Abstract
In this paper, zinc oxide (ZnO) nanoparticles were prepared by laser ablation of Zinc (purity of 99/99 %) target. The effect of solvents, methanol and distilled water on the characterization of ZnO has been investigated. The beam of a Q-switched Nd: Yag laser with the length wave of 1064 nm and pulse duration of 6 ns was used. ZnO nanoparticles which were produced in distilled water and methanol were characterized by transmission electron microscopy, X-ray diffraction (XRD) and the optical absorption spectroscopy–ultraviolet (UV–VIS-IR). The XRD results showed that the ZnO nanoparticles have a hexagonal crystal structure. Different size of ZnO nanoparticles were formed because of different environment of laser pulse generated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanoparticles are of great interest for many technological applications and fundamental research due to their size-dependent physical properties [1–4]. Nanostructured ZnO materials have received broad attention due to their distinguished performance in electronics, optics and photonics. Zinc oxide is a unique material that exhibits semiconducting and piezoelectric dual properties. From the 1960s, synthesis of ZnO thin films has been an active field because of their applications as sensors, transducers and catalysts and in properties [5]. ZnO is an interesting chemically and thermally stable-type semiconductor, wide direct band gap of 3.37 eV at room temperature and high sensitivity to toxic and combustible gases [6]. Compared with other semiconductor materials, ZnO has higher excitation binding energy (60 meV) and has been studied as an optoelectronic, transparent conducting, and piezoelectric material. In the past few years, numerous studies have been conducted on both production as well as electronic and optoelectronic applications of nanostructured ZnO [7, 8]. A wide variety of techniques have been exploited to fabricate ZnO nanostructures. Most well-defined ZnO nanostructures with an abundant variety of shapes, such as nanorods, nanoneedles, nanotubes, Nano belts, flower-, spindle-, and tower-like structures, have been synthesized by traditional methods based on the high-temperature vapor-based techniques or the chemical solution route [9]. Recently, pulsed laser ablation (PLA) in liquid media of a solid target has been proven to provide an efficient approach for preparing various nanoscale materials [10]. The PLA technique for synthesis of nanostructured materials from a solid target in liquid media has many advantages. The first advantage of the PLA technique is inexpensive equipment for controlling the ablation atmosphere. Most importantly, it has been demonstrated that size of synthesized material can be controlled by changing different parameters such as laser wavelength, laser fluence, pulse laser duration, changing the pH of the solution, adding surfactants and changing the temperature of solution [11–13]. The purpose of this work is to investigate the effect of solvents on the properties of Zno nanoparticles that were produced using the first harmonics of Nd-YAG laser.
Experimental
Zn and Zno nanoparticles were synthesized by PLA of zinc target (10 × 10 mm2 and thickness of 1 mm, 99.99 % purity). Two zinc metal plates were placed on the bottom of open glass vessel filled with 30 mL of deionized water and methanol, respectively. The Zn plates had a smooth surface and were first cleaned ultrasonically in acetone and methanol baths consecutively and then rinsed with distilled water for 10 min before the experiment to remove all contaminants. The Zn targets were ablated vertically by a Q-switched, Nd–Yag laser (first harmonic) from spectrum A.N.T. ltd operated at 1064 nm with pulse duration of 6 ns and 10 Hz repetition rate. The laser beam of 6 mm in diameter was loosely focused using a lens with a focal length of 80 mm. The ablation fluency was about of 1 J/cm2 at a repetition rate when 1000 pulses were used. Figure 1 shows Zn and ZnO nanoparticles solution generated by 1064 nm laser pulse wavelength in methanol and water. It is clear that the color of solutions has been changed after ablation. Different analytical methods were applied for the characterization of produced nanoparticles. The solution of Zn–Zno nanoparticles was dried on silicon substrates and then XRD was performed. The crystal structure of the samples was analyzed by X-ray diffraction (XRD) with Cu-Kα radiation (λ¼ 1.54060 Å) (STADIMP: STOE, v = 40 kv, i = 40 Ma) was applied. The optical properties of the nanoparticle solution were examined at room temperature by a UV–VIS-IR absorption Spectrophotometer (model: CARY500 scan, company: Varian, 175–3300 nm). The size and distribution of nanoparticles was performed by transmission electron microscopy (TEM) (Zeiss-EM10c–80 kv) after the solution has dried on the grid.
Results and discussion
Structural properties
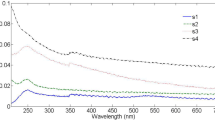
Figure 2 shows a broad absorption spectrum in the Ultraviolet region of the nanoparticles produced by laser ablation of Zn target in methanol and distilled water solution. The absorption spectroscopy system is capable of absorbing solution in the region (175–3300 nm) wavelength range with a resolution of 1 nm. The absorption spectra have peaks centered at 300 nm (methanol) and 335 nm (distilled water) which are assigned to Zn–Zno nanoparticles. The most dramatic property of semiconductor nanoparticles is the size evolution of the optical absorption spectra. Hence, UV–visible absorption spectroscopy is an efficient technique to monitor the optical properties of quantum size particles [14]. When the size of semiconductor nanoparticles is comparable to or below their exciton Bohr radius, they have distinctive electronic and optical behaviors due to exciton quantum confinement phenomena [15]. It is clear that the absorption edge systematically shifts to the lower wavelength with decreasing size of the nanoparticle. This pronounced and systematic shift in the absorption edge is due to the quantum size effect. The blue shift of absorption peak due to quantum confinement effect can be observed in the spectra. Absorption peak intensity increases by changing the type of solution, which could be due to their smaller size and greater number of particles in methanol as compared to distilled water.
XRD studies of nanoparticles
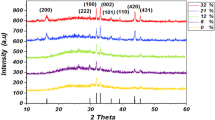
Synthesis of zinc nanoparticles is also verified using X-ray diffraction (XRD). XRD spectrum of samples is presented in Figs. 3 and 4. X-ray diffraction measurement was performed for the dried nanoparticles on silicon substrate. The XRD spectrum clearly shows the crystalline structure of the various peaks of Zn and ZnO nanoparticles. The XRD pattern of the nanoparticles formed by 1064 nm wavelength laser beam in deionized water and methanol at room temperature reveals that they are crystalline and possess the hexagonal Wurtzite structure [16]. The average crystallite size from XRD has been estimated using Scherer formula [17, 18];
where k = constant (0.89< k >1), λ = wavelength of the X-ray, β = full width at half maximum (FWHM) of the diffraction peak, and θ = diffraction angle.
It has been observed from Fig. 3 that the peaks of 34.29°, 47.64°, 56.26° and 61.62° are the characteristic of zinc oxide phase with the crystal size of 20.6, 19.1, 16.3 and 15.1 nm, respectively, and the peak of 54.47° is the characteristic of Zn phase with the crystal size of 16.8 nm for distilled water.
According to Fig. 4 the peak of 36.34° with crystal size of 2.47 nm is the characteristic of zinc oxide phase and the peak of 43.28° with crystal size of 2.08 nm is the characteristic of Zn phase in methanol. X-ray diffraction analysis showed that both samples have a hexagonal crystal structure.
It is clear that the grain size of the nanoparticles decreases by changing the type of solution (of distilled water to the methanol). The pattern reference number (PDF file number) for Zn is 00-004-0831 and for ZnO is 00-021-1486.
Size of nanoparticles
TEM images of the nanoparticles are displayed in Fig. 5. As can be seen, the ZnO nanoparticles are almost spherical in all samples and the particle sizes and size distribution are related to the environment of laser ablation. Figure 6 demonstrates the size distribution graphs of ZnO nanoparticles. It can be seen that with changing solution of target from distilled water to methanol the sizes of nanoparticle decrease. Nanoparticles generated in solution of distilled water are between 1 and 30 nm, but as it is clear in Fig. 6 their size decreases with changing the solution to methanol. We have more uniform nanoparticles in methanol solution.
Conclusion
Zno nanoparticles were successfully fabricated by pulsed laser ablation on a Zn target in methanol and distilled water in a glass vessel. In this work, the effect of laser environment on the structure, morphology and optical properties of ZnO nanoparticles has been investigated. XRD data revealed that these nanoparticles possessed the hexagonal Wurtzite structure. The size distribution of ZnO nanoparticles decreased with changing the solution from distilled water to methanol. The blue shift in the absorption edge indicates the quantum confinement property of nanoparticles.
References
Stach, S., Garczyk, Ż., Ţălu, Ş., Solaymani, S., Ghaderi, A., Moradian, R., Nezafat, N.B., Elahi, S., Gholamali, H.: J. Phys. Chem. C 119, 17887–17898 (2015)
Ţălu, Ş., Stach, S., Ghodselahi, T., Ghaderi, A., Solaymani, S., Boochani, A., Garczyk, Ż.: J. Phys. Chem. B. 119, 5662–5670 (2015)
Ţălu, Ş., Stach, S., Solaymani, S., Moradian, R., Ghaderi, A., Hantehzadeh, M.R., Elahi, S.M., Garczyk, Ż., Izadyar, S.: J. Electroanal. Chem. 749, 31–41 (2015)
Solaymani S., Ghaderi A., Nezafat N.B.: J. Fusion Energy. 31(6), 591 (2012). doi:10.1007/s10894-012-9510-z
Ghaderi, A., Elahi, S.M., Solaymani, S., Naseri, M., Ahmadirad, M., Bahrami, S., Khalili, A.E.: PRAMANA J. Phys. 77(6), 1–8 (2011)
Patil, L.A., Bari, A.R., Shinde, M.D., Deo, V.: Sens. Actuators B 149, 79–86 (2010)
Suliman, A.E., Tang, Y., Xu, L.: Sol. Cells 91, 1658 (2007)
Keis, K., Bauer, C., Boschloo, G., Hagfeldt, A., Westermark, K., Rensmo, H., Siegbahn, H.: J. Photochem. Photobiol. A Chem. 148, 57 (2002)
He, Ch., Sasaki, T., Shimizu, Y., Koshizaki, N.: Appl. Surf. Sci. 254, 2196–2202 (2008)
Huang, C.-C., Yeh, C.-S., Ho, C.-J.: J. Phys. Chem. B 108, 4940 (2004)
Ishikawa, Y., Shimizu, Y., Sasaki, T., Koshizaki, N.: J. Colloid. Interface Sci. 300, 612–615 (2006)
Solati, E., Mashayekh, M., Dorranian, D.: Appl. Phys. A 112, 689–694 (2013)
Drmosh, Q.A., Gondal, M.A., Yamani, Z.H., Saleh, T.A.: Appl. Surf. Sci. 256, 4661–4666
Rao, B.S., Kumar, B.R., Reddy, V.R., Rao, T.S., Venkata Chalapathi, G.: Chalcogenide Lett. 8(1), 39 (2011)
Gondal, M.A., Drmosh, Q.A., Yamani, Z.H., Saleh, T.A.: Appl. Surf. Sci. 256, 298–304 (2009)
Wang, Z. L.: J. Phys. Condens. Mater. 16, R829–R858 (2004)
Suryanarayana, C., Norton, M.G.: X-ray diffraction a practical approach. Plenum Press, New York (1998)
Naderi, S., Ghaderi, A., Solaymani, S., Golzan, M.M.: Eur. Phys. J. Appl. Phys. 58(2), 20401 (2012)
Authors’ contributions
SVF and MG conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgments
This work was supported by Plasma Physics Research Center and Laboratory Complex of Islamic Azad University of Iran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Farahani, S.V., Mahmoodi, A. & Goranneviss, M. The effect of laser environment on the characteristics of ZnO nanoparticles by laser ablation. Int Nano Lett 6, 45–49 (2016). https://doi.org/10.1007/s40089-015-0162-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40089-015-0162-7