Abstract
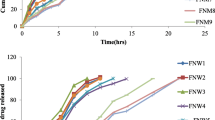
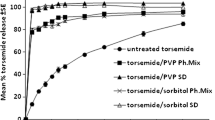
The present work deals with design of zero order sustained release nateglinide matrix tablets by application of statistical design using response surface methodology as a tool. Central composite design was used to investigate the effect of two independent formulation variables (at three levels) such as Kollidon SR (X1), PVP K 30 (X2) on dependent variables viz. time required to release 30 % (T30, Y1), percentage drug released at 6th hour (DR6, Y2) and time required to release 90 % (T90, Y3) of drug. Wet granulation technique was employed for tablets preparation. The result showed that release pattern of the optimized formulation was almost equal to the statistically predicted values. There was no chemical interaction observed between drug and polymer based up on FTIR and DSC results. In vitro release studies were performed in 0.1 N HCl containing 0.5 % SLS for first 2 h followed by pH 6.8 phosphate buffer containing 0.5 % SLS. Stability studies were performed to statistically optimized formulation. The release pattern from statistically optimized formulation was followed zero order kinetics with non-Fickian process as drug release mechanism. Pharmacokinetic studies were performed to optimized formulation in comparison with nateglinide suspension in rabbit as animal model. The results of in vivo studies revealed the % relative bioavailability of statistically optimized formulation was found to be 68.87 %.







Similar content being viewed by others
References
Ai M, Tanaka A, Ogita K, Shimokado K (2011) Favorable effects of early insulin secretion by nateglinide on postprandial hyperlipidemia in patients with type 2 diabetes. Diabetes Care 29:1180. doi:10.2337/dc05-2336
Bhupinder S, Kumar R, Naveen A (2004) Optimizing drug delivery systems using systematic “Design of Experiments” Part I: fundamental aspects. Criti Rev Ther Drug 22(1):27–105. doi:10.1615/CritRevTherDrugCarrierSyst.v22.i1.20
Brahmankar DM (2008) Biopharmaceutics and pharmacokinetics—a treatise, 2nd edn. Vallabh Prakashan, New Delhi, pp 343–350
Chisato M, Nobutaka N, Hidetoshi S, Haruo O, Akira O, Akira Y (2006) Effect of decrease in both postprandial blood glucose (PBG) and fasting blood glucose (FBG) levels in normal beagle dogs with nateglinide enteric coated granules and immediate release tablets. Chem Pharm Bull 54(4):409–414. doi:10.1248/cpb.54.409
Dunn C, Faulds D (2000) Nateglinide. Drugs 60:607
Ferreira SLC, Bruns RE, Ferreira HS, Matos GD, David JM, Brandao GC (2007) Box-Behnken design: an alternative for the optimization of analytical methods. Anal Chim Acta 597(2):179–186. doi:10.1016/j.aca.2007.07.011
Geoffroy JM, Fredrickson JK, Shelton JT (1998) A mixture experiment approach for controlling the dissolution rate of a sustained release tablet. Drug Dev Ind Pharm 24(9):799–806. doi:10.3109/03639049809088524
Ghosh MN (2005) Fundamentals of experimental pharmacology, 3rd edn. Sk Ghosh Publications, Kolkata, pp 192–194
Gribble FM, Manley SE, Levy JC (2001) Randomized dose ranging study of the reduction of fasting and postprandial glucose in type 2 diabetes by nateglinide (A-4166). Diabetes Care 24(7):1221–1225. doi:10.2337/diacare.24.7.1221
Grimm W (1998) Extension of the International Conference on Harmonisation Tripartite Guidelines for stability testing of new drug substances and products to countries of Climatic Zones III and IV. Drug Dev Ind Pharm 24:313–325. doi:10.3109/03639049809085626
Hamed E, Sakr A (2001) Application of multiple response optimization technique to extended release formulation design. J Control Rel 73(2–3):329–338. doi:10.1016/S0168-3659(01)00356-X
Higuchi T (1963) Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci 52:1145–1149. doi:10.1002/jps.2600521210
Hixson AW, Crowell JH (1931) Dependence of reaction velocity upon surface and agitation. I. Theoretical considerations. Ind Eng Chem 23:923–931
ICH (2003) Harmonised tripatite guideline: stability testing of new drug substances and products ICH Q1A(R2). ICH Expert Working Group, Europe, Japan and USA
Jaiprakash NS, Mrinmayee D, Rohidas A, Zaheer Z, Devanand BS (2014) Quality by design approach: regulatory need. Arabian J Chem. doi:10.1016/j.arabjc.2014.01.025
Japanese Pharmacopoeia (2011) 16th edn, The Ministry of Health, Labour and Welfare Ministerial Notification 65:1148
Jolly MS, Mayur GS, Vijay BS, Rajashree CM (2007) Nateglinide quantification in rabbit plasma by HPLC: optimization and application to pharmacokinetic study. J Pharm Biomed Anal 44:196–204
Kolter K, Fraunhofer W, Ruchatz F (2001) Properties of Kollidon SR as a new excipient for sustained release dosage forms. BASF Ex Act 6:5
Korsmeyer R, Gurny R, Peppas N (1983) Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm 15(1):25–35. doi:10.1016/0378-5173(83)90064-9
Lazarus J, Cooper J (1961) Absorption, testing and clinical evaluation of oral prolonged action drugs. J Pharm Sci 50:715–732. doi:10.1002/jps.2600500902
Long W, Kitlun Y, Sunpui N, Joanne Y (2012) Application of the Box-Behnken design to the optimization of process parameters in foam cup molding. Expert Syst Appl 39(9):8059–8065. doi:10.1016/j.eswa.2012.01.137
Makino C, Sakai H, Okano A, Yabuki A (2009) Design of nateglinide controlled release tablet containing erosion matrix tablet and multiple administration study in normal beagle dogs. Chem Pharm Bull 57:907–913. doi:10.1248/cpb.57.907
McLeod JF (2004) Clinical pharmacokinetics of nateglinide: a rapidly-absorbed, short-acting insulinotropic agent. Clin Pharmacokinet 43(2):97–120
Montgomery DC (2001) Design and analysis of experiments, 5th edn. Wiley, New York
Peppas NA (1985) Analysis of Fickian and non-Fickian drug release from polymers. Pharm Acta Helv 60:110–111
Porter SC, Verseput RP, Cunnigaham CR (1997) Process optimization using design of experiments. Pharm Technol 21:60–70
Shargel L, Wu-Pong S, Andrew BCY (1999) Applied Biopharmaceutics and pharmacokinetics, 6th edn. The McGraw Hill Companies, New York, pp 476–478
Tentolouris N, Voulgari C, Katsilambros N (2007) A review of nateglinide in the management of patients with type 2 diabetes. Vasc Health Risk Manag 3:797–807
Wagner JG (1969) Interpretation of percent dissolved-time plots derived from in vitro testing of conventional tablets and capsules. J Pharm Sci 58(10):1253–1257. doi:10.1002/jps.2600581021
Acknowledgments
This article does not contain any studies with human and animal subjects performed by any of authors. All authors (S. Betha1, B. P. Reddy, P. V. Swamy, M. M. Varma, D. B. Raju, V. R. M. Kolapalli) declare that they have no conflict of interest. The authors are very much thankful to Ajinomoto Co. In. for providing nateglinide as a gift sample. The authors extend acknowledgements to B.V. Raju educational institutions for providing the necessary facilities to complete the present research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Betha, S., Pamula Reddy, B., Swamy, P.V. et al. Dose calculation, design and development of nateglinide matrix tablets using quality by design approach and its pharmacokinetic evaluation in animal model. Journal of Pharmaceutical Investigation 45, 515–528 (2015). https://doi.org/10.1007/s40005-015-0200-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-015-0200-5