Abstract
BACKGROUND:
Meshes play a crucial role in hernia repair. However, the displacement of mesh inevitably leads to various associated complications. This process is difficult to be traced by conventional imaging means. The purpose of this study is to create a contrast-enhanced material with high-density property that can be detected by computed tomography (CT).
METHODS:
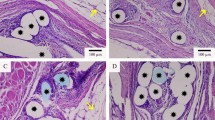
The contrast-enhanced monofilament was manufactured from barium sulfate nanoparticles and medical polypropylene (PP/Ba). To characterize the composite, stress tensile tests and scanning electron microscopy (SEM) was performed. Toxicity and biocompatibility of PP/Ba materials was verified by in vitro cellular assays. Meanwhile, the inflammatory response was tested by protein adsorption assay. In addition, an animal model was established to demonstrate the long-term radiographic effect of the composite material in vivo. Subsequent pathological tests confirmed its in vivo compatibility.
RESULTS:
The SEM revealed that the main component of the monofilament is carbon. In vitro cell experiments demonstrated that novel material does not affect cell activity and proliferation. Protein adsorption assays indicated that the contrast-enhanced material does not cause additional inflammatory responses. In addition, in vivo experiments illustrated that PP/Ba mesh can be detected by CT and has good in vivo compatibility.
CONCLUSION:
These results highlight the excellent biocompatibility of the contrast-enhanced material, which is suitable for human abdominal wall tissue engineering.







Similar content being viewed by others
References
Kingsnorth A, LeBlanc K. Hernias: inguinal and incisional. Lancet. 2003;362:1561–71.
Barbaros U, Asoglu O, Seven R, Erbil Y, Dinccag A, Deveci U, et al. The comparison of laparoscopic and open ventral hernia repairs: a prospective randomized study. Hernia. 2007;11:51–6.
Chatterjee A, Krishnan NM, Rosen JM. Complex ventral hernia repair using components separation with or without synthetic mesh: a cost-utility analysis. Plast Reconstr Surg. 2014;133:137–46.
HerniaSurge G. International guidelines for groin hernia management. Hernia. 2018;22:1–165.
Picchio M, Muggianu A, Mancini F, Tintisona O, Spaziani E. Complete mesh migration into the small bowel after incisional hernia repair: a case report and literature review. Acta Chir Belg. 2017;117:118–21.
Govindraj R, Decadt BJ, Thomson K. The British-flag technique of mesh insertion in transabdominal preperitonial laparoscopic inguinal hernia repair. J Laparoendosc Adv Surg Tech A. 2010;20:239–40.
Losanoff JE, Salwen WA, Basson MD, Levi E. Large neomucosal space 25 years after mesh repair of ventral hernia. Am J Surg. 2010;199:e39-41.
Aubé C, Pessaux P, Tuech JJ, du Plessis R, Becker P, Caron C, et al. Detection of peritoneal adhesions using ultrasound examination for the evaluation of an innovative intraperitoneal mesh. Surg Endosc. 2004;18:131–5.
Rakic S, LeBlanc KA. The radiologic appearance of prosthetic materials used in hernia repair and a recommended classification. AJR Am J Roentgenol. 2013;201:1180–3.
Chen L, Lenz F, Alt CD, Sohn C, De Lancey JO, Brocker KA. MRI visible Fe3O4 polypropylene mesh: 3D reconstruction of spatial relation to bony pelvis and neurovascular structures. Int Urogynecol J. 2017;28:1131–8.
Krämer NA, Donker HC, Otto J, Hodenius M, Sénégas J, Slabu I, et al. A concept for magnetic resonance visualization of surgical textile implants. Invest Radiol. 2010;45:477–83.
Köhler G, Pallwein-Prettner L, Lechner M, Spaun GO, Koch OO, Emmanuel K. First human magnetic resonance visualisation of prosthetics for laparoscopic large hiatal hernia repair. Hernia. 2015;19:975–82.
Özveri E, Şanlı DET, Yıldırım D, Gök H, Ertem M. Magnetic resonance visualization of iron-loaded meshes in patients with pain after inguinal hernia repair. Hernia. 2021;25:727–32.
DeLancey JO. Anatomy and biomechanics of genital prolapse. Clin Obstet Gynecol. 1993;36:897–909.
Kim EY, Shim YS, Kim YS, Lee SP, Ko KD, Choi WJ. Adherence to general medical checkup and cancer screening guidelines according to self-reported smoking status: Korea National Health and Nutrition Examination Survey (KNHANES) 2010–2012. PLoS One. 2019;14:e0224224.
Ballard DH, Jammalamadaka U, Tappa K, Weisman JA, Boyer CJ, Alexander JS, et al. 3D printing of surgical hernia meshes impregnated with contrast agents: in vitro proof of concept with imaging characteristics on computed tomography. 3D Print Med. 2018;4:13.
Li H, Shu H, Qiao G, Dai Z. Visualization of implanted mesh in the pelvic reconstructive surgery using an X-ray-detectable thread. Arch Gynecol Obstet. 2021;304:965–73.
Karapınar-Kazandağ M, Bayrak OF, Yalvaç ME, Ersev H, Tanalp J, Sahin F, et al. Cytotoxicity of 5 endodontic sealers on L929 cell line and human dental pulp cells. Int Endod J. 2011;44:626–34.
Dievernich A, Achenbach P, Davies L, Klinge U. Characterization of innate and adaptive immune cells involved in the foreign body reaction to polypropylene meshes in the human abdomen. Hernia. 2022;26:309–23.
Todros S, Pavan PG, Natali AN. Biomechanical properties of synthetic surgical meshes for pelvic prolapse repair. J Mech Behav Biomed Mater. 2015;55:271–85.
Kalaba S, Gerhard E, Winder JS, Pauli EM, Haluck RS, Yang J. Design strategies and applications of biomaterials and devices for hernia repair. Bioact Mater. 2016;1:2–17.
Eickhoff RM, Kroh A, Eickhoff S, Heise D, Helmedag MJ, Tolba RH, et al. A peritoneal defect covered by intraperitoneal mesh prosthesis effects an increased and distinctive foreign body reaction in a minipig model. J Biomater Appl. 2021;35:732–9.
Aquino F, Stribeck A, Li X, Zeinolebadi A, Buchner S, Santoro G. Variation of poly(vinylidene fluoride) morphology due to radial cold flow in a flexible pipe. Polym Eng Sci. 2015;55:2869–77.
Choi SY, Hur W, Kim BK, Shasteen C, Kim MH, Choi LM, et al. Bioabsorbable bone fixation plates for X-ray imaging diagnosis by a radiopaque layer of barium sulfate and poly(lactic-co-glycolic acid). J Biomed Mater Res B Appl Biomater. 2015;103:596–607.
Ghosh P, Das M, Rameshbabu AP, Das D, Datta S, Pal S, et al. Chitosan derivatives cross-linked with iodinated 2,5-dimethoxy-2,5-dihydrofuran for non-invasive imaging. ACS Appl Mater Interfaces. 2014;6:17926–36.
Guillaume O, Blanquer S, Letouzey V, Cornille A, Huberlant S, Lemaire L, et al. Permanent polymer coating for in vivo MRI visualization of tissue reinforcement prostheses. Macromol Biosci. 2012;12:1364–74.
Blanquer S, Guillaume O, Letouzey V, Lemaire L, Franconi F, Paniagua C, et al. New magnetic-resonance-imaging-visible poly(epsilon-caprolactone)-based polyester for biomedical applications. Acta Biomater. 2012;8:1339–47.
Mottu F, Rüfenacht DA, Doelker E. Radiopaque polymeric materials for medical applications. Current aspects of biomaterial research. Invest Radiol. 1999;34:323–35.
Pepiol A, Teixidor F, Saralidze K, van der Marel C, Willems P, Voss L, et al. A highly radiopaque vertebroplasty cement using tetraiodinated o-carborane additive. Biomaterials. 2011;32:6389–98.
Aganovic L, Ishioka KM, Hughes Cassidy F, Chu PK, Cosman BC. Plugoma: CT findings after prosthetic plug inguinal hernia repairs. J Am Coll Surg. 2010;211:481–4.
Houshyar S, Sarker A, Jadhav A, Kumar GS, Bhattacharyya A, Nayak R, et al. Polypropylene-nanodiamond composite for hernia mesh. Mater Sci Eng C Mater Biol Appl. 2020;111: 110780.
Acknowledgements
This work was supported by the National Youth Science Foundation Project (Award number 150141).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical statement
All animal surgical experiments were performed under the approval by Laboratory Animal Center of Nantong University (Approval No. S20201230-334).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ding, X., Zhu, J., Liu, A. et al. Preparation and Biocompatibility Study of Contrast-Enhanced Hernia Mesh Material. Tissue Eng Regen Med 19, 703–715 (2022). https://doi.org/10.1007/s13770-022-00460-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-022-00460-6