Abstract
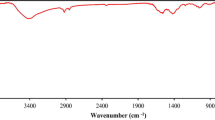
ZnMnO3/Fe3O4 magnetic nanocomposites were fabricated via facile co-precipitation route and were calcined at 400 °C for 3 h. Synthesis of ZnMnO3/Fe3O4 magnetic nanocomposites were optimized by different weight percentages. Then, the as-synthesized sample was characterized by X-ray diffraction (XRD), Fourier transform infrared (FT-IR), photoluminescence(PL), vibrating Sample Magnetometer (VSM), EDAX (Energy dispersive X-ray analysis), diffuse reflectance UV–Vis spectroscopy (DRS),ultraviolet–visible (UV–Vis) spectrometry, Bruner-Emmett-Teller (BET), transmission electron microscopy (TEM) and field emission scanning electron microscopy (FESEM). Based on the results, elemental analyses of the samples were similar to those expected from the initial concentrations of the solutions used during synthesis. The x-ray diffraction pattern revealed that ZnMnO3/Fe3O4 has a cubic structure and average particle size of the catalyst was found 27.43 nm. In addition, Fourier transform infrared spectra could confirm the presence of hydroxyl group and Fe–O bond vibration in the catalyst. Further, the superparamagnetic behavior of the synthesized nanocomposite at room temperature was confirmed by VSM studies. Furthermore, the photocatalytic performance of ZnMnO3/Fe3O4 samples were evaluated based on the removal of Congo red (CR) in aqueous solution in 60 min of under visible light irradiation. The experiment demonstrated that 0.10 g of ZnMnO3/Fe3O4 nanocomposites can degrade (98.17%) 50 mg l−1 of Congo red (CR) solution. The mechanistic study using scavengers propose that the superoxide (O2·−) is the most reactive species involved in the photodegradation of organic dyes. The photocatalytic degradation of Congo red conformed the pseudo-first-order kinetic model and the rate constant achieved for 0.10 g l−1 of ZnMnO3/Fe3O4 was (k = 0.0384 min−1). Finally, the effect of reaction time, pH, and loading of ZnMnO3/Fe3O4 on degrading Congo red was studied. The synthesized ZnMnO3/Fe3O4 nanocomposite can be potentially applied as a magnetically separable photocatalyst to deal with water pollution problems.


















Similar content being viewed by others
References
Forgacs E, Cserháti T, Oros G (2004) Removal of synthetic dyes from wastewaters: a review. Environ Int 30:953–971. https://doi.org/10.1016/j.envint2004.02.001
Xu Y, Xu H, Li H, Xia J, Liu C, Liu L (2011) Enhanced photocatalytic activity of new photocatalyst Ag/AgCl/ZnO. J AlloysCompd 509:3286–3292. https://doi.org/10.1016/j.jallcom.2010.11.193
Firooz AA, Mahjoub AR, Khodadadi AA, Movahedi M (2010) High photocatalytic activity of Zn-2SnO4 among various nanostructures of Zn2xSn1−xO2 prepared by a hydrothermal method. Chem Eng J 165:735–739. https://doi.org/10.1016/j.cej.2010.09.052
Yasstepe E, Yatmaz HC, Ozturk C, Ozturk O, Duran C (2008) Photocatalytic efficiency of ZnO plates in degradation of azo dye solutions. J Photochem Photobiol A Chem 198:1–6. https://doi.org/10.1016/jphotochem.2008.02.007
Namasivayam C, Arasi DJSE (1997) Removal of Congo red from wastewater by adsorption onto waste red mud. Chemosphere 34:401–417. https://doi.org/10.1016/S0045-6535(96)00385-2
Mittal A, Mittal J, Malviya A, Gupta VK (2009) Adsorptive removal of hazardous anionic dye Congo red from wastewater using waste materials and recovery by desorption. J Colloid Interface Sci 340:16–26. https://doi.org/10.1016/j.jcis.2009.08.019
Hairom NHH, Mohammad AW, Kadhum AAH (2014) Nanofiltration of hazardous Congo red dye: performance andflux decline analysis. J Water Process Eng 4:99–106. https://doi.org/10.1016/j.jwpe.2014.09.008
Walker GM, Weatherley LR (1997) Adsorption of acid dyes on togranular activated carbon in fixed beds. Water Res 31:2093–2101. https://doi.org/10.1016/S0043-1354(97)00039-0
Malik PK, Sanyal SK (2004) Kinetics of decolourisation of azodyes in wastewater by UV/H2O2 process. Sep Purif Technol 36:167–175. https://doi.org/10.1016/S1383-5866(03)00212-0
Liu X, Zhao C, Zhang H, Shen Q (2015) Facile synthesis of porous ZnMnO3 spherulites with a high lithium storage capability. Electrochim Acta 151:56–62. https://doi.org/10.1016/j.electacta.2014.11.020
Peiteado M, Caballero AC, Makovec D (2007) Phase evolution of Zn1−xMnxO system synthesized via oxalate precursors. J Euro Ceram ScI 27:3915–3918. https://doi.org/10.1016/j.jeurceramsoc.2007.02.062
Jacimovi J, Mickovi Z, Gaal R, Smajda R, Vaju C, Sienkiewicz C, Forro A, Magrez A (2011) Synthesis, electrical resistivity, thermo-electric power and magnetization of cubic ZnMnO3. Solid State Commun 151:487–490. https://doi.org/10.1016/j.ssc.2010.12.025
Zhou L, Wu HB, Zhu T, Lou XW (2012) Facile preparation of ZnMn2O4 hollow microspheres as high-capacity anodes for lithium-ion batteries. J Mater Chem 22:827–829. https://doi.org/10.1039/C1JM15054E
Kang M, Park ED, Kim JM, Yie JE (2007) Manganese oxide catalysts for NOx reduction with NH3 at low temperatures. Appl Cats A Gen 327:261–269. https://doi.org/10.1016/j.apcata.2007.05.024
Gupta VK, Yola ML, Atar N (2014) A novel molecular imprinted nanosensor based quartz crystal microbalance for determination of kaempferol. Sensors Actuators B Chem 194:79–85
Balu AM, Baruwati B, Serrano E, Cot J, Garcia-Martinez J, Varma RS, Luque R (2011) Magnetically separable nanocomposites with photocatalytic activity under visible light for the selective transformation of biomass-derived platform molecules. Green Chem 13:2750–2758
Gupta VK, Yola ML, Eren T, Kartal F, Calayan MO, Atar N (2014) Catalytic activity of Fe@ Ag nanoparticle involved calcium alginate beads for the reduction of nitrophenol. J Mol Liq 190:133–138
Sobhani-Nasab A, Pourmasoud S, Ahmadi F, Wysokowski M, Jesionowski T, Ehrlich H, Rahimi-Nasrabadi M (2019) Synthesis and characterization of MnWO4/TmVO4 ternary nano-hybrids by an ultrasonic method for enhanced photocatalytic activity in the degradation of organic dyes. Mater Lett 238:159–162
Kooshki H, Sobhani-Nasab A, Eghbali-Arani M, Ahmadi F, Ameri V, Rahimi-Nasrabadi M (2019) Eco-friendly synthesis of PbTiO3 nanoparticles and PbTiO3/carbon quantum dots binary nano-hybrids for enhanced photocatalytic performance under visible light. Sep Purif Technol 211:873–881
Sobhani-Nasab A, Behpour M, Rahimi-Nasrabadi M, Ahmadi F, Pourmasoud S, Sedighi F (2019) Preparation, characterization and investigation of sonophotocatalytic activity of thulium titanate/polyaniline nanocomposites in degradation of dyes. Ultrason Sonochem 50:46–58
Sedighi F, Esmaeili-Zare M, Sobhani-Nasab A, Behpour M (2018) Synthesis and characterization of CuWO4 nanoparticle and CuWO4/NiO nanocomposite using co-precipitation method; application in photodegradation of organic dye in water. J Mater Sci Mater Electron 29(16):13737–13745
Eghbali-Arani M, Sobhani-Nasab A, Rahimi-Nasrabadi M, Ahmadi F, Pourmasoud S (2018) Ultrasound-assisted synthesis of YbVO4 nanostructure and YbVO4/CuWO4 nanocomposites for enhanced photocatalytic degradation of organic dyes under visible light. Ultrason Sonochem 43:120–135
Das RS, Warkhade SK, Kumar A, Wankhade AV (2019) Graphene oxide-based zirconium oxide nanocomposite for enhanced visible light-driven photocatalytic activity. Res Chem Intermed 45(4):1689–1705
Borhade AV, Tope DR, Dabhade GB (2017) Removal of erioglaucine dye from aqueous medium using ecofriendly synthesized ZnMnO3 photocatalyst. J Surface Sci nanotech 15:74–80. https://doi.org/10.1380/ejssnt.2017.74
Gupta VK, Eren T, Atar N, Lütfi Yola M, Parlak C, Karimi-Maleh H (2015) CoFe2O4@TiO2 decorated reduced graphene oxide nanocomposite for photocatalytic degradation of chlorpyrifos. J Mol Liq 208:122–129
Aslibeiki B, Kameli P, Salamati H (2013) The role of Ag on dynamics of superspins in MnFe2−xAg xO4 nanoparticles. J Nanoparticle Res 15:1–12. https://doi.org/10.1007/s11051-013-1430-y
Finocchio E, Cristiani C, Dotelli G, Stampino PG, Zampori L (2014) Thermal evolution of PEG-based andBRIJ-based hybrid organo-inorganic materials. FT-IR Stud Vib Spectrosc 71:47–56. https://doi.org/10.1016/j.vibspec.2013.12.010
Ba-Abbad MM, Kadhum AAH, Mohamad AB, Takriff MS, Sopian K (2013) The effect of process parameters on the size of ZnO nanoparticles synthesized via the sol–gel technique. J Alloys Compd 550:63–70. https://doi.org/10.1016/j.jallcom.2012.09.076
Waldron RD (1955) Infrared spectra of ferrites. Phys Rev J Archive Rev 99:1727
Naseri MG, Saion EB (2012) Crystalization in spinel ferrite nanoparticles. Adv Crystall Process. https://doi.org/10.5772/35731
Asgari S, Fakhari Z, Berijani S (2014) Synthesis and characterization of Fe3O4 magnetic nanoparticles coated with carboxymethyl chitosan grafted sodium methacrylate. J Nanostruct 4(1):55–63
El-Sayed AM, Hamzawy EMA (2006) Structure and magnetic properties of nickel-zinc ferrite nanoparticles prepared by glass crystallization method. Chem Mon 137:1119–1125. https://doi.org/10.1007/s00706-006-0521-1
Sathishkumar P, Mangalaraja RV, Anandan S, Ashokkumar M (2013) CoFe2O4/TiO2 nanocatalysts for the photocatalytic degradation of reactive Red 120 in aqueous solutions in the presence and absence of electron acceptors. Chem Eng J 220:302–310
Guo H, Chen J, Weng W, Wang Q, Li S (2014) Facile template-free one-pot fabrication of ZnCo2O4 microspheres with enhanced photocatalytic activities under visible-light illumination. Chem Eng J 239:192–199
Kamakshi T, Sundari GS, Erothu H, Rao TP (2018) Synthesis and characterization of graphene based iron oxide (Fe3O4) nanocomposites. Rasayan J Chem 11(3):1113–1119
Liu X, Chen C, Zhao Y, Jia B (2013) A review on the synthesis of manganese oxide nanomaterials and their applications on lithium-ion batteries. J Nanomater 2013:736375
Srivastava V, Singh P, Weng C, Sharma Y (2011) Economically viable synthesis of Fe3O4 nanoparticles and their characterization. Polish J Chem Technol 13(2):1–5
Karthik R, Kumar JV, Chen S-M, Kumar PS, Selvam V, Muthuraj V (2017) A selective electrochemical sensor for caffeic acid and photocatalyst for metronidazole drug pollutant—a dual role by rodlikeSrV 2 O 6. Sci Rep 7:7254
Arunadevi A, Kavitha B, Rajarajan M, Suganthi A, Jeyamurugan A (2018) Investigation of the drastic improvement of photocatalytic degradation of Congo red by monoclinic Cd, Ba-CuO nanoparticles and its antimicrobial activities. Surf Interfaces 10:32
Chin BO, Mohammad AW, Rohani R, Ba-Abbad MM, Hairom NHH (2016) solar photocatalytic degration of hazardous congo red using low- temperature synthesise of zinc oxide nanoparticles. Process Saf Environ Protect 104:549–557. https://doi.org/10.1016/j.psep.2016-04-006
Shinde DR, Tambade PS, Chaskar MG, Gadave KM (2017) Photocatalytic degradation of dyes in water by analytical reagent grades ZnO, TiO2 and SnO2: a comparative study. Drink Water Eng Sci 10(2):109–117
Acknowledgements
We thank science and research Branch, Islamic Azad University Tehran for supporting this study and Iran Nanotechnology Initiative.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zamani, A., Sadjadi, M.S., Mahjoub, A. et al. Synthesis, characterization and investigation of photocatalytic activity of ZnMnO3/Fe3O4 nanocomposite for degradation of dye Congo red under visible light irradiation. Int J Ind Chem 11, 205–216 (2020). https://doi.org/10.1007/s40090-020-00215-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40090-020-00215-z