Abstract
Ion mobility spectrometry with corona discharge ion source was used for the measurements of selected pesticides cybutryne, terbutryn, dichlorvos, and quinoxyfen in water. The analyses were carried out by (i) direct injection of the aqueous solution of the pesticides and (ii) extraction using solid phase microextraction by commercial SPME arrows. The instrumental and the experimental extraction parameters including the drift tube temperature, injection port temperature, and the SPME fiber type were studied and optimized to achieve the maximum signal intensity. The limit of detections obtained by the direct injection were about 0.1–1.0 mg L−1. Using the SPME arrow-ion mobility spectrometry method, the detection limits were improved 7–50 times and obtained as 0.01, 0.015, 0.05, and 0.1 mg L−1 for terbutryn, dichlorvos, cybutryne, and quinoxyfen, respectively. The recovery rate for analysis in surface water (real samples) was approximately 90–110% for both the direct injection and solid phase microextraction method. The capability of ion mobility spectrometry for the simultaneous detection of a mixture of the studied pesticides was assessed, and it was found that terbutryn, dichlorvos, and quinoxyfen can be detected simultaneously without peak overlapping.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pesticides are a wide range of compounds used worldwide in agriculture to protect plants and their crops against different types of pests. Although these compounds are necessary for production of vegetables and fruits and consequently in food security, pesticides are harmful substances for human and environment (Bassil et al. 2007; Pimentel et al. 1992). Approximately, 2 million tones pesticides are used worldwide annually without programs or with limited programs to control exposure (Sharma et al. 2019). Structurally, pesticides are classified to several groups such as organophosphates, organochlorines, triazines, and carbamates. Another classification is based on their applications including herbicides, biocides, fungicides, and insecticides. One pesticide from each group, namely cybutryne (biocide), terbutryn (herbicide), dichlorvos (insecticide), and quinoxyfen (fungicide), was selected for this work. Chemical structures of these pesticides are shown in Scheme 1.
The pesticide residues remain in the fruits and leaves of plants or enter the surface and groundwater. The United State agricultural health study found that approximately 50 million people in U.S access their water consumption through the groundwater which is contaminated by pesticides (Alavanja 2009). Also, in the year 2000, German Federal Environmental Agency (UBA) concluded that annually approximately 30 tons of different pesticides enter to the surface water (Weber and Smolka 2005). Hence, detection and measurement of the pesticide residues in water are of importance. During the last decades, several methods have been developed for detection and quantification of pesticides in different samples. Although most of the reported methods are chromatographic based methods (Grimalt and Dehouck 2016; Pitarch-Anderson et al. 2017), other standard techniques such as immunochemical electrochemical methods have been occasionally employed (Duncan et al. 2018; Maltman et al. 2012; Elshahawi and Kamal 1998).
In 1990, solid phase microextraction (SPME), as an effective alternative to liquid–liquid extraction, was invented by Pawliszyn et al. (1990). The advantages of this method were integration of sampling, extraction, concentration, and sample introduction steps as well as saving preparation time (Vas and Vekey 2004). SPME coupled to GC and MS has been used for determination of different classes of pesticides in water and other liquid samples (Rickert et al. 2020; Albains et al. 2004).
Ion mobility spectrometry (IMS) is a fast, simple, and low-cost techniques having vast application in detection of volatile organic compounds (VOCs), chemical warfare agents, food ingredients, abused drugs and opiates, explosives, and environmental monitoring (Eiceman et al. 2014). IMS has been directly used for determination of different types of pesticides (Khademi et al. 2019; Touvinen et al. 2000; Sadat et al. 2020; Goscinny et al. 2019; Chen et al. 2019; Weickhardt et al. 2012; Gallart-Mateu et al. 2016). To achieve better sensitivity, solid phase extraction (SPE) and microextraction (Classical SPME) coupled with IMS have been used for determination of different pesticides (Walendzik et al. 2005; Mohammadi et al. 2009; Holopainen et al. 2013; Saraji et al. 2018; Wang et al. 2015). The pesticide diazinon was measured in water using SPME-IMS and sol–gel/nanoclay composite as a sorbent with LOD of 0.07 μg L−1 (Saraji et al. 2018). Also, using SPME-IMS, the pesticide dichlorvos was determined in tea drinks with LOD of 6 μg L−1 (Wang et al. 2015).
The conventional methods for pesticide measurements such as LC–MS and GC–MS involve costly apparatus and require a high degree of technical knowledge. Furthermore, the run time of GC and LC is usually more longer than few minutes. This work presents development of a fast (response time of millisecond) and low-cost analytical method for detection of the selected pesticides, cybutryne, terbutryn, dichlorvos, and quinoxyfen using direct immersion-arrow SPME coupled with the corona discharge ion mobility spectrometry (SPME arrow-IMS). The capability of the method to extract and detection of the studied pesticides in water sample as fast and sensitive method are investigated. The water samples were obtained from Taleqan Dam (Alborz Province, Iran) in November and December 2020 and analyzed within 1–2 days after sampling in Imam Khomeini International University of Qazvin, Iran.
Materials and methods
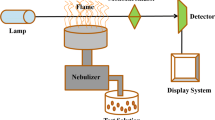
The ion mobility spectrometer (IMS) used in this work was an atmospheric pressure drift tube with a corona discharge as the ionization source (IMS-400, TOF Tech. Pars Co., Iran). The IMS cell consisted of two parts: an ionization region and a drift tube, separated by an ion gate (shutter grid). The shutter grid pulses the ions from the ionization region to the drift tube with an opening time of about 50 μs at 20 ms intervals. The applied voltage on the corona discharge with a point to plane geometry was 2.3 kV, and the applied voltage on the IMS cell was 8 kV providing a drift field of 500 V/cm. Dry air was used as both the drift and carrier gases with flow rates of 700 ml/min and 350 ml/min, respectively. In the positive polarity of the corona discharge, H3O+(H2O)n is formed as the main reactant ion; hence, the mechanism of ionization is protonation of the analyte (Valadbeigi et al. 2019). The sample is vaporized in the injection port, transferred to the ionization region by the carrier gas. The cell temperature can be adjusted from ambient temperature to 200 °C; however, the cell temperature for the determination of the pesticides was optimized. The optimum temperature of the injection port of IMS was also determined (see next section).
Dichlorvos, terbutryn, cybutryne, and quinoxyfen standards were purchased from Sigma-Aldrich (Steinheim, Germany). Physicochemical properties of these pesticides are summarized in Table S1 (Supplementary Materials). To obtain calibration curves, the standard solutions in distilled water were prepared. Measurements were carried out in two different ways: (i) direct injection of the solutions of pesticides in water solvent (2 μL) into the injection port of IMS using a 10-μL-Hamilton syringe and (ii) extraction of the pesticides by immersion of commercially available PAL SPME Arrows (BGB Analytik AG, Restek, Switzerland). The composition of the SPME fibers, purity of the pesticides, and the provider companies are summarized in Table S2. Pre and reconditioning of the arrow fibers was done thermally in the injection port of IMS according to the manufacture’s instruction. After adsorption of the pesticides, the SPME arrow was put in the injection port of IMS to introduce the pesticides to the IMS via thermal desorption. The optimum extraction conditions obtained in a previous study conducted by GC–MS, i.e., extraction time of 70 min, pH of 7, extraction temperature of 60 °C, and the level of 500 rpm agitation, in the presence of 17% NaCl were used (Khademi et al. 2021). Three SPME arrows with different solid phases were checked for adsorption of the pesticides. The images of the SPME arrows with colors gray, red, and violet are shown in Fig. S1 (Supplementary Materials). The solid phases of the SPME arrows were divinylbenzene/carbon wide range/polydimethylsiloxane (DVB/CWR/PMDS), PDMS, and DVB/PMDS, respectively. To assess recovery rates of the method, additional measurements were carried out in a water sample obtained from Taleqan Dam (Alborz, Iran).
Computational details
Since the ionization of the pesticides in the ionization source is based on proton transfer, gas phase basicity of the pesticide is one of the main factors influencing their ionization. Hence, proton affinities (PAs) and gas phase basicities (GBs) of the pesticides were computed, theoretically. Density functional theory (DFT) methods have been used for benchmark calculations of PA and GB values, and it has been proved that they produce acceptable agreement with the experimental PA and GB values (Safi and Wazzan 2021; Valadbeigi and Farrokhpour 2013). Also, DFT methods can be employed for calculations with a lower computational cost compared with the other ab initio and multilevel methods (Safi and Wazzan 2021). Hence, DFT method with the hybrid functional of B3LYP was used for structural optimization of the pesticides and their protonated forms in the gas phase. The basis set 6–311++G(d, p) which includes both polarization and diffuse functions for hydrogen and heavy atoms was used for all calculations. Frequency calculations were performed at the same level of theory to obtained PA and GB values of the pesticides at 298 K. PA and GB of a molecule, M, are defined, respectively, as − ∆H and − ∆G of its protonation in gas phase. Gaussian 09 software was used for all calculations (Frisch et al. 2009).
Results and discussion
Direct injection of pesticide solutions
Figure 1 compares the ion mobility spectra obtained by direct injection of the aqueous solution of pesticides dichlorvos, terbutryn, cybutryne, and quinoxyfen recorded at drift tube temperature of 200 °C. One peak is observed per each pesticide at drift times of 8.1, 10.3, 10.5, and 10.8 ms for dichlorvos, terbutryn, cybutryne, and quinoxyfen, respectively. The drift times correspond with the molecular weight of the pesticides, 230, 241, 253, and 308 g mol−1, respectively.
Since the main reactant ion in the ionization region of IMS is hydronium ion H3O+(H2O)n, the mechanism of ionization is based on proton transfer from the hydronium ion and formation of the protonated forms of the pesticides. The optimized structures of the protonated forms of the pesticides are shown in Fig. S2 (Supplementary Materials). Terbutryn and cybutryne have different sites of protonation (shown by circles). Protonation of terbutryn and cybutryne at these sites leads to formation of different protonated isomers (protomers). The peak broadening of cybutryne can be attributed to formation of these protomers with slightly different mobilities (drift times). The calculated PA and GB for different sites of the pesticides are summarized in Table S3. Comparison of the PAs shows that in the case of terbutryn, cybutryne, and quinoxyfen, the nitrogen atom of the six-membered ring is the most preferred site of protonation. In dichlorvos, oxygen atom of the P=O group is the most basic site for protonation.
To obtain the optimum operational condition of the IMS, the ion mobility spectra of the pesticides were recorded at different temperatures of the drift tube. The ion mobility spectra of the pesticides at drift tube temperature range of 120–200 °C are provided in Fig. S3. The decrease in the signal intensity at lower temperatures may be due to more ion loss as the ions spend more time in the drift tube at lower temperatures. Furthermore, as temperature decreases, hydronium ion, H3O+(H2O)n, is more hydrated (Valadbeigi et al. 2019). As the number of water molecules in H3O+(H2O)n increases, its tendency to lose a proton decreases (Goebbert and Wenthold 2004), leading to its lower efficiency for protonation of the pesticides. Hence, the pesticides exhibit the highest signal intensity at drift tube temperature of 200 °C.
Selection of the optimum SPME arrow sorbent
The adsorption efficiencies of three SPME arrows with fiber compositions of (i) divinylbenzene/carbon wide range/polydimethylsiloxane (DVB/CWR/PMDS), (ii) PDMS, and (iii) DVB/PMDS were investigated for adsorption of the pesticides. The responses of these SPME arrows or their capability for adsorption of the pesticides are compared in Fig. 2. For the SPME adsorption, the concentration of cybutryne, dichlorvos, quinoxyfen, and terbutryn in the extraction vial was 0.8, 0.7, 1.0, and 0.3 mg L−1, respectively. The extraction time was 70 min, and the temperature of the solution was kept at 60 °C during the extraction. After extraction, the SPME needle was put in the injection port with the temperature of 260 °C to desorbed the adsorbed pesticides from its fiber. In the case of cybutryne, the SPME fiber coated with DVB/CWR/PMDS and DVB/PMDS exhibited comparable adsorption while adsorption of cybutryne on the SPME arrow with the PMDS fiber composition was less than the two others. Figure 2b shows that the SPME arrow fiber coated with the DVB/CWR/PMDS exhibits the highest response toward the sorption of dichlorvos while the PDMS one did not adsorb any dichlorvos. This is in agreement with the literatures reporting that PDMS fiber has a low affinity toward highly volatile and polar compounds (Pawlizsyn 2009). In the case of quinoxyfen and terbutryn, the response (adsorption efficiency) of the SPME arrows toward these pesticides was as (DVB/CWR/PMDS) > (PMDS) > (DVB/PMDS). Hence, the SPME arrow coated with the DVB/CWR/PMDS was selected as the optimum SPME arrow for detection of the selected pesticides in water. The highest efficiency of the DVB/CWR/PMDS fiber in the extraction of the pesticides is due to micropores in the surface of the fiber that is more effective at trapping low-molecular weight compounds (Helin et al. 2015; Song et al. 2019).
Comparison of performance of the three SPME arrows with solid phases of DVB/CWR/PMDS (gray), PDMS (red), and DVB/PMDS (violet) for adsorption of the pesticides a cybutryne (0.8 mg L−1), b dichlorvos (0.7 mg L−1), c quinoxyfen (1.0 mg L−1), and d terbutryn (0.3 mg L−1). The extraction temperature and time were 60 °C and 70 min, respectively
Effect of injection port temperature
After immersion of the SMPE arrow in the solution and 70-min extraction, it was put in the injection port of the IMS. The adsorbed pesticides were desorbed from the fiber of the SPME arrow in the heated injection port of IMS. For the commercial SPME fibers, the maximum desorption temperatures recommend by the manufacturer are 280–320 °C (Li et al. 2012; Gaffke and Alborn 2021). Higher desorption temperatures can increase the sensitivity and decrease the analysis time (Li et al. 2012); however, it may lead to dissociation of the adsorbed analyte (Gaffke and Alborn 2021). Hence, the temperature of the injection port should be optimized to ensure efficient transfer of the desorbed pesticides to the ionization region of IMS. For this purpose, effect of the injection port temperature on desorption time and signal intensity of the adsorbed pesticides on the SPME arrow coated with DVB/CWR/PMDS were investigated. Figure 3 shows the time evolution of the signal intensity of the IMS-peaks of the pesticides at different temperatures of the injection port up to 260 °C (the maximum possible temperature of the injection port). These plots have been obtained by recording the height of the IMS peak of the pesticides (those shown in Fig. 1) since the SPME needle was put in the injection port. The injection temperature influences both the rate of desorption and the signal intensity. For the selected pesticides, the maximum signal intensity and the shortest analysis time were achieved at the injection port temperature of 260 °C. For cybutryne and terbutryn, at temperatures lower than 200 °C, no signal was observed (Fig. 3a, d), indicating that their interaction with the SPME surface is strong and higher temperatures is needed for their desorption from the SPME fiber. The signal of dichlorvos appeared immediately after putting the SPME device in the injection port with the temperature of 260 °C (Fig. 3b). Desorption of dichlorvos occurred even at 150 °C. This may be attributed to both its low-molecular weight and weak interaction with the SPME surface. Using the data in Fig. 3, the injection port temperature of 260 °C was selected as the optimum temperature for the quantitative analysis.
Simultaneous detection of selected pesticides in aqueous solutions
Although IMS is not as efficient as GC and LC for separation of analytes in a complex mixture, we investigated its performance for simultaneous detection of a mixture of the pesticides. In Fig. 4, the IMS spectra of individually injected pesticides into the IMS injection port (the top spectra) are compared with the IMS spectra of a mixture of the same pesticides extracted from the using the SPME arrow (black below spectrum). Figure 4a compares the IMS spectra for four pesticides cybutryne, dichlorvos, terbutryn, and quinoxyfen and their mixture, and Fig. 4b shows the IMS spectra of three of the pesticides of their mixture (dichlorvos, terbutryn, and quinoxyfen). Since cybutryne shows a broad IMS peak, it overlaps with terbutryn and quinoxyfen peaks at a drift time of 10.5 ms (Fig. 4a); hence, simultaneous detection of these pesticides by IMS is not possible. Other than peak overlapping, because of the high basicity of cybutryne (Table S3), it may suppress the ionization and consequently the signal of other pesticides. Hence, simultaneous detection of three pesticides in a ternary mixture solution without cybutryne was examined. The IMS spectra of the individually injected pesticides dichlorvos, terbutryn, and quinoxyfen are compared with the IMS spectrum of their mixture extracted using SPME arrow. The IMS spectrum of the ternary mixture in Fig. 4b shows that dichlorvos, terbutryn, and quinoxyfen can be separated by MS and detected simultaneously in their mixture. The peak observed at a drift time greater than 12 ms probably corresponds to a proton-bound dimer of dichlorvos.
a Comparison of ion mobility spectra of the liquid injection of analyte standards and their mixture extracted using SPME arrow for a quinoxyfen, cybutryne, terbutryn, and dichlorvos and b quinoxyfen, terbutryn, and dichlorvos. The concentrations of quinoxyfen, cybutryne, terbutryn, and dichlorvos in the mixture are 1.0, 0.5, 0.1, and 0.3 mg L−1, respectively
Quantitative analysis by the direct injection and SPME arrow-IMS
The calibration curves obtained by direct injection of the pesticides into IMS are shown in Fig. S4 (Supplementary Materials). The linear ranges are 1–20, 0.1–5, 1–100, and 0.5–10 mg L−1, for cybutryne, dichlorvos, quinoxyfen, and terbutryn, respectively. The determined relative standard deviation (RSD), accuracy (recovery mean), and limits of detections (LODs, signal to noise > 3) for direct injection of the pesticides are summarized in Table 1. The LODs are also compared with those reported in literature. Table 1 shows that the LODs obtained by the direct injection are higher than those obtained by the SPME arrow-GC/MS, SPME-IMS, immunochemistry, and LC-UV methods. Hence, SPME arrow method was applied to achieve lower LODs.
Using the optimum instrumental and extraction conditions, the calibration curves for the pesticides were obtained by SPME arrow-IMS. Figure S5 shows the calibration curves for (a) cybutryne, (b) dichlorvos, (c) quinoxyfen, and (d) terbutryn (× 3 replicates for each concentration). The linear range of the calibration curves is 0.05–1.0, 0.015–0.8, 0.1–4.0, and 0.01–0.4 mg L−1 for cybutryne, dichlorvos, quinoxyfen, and terbutryn, respectively. The determined recovery mean, RSD, and LOD values by SPME arrow-IMS (signal to noise > 3) are summarized in Table 1. The RSD values for the SPME arrow-IMS method are smaller than those determined for the direct injection method. Also, comparison of the LODs obtained by direct injection into IMS and the SPME arrow-IMS method shows that by using the SPME arrow, the LODs can be improved 7–50 times. The most improvement in LOD was observed for terbutryn and cybutryne with 50 and 20 times, respectively. This can be attributed to the higher tendency of the SPME surface for adsorption of terbutryn and cybutryne. This finding is in good agreement with those obtained in the previous section (Fig. 3). Interactions of terbutryn and cybutryne with the SPME surface are stronger than those of dichlorvos and quinoxyfen. The apparent difference in the structures of cybutryne, terbutryn, dichlorvos, and quinoxyfen is existence of the sulfur atoms in the structures of cybutryne and terbutryn which may be responsible for their stronger interaction with the SPME surface (Gatidou et al. 2007). It is worth noting that other than interaction, the desorption temperature plays an important role in the LOD improvement, as at temperatures lower than 200 °C, cybutryne and terbutryn did not desorb from the SPME surface (Fig. 4).
The LODs obtained by SPME arrow-IMS are compared with those reported for other standard techniques in Table 1. These data show that the SPME arrow-IMS method is more sensitive than LC-UV (Pitarch-Andres et al. 2017) and has a comparable sensitivity with the immunochemical method (Duncan et al. 2018), while its sensitivity is less than SPME arrow-GC–MS (Khademi et al. 2021). HPLC with diode-array detection (DAD) has been used for detection of carbamate pesticides with LOD of 0.017–0.29 µg L−1 (Wu et al. 2019) indicating a higher or comparable sensitivity of HPLC–DAD relative to the SPME-IMS. Flame ionization detector (FID) coupled with GC provides LODs of 0.1–5 µg L−1 for pesticides (Farajzadeh et al. 2015) and 0.2–7 µg L−1 for herbicides (Sorouraddin and Moghadam 2016). Also, the reported LOD for measurement of carbendazim fungicides in water and soil by UV–Vis technique is 2.1 µg L−1 (Pourreza et al 2015). Comparison of these LODs reveals that sensitivity of the SPME-IMS method toward terbutryn is comparable with the sensitivity of GC-FID and UV–Vis techniques.
The matrix effect (%ME) on the analysis of the real samples was evaluated by comparing the maximum peak intensity of the analytes spiked in distilled water as the blank and river water (Taleqan Dam in Alborz province of Iran) as the matrix-spiked sample. Table 2 summarizes the matrix effect of the real samples on the determination of the pesticides with and without SPME. The calculated matrix effect is in the range of 92–110% and 87–111% for the SPME arrow-IMS method and direct injection of the target pesticides, respectively. The SPME arrow-IMS method shows a lower matrix effect (max–min = 18%) than analysis by direct injection (max–min = 24%) of the pesticides indicating that SPME arrow affords a higher recovery percentage.
To validate and highlight the advantages and drawbacks of the developed SPME-IMS method, the performance of this method for the analysis of the pesticides is compared with the SPME–GC–MS method that we have recently developed for the analysis of the same pesticides (Khademi et al. 2021). In the case of optimization of the SPME fiber types, both the SPME-IMS and SPME–GC–MS methods exhibited similar results, and DVB/CWR/PMDS fiber was selected as the optimal sorbent by both the methods. The LODs obtained by the SPME–GC–MS method are about three order smaller than those obtained by the SPME-IMS, because of the high sensitivity of the mass spectrometer detector of the SPME–GC–MS. However, both the methods showed similar RSD and recovery range. The GC-based method was able to separate a mixture of four pesticides without peak overlapping while by the IMS method, separation of three pesticides was possible. The total analysis time by the SPME-IMS is shorter than that by the SPME–GC–MS, as the drift times of IMS are in ms (~ 11 ms for quinoxyfen) while the retention times of GC are in min (~ 14 min for quinoxyfen, for example). Hence, the SPME-IMS provided a much faster analysis of the pesticides, and it can be applied where analysis speed is more important than the sensitivity. Finally, IMS is more cost-efficient than GC–MS, and also it can be used as both a portable analyzer for in-site measurements and a benchtop instrument for laboratory analyses.
Conclusion
An IMS-based method was developed and validated for qualitative and quantitative analysis of different classes of pesticides in water samples. In this work, the direct immersion SPME arrow as a simple and compatible preconcentration and sample introduction technique was coupled with CD-IMS. The operational parameters including the drift tube temperature, the injection port temperature, and the fiber coating type of the SPME arrow were optimized. As the detection time in IMS is of the order of milliseconds (ms), this developed method provided a fast detection and analysis of the pesticides compared to GC with retention times from a few to tens of minutes. The proposed SPME arrow-IMS method was used for the quantification of pesticides in both standard and real water samples, and the detection limits were obtained at the level of low micrograms per liter (µg L−1). Small matrix effect (about 10%) and high recovery were observed for measurements of the pesticides by the SPME arrow-IMS method. The developed method was applied for simultaneous analysis of mixtures of the target pesticides in water samples. Complete separation was achieved for a mixture of three pesticides dichlorvos, terbutryn, and quinoxyfen; however, because of the peak overlapping, IMS could not separate the pesticides in a mixture of the four pesticides.
References
Alavanja MCR (2009) Introduction: pesticides use and exposure extensive worldwide. Rev Environ Health 24:303–309
Albanis TA, Hela DG, Lambropoulou DA, Vasilios AS (2004) Gas chromatographic–mass spectrometric methodology using solid-phase microextraction for the multiresidue determination of pesticides in surface waters. Int J Environ Anal Chem 84:1079–1092
Arthur CL, Pawliszyn J (1990) Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal Chem 62:2145–2148
Bassil KL, Vakil C, Sanborn M, Cole DC, Kaur JS, Kerr KJ (2007) Cancer health effects of pesticides. Systematic review. Can Fam Phys 53:1704–1711
Chen XP, Zhang F, Guo YL (2019) Validating an ion mobility spectrometry-quadrupole time of flight mass spectrometry method for high-throughput pesticide screening. Analyst 144:4835–4840
Duncan H, Abad-Somovilla A, Abad-Fuentes A, Agullo C, Mercader JV (2018) Immunochemical rapid determination of quinoxyfen, a priority hazardous pollutant. Chemosphere 211:302–307
Eiceman GA, Karpas Z, Hill HH Jr (2014) Ion mobility spectrometry, 3rd edn. CRC Press, Taylor & Francis Group, Boca Raton
El-Shahawi MS, Kamal MM (1998) Determination of the pesticide chlorpyrifos by cathodic adsorptive stripping voltammetry. Fresenius J Anal Chem 362:344–347
Farajzadeh MA, Feriduni B, Moghadam MRA (2015) Development of counter current salting-out homogenous liquid–liquid extraction for isolation and preconcentration of some pesticides from aqueous samples. Anal Chim Acta 885:122–131
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B et al (2009) Gaussian 09, Revision A.1. Gaussian Inc., Wallingford
Gaffke AM, Alborn HT (2021) Desorption temperature, solid-phase microextraction (SPME), and natural product analyses, how low can we go? J Chem Ecol 47:134–138
Gallart-Mateu D, Armenta S, de la Guardia M (2016) Indoor and outdoor determination of pesticides in air by ion mobility spectrometry. Talanta 161:632–639
Gatidou G, Thomaidis NS, Zhou JL (2007) Fate of Irgarol 1051, diuron and their main metabolites in two UK marine systems after restrictions in antifouling paints. Environ Int 33:70–77
Goebbert DJ, Wenthold PG (2004) Water dimer proton affinity from the kinetic method: dissociation energy of the water dimer. Eur J Mass Spectrom 10:837–845
Goscinny S, McCullagh M, Far J, De Pauw E, Eppe G (2019) Towards the use of ion mobility mass spectrometry derived collision cross section as a screening approach for unambiguous identification of targeted pesticides in food. Rapid Commun Mass Spectrom 33:34–48
Grimalt S, Dehouck P (2016) Review of analytical methods for the determination of pesticide residues in grapes. J Chromatogr A 1433:1–23
Helin A, Rönkkö T, Parshintsev J, Hartonen K, Schilling B, Läubli T, Riekkola ML (2015) Solid phase microextraction Arrow for the sampling of volatile amines in wastewater and atmosphere. J Chromatogr A 1426:56–63
Holopainen S, Luukkonen V, Nousiainen M, Sillanpää M (2013) Determination of chlorophenols in water by headspace solid phase microextraction ion mobility spectrometry (HS-SPME-IMS). Talanta 114:176–182. https://doi.org/10.1016/j.talanta.2013.04.023
Khademi SMS, Salemi A, Jochmann M, Jaksimoski S, Telgheder U (2021) Development and comparison of direct immersion solid-phase micro extraction arrow-GC-MS for the determination of selected pesticides in water. Michrochem J 164:106006. https://doi.org/10.1016/j.microc.2021.106006
Khademi SMS, Telgheder U, Valadbeigi Y, Ilbeigi V, Tabrizchi M (2019) Direct detection of glyphosate in drinking water using corona-discharge ion mobility spectrometry: a theoretical and experimental study. Int J Mass Spectrom 442:29–34
Li Y, Wang Y, Zhang Y, Sun C (2012) Determination of 16 polycyclic aromatic hydrocarbons in water using fluorinated polyaniline-based solid-phase microextraction coupled with gas chromatography. Environ Monitor Assess 184:4345–4353
Maltzman SL, Minteer SD (2012) Mitocondrial-based voltammetric sensor for pesticides. Anal Methods 4:1202–1206
Mohammadi A, Ameli A, Alizadeh N (2009) Headspace solid-phase microextraction using a dodecylsulfate-doped polypyrrole film coupled to ion mobility spectrometry for the simultaneous determination of atrazine and ametryn in soil and water samples. Talanta 78:1107–1114
Pawlizsyn J (2009) Handbook of solid phase microextraction. Chemical Industry Press, Beijing
Pimentel D, Acquay H, Biltonen M, Rice P, Silva M, Nelson J, Lipner V, Giordano S, Horowitz A, D’Amore M (1992) Environmental and economic costs of pesticide use. Bioscience 42:750–760
Pitarch-Andres S, Roca-Genoves P, Pris-Vicente J, Esteve-Romero J (2017) Determination of diuron, terbuthylazine, and terbutryn in wastewater and soil by micellar liquid chromatography. Anal Bioanal Chem 409:2037–2049
Pourreza N, Rastegarzadeh S, Larki A (2015) Determination of fungicide carbendazim in water and soil samples using dispersive liquid–liquid microextraction and microvolume UV–vis spectrophotometry. Talanta 134:24–29
Rickert DA, Singh V, Thirukumaran M, Grandy JJ, Belinato JR, Lashgary M, Pawliszyn J (2020) Comprehensive analysis of multiresidue pesticides from process water obtained from wastewater treatment facilities using solid phase microextraction. Environ Sci Technol 54:15789–15799
Sadat SAA, Ilbeigi V, Valadbeigi Y, Soleimani M (2020) Determination of pesticides phosalone and diazinon in pistachio using ion mobility spectrometry. Int J Ion Mobil Spectrom 23:127–131
Safi ZS, Wazzan N (2021) Benchmark calculations of proton affinity and gas-phase basicity using multilevel (G4 and G3B3), B3LYP and MP2 computational methods of para-substituted benzaldehyde compounds. J Comput Chem 42:1106–1117
Saraji M, Jafari MT, Amooshahi MM (2018) Sol–gel/nanoclay composite as a sorbent for microextraction in packed syringe combined with corona discharge ionization ion mobility spectrometry for the determination of diazinon in water samples. J Sep Sci 41:493–500
Sharma A, Kumar V, Shahzad B et al (2019) Worldwide pesticide usage and its impacts on ecosystem. SN Appl Sci 1:1446
Song NE, Lee JY, Lee YY, Park JD, Jang HW (2019) Comparison of headspace-SPME and SPME-Arrow-GC-MS methods for the determination of volatile compounds in Korean salt-fermented fish sauce. Appl Biol Chem 62:16. https://doi.org/10.1186/s13765-019-0424-6
Sorouraddin SM, Moghaddam MRA (2016) Development of molecularly imprinted-solid phase extraction combined with dispersive liquid–liquid microextraction for selective extraction and preconcentration of triazine herbicides from aqueous samples. J Iran Chem Soc 13:1093–1104
Tuovinen K, Paakkanen H, Hanninen O (2000) Detection of pesticides from liquid matrices by ion mobility spectrometry. Anal Chim Acta 404:7–17
Valadbeigi Y, Farrokhpour H (2013) DFT, CBS-Q, W1BD and G4MP2 calculation of the proton and electron affinities, gas phase basicities and ionization energies of saturated and unsaturated carboxylic acids (C1–C4). Int J Quantum Chem 113:1717–1721
Valadbeigi Y, Ilbeigi V, Michalczuk B, Sabo M, Matejcik S (2019) Study of atmospheric pressure chemical ionization mechanism in corona discharge ion source with and without NH3 dopant by ion mobility spectrometry combined with mass spectrometry: a theoretical and experimental study. J Phys Chem A 123:313–322
Vas G, Vékey K (2004) Solid-phase microextraction: a powerful sample preparation tool prior to mass spectrometric analysis. J Mass Spectrom 39:233–254
Walendzik G, Baumbach JI, Klockow D (2005) Coupling of SPME with MCC/UV-IMS as a tool for rapid on-site detection of groundwater and surface water contamination. Anal Bioanal Chem 382:1842–1847
Wang JX, Gao XG, Jia J, Li JP, He XL (2015) Solid phase microextraction-ion mobility spectrometry for rapid determination of trace dichlorovos in tea drinks. Chin J Anal Chem 43:1193–1197
Weber C, Smolka S (2005) Towards pesticide use reduction in Germany, Pan Ger. 1–79. http://ww.pan.germany.org
Weickhardt C, Kaiser N, Borsdorf H (2012) Ion mobility spectrometry of laser desorbed pesticides from fruit surfaces. Int J Ion Mobil Spectrom 15:55–62
Wu J, Mei M, Huang X (2019) Fabrication of boron-rich multiple monolithic fibers for the solid-phase microextraction of carbamate pesticide residues in complex samples. J Sep Sci 42:878–887
Yu M, Zhang N, Fan L, Zhang C, He X, Zheng M, Li Z (2015) Removal of organic sulfur compounds from diesel by adsorption on carbon materials. Rev Chem Eng 31:27–43
Acknowledgements
The authors thank HPC Computing Facility of IKIU, Iran, for computational resources.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Editorial responsibility: Samareh Mirkia.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Seyed Khademi, S.M., Ilbeigi, V., Valadbeigi, Y. et al. Solid phase microextraction arrow-ion mobility spectrometry for determination of selected pesticides in water. Int. J. Environ. Sci. Technol. 21, 6925–6934 (2024). https://doi.org/10.1007/s13762-024-05469-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-024-05469-z