in their SDMT score from baseline was similar over the course of the study with the exception of year 1, where numerically more patients in the 4-year natalizumab completer subgroup experienced clinically meaningful improvement than in the ITT subgroup (83.3% vs. 50.0%). Although the reason for this discrepancy is unknown, it may be partly because the median SDMT score at baseline was lower in the 4-year natalizumab completer subgroup compared to the ITT subgroup (data not shown). A recent exploratory analysis of the complete ITT STRIVE data set found that a higher proportion of patients with baseline SDMT scores below the lowest quartile experienced clinically meaningful improvements in their SDMT scores compared with the other two quartile subgroups, the difference most evident at years 1 and 2 [13]. The percentage of patients experiencing stability or improvement in their annual MSIS-29 physical and psychological score from screening was similar between the Hispanic/Latino ITT and 4-year natalizumab completer subgroups.
Safety over 4 years in Hispanic/Latino patients with RRMS was consistent with that observed in the overall STRIVE population and with natalizumab’s well-established safety profile [9, 10, 12, 23, 24].
In addition to the limitations discussed in the previous STRIVE manuscripts [12, 13], including the open-label single-arm study design and missing data, the small sizes of the racial/ethnic subgroups limited conclusions from being drawn from the current analysis. Also, STRIVE did not capture social determinants of health, including annual income, education, and access to healthcare (e.g., insurance) [25], which are known to impact clinical outcomes.
Conclusion
Overall, the results from this STRIVE post hoc racial/ethnic minority subgroup analysis highlight the long-term effectiveness and safety of natalizumab treatment in Black/AA and Hispanic/Latino patients when administered early in the RRMS disease course. This evidence-based data on natalizumab treatment in these minority underrepresented populations informs clinicians making treatment decisions for patients with RRMS who self-identify as Black/AA or Hispanic/Latino.
References
Cree BAC, Pradhan A, Pei J, Williams MJ, OPERA I and OPERA II clinical investigators. Efficacy and safety of ocrelizumab vs interferon beta-1a in participants of African descent with relapsing multiple sclerosis in the phase III OPERA I and OPERA II studies. Mult Scler Relat Disord. 2021;52:103010.
Cree BA, Stuart WH, Tornatore CS, Jeffery DR, Pace AL, Cha CH. Efficacy of natalizumab therapy in patients of African descent with relapsing multiple sclerosis: analysis of AFFIRM and SENTINEL data. Arch Neurol. 2011;68(4):464–8.
Herbert J, Rivera V, Ford C, et al. Effects of natalizumab on relapses and MRI outcomes in Hispanic patients with relapsing MS. 25th Congress of the European Committee of Treatment and Research in Multiple Sclerosis; 2009.
Onuorah HM, Charron O, Meltzer E, et al. Enrollment of non-white participants and reporting of race and ethnicity in phase III trials of multiple sclerosis DMTs: a systematic review. Neurology. 2022;98(9):e880–92.
Williams MJ, Amezcua L, Okai A, et al. Real-world safety and effectiveness of dimethyl fumarate in black or African American patients with multiple sclerosis: 3-year results from ESTEEM. Neurol Ther. 2020;9(2):483–93.
Stephensen J, Hou L, Agarwal S, et al. African American MSIS-29 scores improve after 6 natalizumab infusions. Consortium of Multiple Sclerosis Centers 2010 Annual Meeting; 2010.
Stephensen J, Hou L, Agarwal S, et al. Early effects of natalizumab on the cognitive functioning, fatigue and quality of life of African American patients with multiple sclerosis. American Academy of Neurology 2010 Annual Meeting; 2010.
Pimentel Maldonado DA, Moreno A, Williams MJ, et al. Perceptions and preferences regarding multiple sclerosis research among racial and ethnic groups. Int J MS Care. 2021;23(4):170–7.
Tysabri Prescribing Information. TYSABRI® (natalizumab) [prescribing information]. Cambridge, MA: Biogen; 2021.
Polman CH, O’Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899–910.
Rudick RA, Stuart WH, Calabresi PA, et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):911–23.
Perumal J, Balabanov R, Su R, et al. Natalizumab in early relapsing-remitting multiple sclerosis: a 4-year, open-label study. Adv Ther. 2021;38(7):3724–42.
Perumal J, Balabanov R, Su R, et al. Improvements in cognitive processing speed, disability, and patient-reported outcomes in patients with early relapsing-remitting multiple sclerosis treated with natalizumab: results of a 4-year, real-world, open-label study. CNS Drugs. 2022;36:977–93.
Benedict RH, DeLuca J, Phillips G, et al. Validity of the symbol digit modalities test as a cognition performance outcome measure for multiple sclerosis. Mult Scler. 2017;23(5):721–33.
Hobart J, Lamping D, Fitzpatrick R, Riazi A, Thompson A. The Multiple Sclerosis Impact Scale (MSIS-29): a new patient-based outcome measure. Brain. 2001;124(Pt 5):962–73.
Stephenson JJ, Kern DM, Agarwal SS, et al. Impact of natalizumab on patient-reported outcomes in multiple sclerosis: a longitudinal study. Health Qual Life Outcomes. 2012;10:155.
Caldito NG, Saidha S, Sotirchos ES, et al. Brain and retinal atrophy in African-Americans versus Caucasian-Americans with multiple sclerosis: a longitudinal study. Brain. 2018;141(11):3115–29.
Kister I, Chamot E, Bacon JH, et al. Rapid disease course in African Americans with multiple sclerosis. Neurology. 2010;75(3):217–23.
Naismith RT, Trinkaus K, Cross AH. Phenotype and prognosis in African-Americans with multiple sclerosis: a retrospective chart review. Mult Scler. 2006;12(6):775–81.
Ventura RE, Antezana AO, Bacon T, Kister I. Hispanic Americans and African Americans with multiple sclerosis have more severe disease course than Caucasian Americans. Mult Scler. 2017;23(11):1554–7.
Petracca M, Palladino R, Droby A, et al. Disability outcomes in early-stage African American and White people with multiple sclerosis. Mult Scler Relat Disord. 2022;69: 104413.
Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221–34.
Butzkueven H, Kappos L, Wiendl H, et al. Long-term safety and effectiveness of natalizumab treatment in clinical practice: 10 years of real-world data from the Tysabri Observational Program (TOP). J Neurol Neurosurg Psychiatry. 2020;91(6):660–8.
Foley J, Carrillo-Infante C, Smith J, et al. The 5-year Tysabri global observational program in safety (TYGRIS) study confirms the long-term safety profile of natalizumab treatment in multiple sclerosis. Mult Scler Relat Disord. 2019;39:101863.
Amezcua L, Rivera VM, Vazquez TC, Baezconde-Garbanati L, Langer-Gould A. Health disparities, inequities, and social determinants of health in multiple sclerosis and related disorders in the US: a review. JAMA Neurol. 2021;78(12):1515–24.
Acknowledgements
The authors would like to gratefully acknowledge the STRIVE investigators, listed below, for their efforts and contributions, as well as the patients. STRIVE investigators: Bridget Bagert, MD, Roumen Balabanov, MD, Margaret Burnett, MD, Claudia Chaves, MD, Stanley Cohan, MD, PhD, Joanna Cooper, MD, Eric Eggenberger, DO, John Foley, MD, Edward Fox, MD, PhD, Robert Fox, MD, Dennis Garwacki, MD, Lawrence Goldstick, MD, Benjamin Greenberg, MD, MHS, Mark Gudesblatt, MD, Craig Herrman, MD, Jonathan Howard, MD, John Huddlestone, MD, Mark Janicki, MD, Jeffrey Kaplan, MD, George Katsamakis, MD, Amos Katz, MD, Mariko Kita, MD, Lauren Krupp, MD, Ellen Lathi, MD, Kermit Lloyd, MD, Kenneth Mankowski, DO, Tamara Miller, MD, Stephen Newman, MD, Scott Newsome, DO, Allan Perel, MD, Jai Perumal, MD, John Puente, MD, Marcus Rice, MD, Emily Riser, MD, Peter Riskind, MD, PhD, Teri Schreiner, MD, MPH, Christopher Sheppard, MD, Scott Silliman, MD, Jason Silversteen, DO, Jacob Sloane, MD, PhD, Charles Smith, MD, Ben Thrower, MD, Robert Tillett, MD, Carlo Tornatore, MD.
Funding
Funding for this study, preparation of the manuscript, Rapid Service fee, and Open Access fee was provided by Biogen.
Medical Writing and Editorial Assistance
Writing and editorial support for the preparation of this article were provided by Shelly Lim, PhD, and Celia Nelson of Ashfield MedComms (Middletown, CT, USA), an Inizio Company.
Author Contributions
Study conception and design: Jai Perumal, Roumen Balabanov, Laura Balcer, Steven Galetta, Robert J. Fox. Data collection: Jai Perumal, Roumen Balabanov, Laura Balcer, Steven Galetta, Robert J. Fox. Statistical analysis: Zhaonan Sun, Hanyue Li. All authors were involved in discussing the results, drafting and critically reviewing the manuscript for intellectual content, and provided final approval of the submitted version. All authors agree to be accountable for this work.
Disclosures
Jai Perumal has received fees from Acorda, Biogen, Genzyme, and Teva. Roumen Balabanov has received consulting fees from Biogen, Sanofi, and Teva and grant/research support from Biogen. Laura Balcer has received consulting fees from Biogen and Genzyme. Steven Galetta has received consulting fees from Biogen. Zhaonan Sun, Hanyue Li, Danette Rutledge, and Robin L. Avila are employees of and may hold stock and/or stock options in Biogen. Robert J. Fox has received personal consulting fees from AB Science, Biogen, Bristol Myers Squib, EMD Serono, Genentech, Genzyme, Greenwich Biosciences, Immunic, Janssen, Novartis, Sanofi, Siemens, and TG Therapeutics; has served on advisory committees for AB Science, Biogen, Immunic, Janssen, Novartis, and Sanofi; and received clinical trial contract and research grant funding from Biogen, Novartis, and Sanofi.
Compliance with Ethics Guidelines
All patients provided written informed consent prior to enrollment. Approval was granted by the Copernicus Group IRB #1 (reference number IRB00001313) and, at the rest of the study sites, by an independent ethics committee. The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Data Availability
The data sets generated and/or analyzed during the current study are not publicly available. The authors fully support sharing data whenever possible. Requests for deidentified data should be made to Biogen via established company data-sharing policies and processes detailed on the website http://clinicalresearch.biogen.com/.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Perumal, J., Balabanov, R., Balcer, L. et al. Long-Term Effectiveness and Safety of Natalizumab in African American and Hispanic/Latino Patients with Early Relapsing–Remitting Multiple Sclerosis: STRIVE Data Analysis. Neurol Ther 12, 833–848 (2023). https://doi.org/10.1007/s40120-023-00461-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-023-00461-0