Abstract
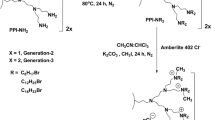
One-pot synthesis of novel N-methyl pyrrolidine dendrimers has been accomplished in good yield via a facile [3+2] cycloaddition of azomethine ylide with chalcone moiety of the dendrimer as a dipolarophile. All the pyrrolidine dendrimers exhibited moderate and comparable antibacterial activity as that of the standard viz., chloramphenicol against Bacillus subtilis, E. coli, Klebsiella pneumoniae and species of Streptococcus by agar well diffusion method.






Similar content being viewed by others
References
Gilat SL, Adronov A, Fréchet JMJ (1999) Light harvesting and energy transfer in novel convergently constructed dendrimers. Angew Chem Int Ed 38:27–1422
Adronov A, Fréchet JMJ (2000) Light harvesting dendrimers. Chem Commun 18:1701–1710
Kleij AW, Gossage RA, Gebbink RJMK, Brinkmann N, Reijerse EJ, Kragl U, Luts M, Spek AL, Koten GV (2000) A dendritic effect in homogeneous catalysis with carbosilane-supported arylnickel(II) Catalysts: observation of active-site proximity effects in atom-transfer radical addition. J Am Chem Soc 122:12112–12124
Mager M, Becke S, Windisch H, Denninger U (2001) Noncoordinating dendrimer polyanions: cocatalysts for the metallocene17 catalyzed olefin polymerization. Angew Chem Int Ed 40:1898–1902
Hahn U, Gorka M, Vögtle F, Vicinelli V, Ceroni P, Maestri M, Balsani V (2002) Light-harvesting dendrimers: efficient intra and intermolecular energy-transfer processes in a species containing 65 chromophoric groups of four different types. Angew Chem Int Ed 41:3595–3598
Rajakumar P, Ganesan K, Jayavelu S, Murugesan K (2005) Synthesis and bactericidal efficacy of novel dendrimers. Synlett 7:1121–1124
Gillies ER, Fréchet JMJ (2002) Designing macromolecules for therapeutic applications: polyester DendrimersPoly(ethylene oxide) ‘‘Bow-Tie’’ hybrids with tunable molecular weight and architecture. J Am Chem Soc 124:14137–14146
Padilla De Jesus OL, Ihre HR, Gagne L, Fréchet JMJ, Szoka FC Jr (2002) Polyester dendritic systems for drug delivery applications: inVitro and in vivo evaluation. Bioconjugate Chem 13:453–461
Wang Q, Chan TR, Hilgraf R, Fokin VV, Sharpless KB, Finn MG (2003) Bioconjugation by copper (I)-catalyzed azide-alkyne [3+2] cycloaddition. J Am Chem Soc 125:31923193
Bureley GA, Gierlich J, Mofid MR, Nir H, Tal S, Eichen Y, Carell TJ (2006) Directed DNA metallization. J Am Chem Soc 128:1398–1399
Fuchs S, Otto H, Jehle S, Henklein P, Schlüter AD (2005) Fluorescent dendrimers with a peptide cathepsin B cleavage site for drug delivery applications. Chem Comm 1830–1832
Majoros IJ, Myc A, Thomas T, Mehta CB, Baker JR Jr (2006) PAMAM dendrimer-based multifunctional conjugate for cancer therapy: synthesis, characterization, and functionality. Biomacromolecules 7:572–579
Cloninger MJ (2002) Biological applications of dendrimers. Curr Opin Chem 18 Biol 6:742–748
Boas U, Heegaard PMH (2004) Dendrimers in drug research. Chem Soc Rev 33:43–63
Shaunak S, Thomas S, Gianasi E, Godwin A, Jones E (2004) Polyvalent dendrimer glucosamine conjugates prevent scar tissue formation. Nat Biotechnol 22:84–977
Zhang Q, Ning Z, Yan Y, Qian S, Tian H (2008) Photochromic spiropyran dendrimers:‘Click’ syntheses, characterization, and optical properties. Macromol Rapid Commun 29:193–201
Goodwin AP, Lam SS, Frechet JMJ (2007) Rapid, efficient synthesis of heterobifunctional biodegradable dendrimers. J Am Chem Soc 129:6994–6995
Antoni P, Hed Y, Nordberg A, Nystrom D, von Holst H, Hult A, Malkoch M (2009) Bifunctional dendrimers: from robust synthesis and accelerated one-pot postfunctionalization strategy to potential applications. Angew Chem Int Ed 48:2126–2130
Navath RS, Menjoge AR, Wang B, Romero R, Kannan S, Kannan RM (2010) Amino acid-functionalized dendrimers with heterobifunctional chemoselective peripheral groups for drug delivery applications. Biomacromolecules 11:1544–1563
Chavan P, Mane AS, Shingare MS (2001) ChemInform Abstract: synthesis of New O, O-Dialkyl-O-coumarinophosphorothioates and their pesticidal bioassay against helicoverpa armigera. Indian J Chem 40B:339–341
Rajakumar P, Raja S, Thirunarayanan A (2010) A facile synthesis of novel pyrrolidine dendrimers by terminal group modification through 1,3-dipolar cycloaddition reaction. Synlett 11:1669–1673
Rajakumar P, Raja S (2009) Synthesis and characterization of some novel dendritic architectures bearing chalcone at the periphery through click approach 19. Synth Commun 39:3888–3897
Rajakumar P, Anandhan R (2011) Synthesis and in-vitro anti-inflammatory activity of novel glycodendrimers with benzene 1,3,5 carboxamide core and triazole as branching unit. Eur J Med Chem 46:4687–4693
Tsuge O, Kanemasa S (1989) Recent Advances in Azomethine Ylide Chemistry. Advances in heterocyclic chemistry; Katritzky AR Ed. Academic Press: San Diego 45:231
Padwa A (1991) Intramolecular 1,3-dipolar cycloaddition. In: Trost BM, Fleming I (eds) Comprehensive organic synthesis. Pergamon Press, Oxford, pp 1069–1109
Grigg R, Sridharan V (1993) In: Curran DP (ed) Advances in Cycloaddition, vol 3. Jai Press, London, p 161
Boger DL (1983) Diels-alder reactions of azadiens. Tetrahedron 39:2869
Dessimoni G, Tacconi G (1975) Heterodiene syntheses with á, â,-unsaturated carbonyl compounds. Chem Rev 75:651–692
Polyak F, Lubell WD (2001) Mimicry of peptide backbone geometry and heteroatomic side-chain functionality: synthesis of enantiopure indolizidin-2-one amino acids possessing alcohol, acid, and azide functional groups. J Org Chem 66:1171–1180
Feng Z, Lubell WD (2001) Synthesis of enantiopure 7-[3-Azidopropyl] indolizidin-2-one amino acid. A constrained mimic of the peptide backbone geometry and heteroatomic side-chain functionality of the Ala-Lys dipeptide. J Org Chem 66:1181–1185
Kolodziej SA, Nikiforovich GV, Skeean R, Lignon MF, Martinez J, Marshall GR (1995) Ac-[3- and 4-Alkylthioproline31]-CCK4 analogs: synthesis and implications for the CCK-B receptor-bound conformation. J Med Chem 38:137–149
Boyanova L, Gergova G, Nikolov R, Derejian S, Lazarova E, Katsarov N, Mitov I, Krastev Z (2005) Activity of bulgarialn propolis against 94 Helicobacter pylori strains in vitro by agar well diffusion, agar dilution and disc diffusion methods. J Med Microbiol 54:481–483
Nyerges M, Ballás L, Kádas I, Bitter I, Kovesdi I, To ke L (1995) trans-3-Aryl-4-nitro-pyrrolidines via 1,3-dipolar cycioaddition of nonstabilized azomethine ylide to â -nitro styrenes. Tetrahedron 51:6783–6788
Rajakumar P, Raja S (2008) Synthesis, optical and thermal studies of dendritic architectures with chalcone surface groups. Tetrahedron Lett 49:6539–6542
Acknowledgments
The authors thank CSIR, New Delhi, for financial assistance and DST-FIST for providing NMR facilities to the department and also grateful to the department of biotechnology, Vels University, Chennai, India. For anti bacterial studies. AT thanks CSIR-UGC for fellowship in the form of SRF.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rajakumar, P., Thirunarayanan, A. & Raja, S. Synthesis and Antibacterial Activity of Novel N-Methyl Pyrrolidine Dendrimers via [3+2] Cycloaddition. Proc. Natl. Acad. Sci., India, Sect. A Phys. Sci. 84, 371–379 (2014). https://doi.org/10.1007/s40010-013-0120-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40010-013-0120-6