Abstract
Central composite rotatable design (CCRD) was employed to optimize initial temperature (ºC), ramp function (ºC/min) and salt addition for trihalomethane extraction/quantification from the drinking water distribution network in Ratta Amral, Rawalpindi., Pakistan. Drinking water samples were collected from the treatment plant, overhead reservoir and consumer’s taps. The USEPA method for trihalomethane detection 551.1 via gas chromatography was applied using liquid–liquid extraction. The experiments with input variables for sample preparation and operational conditions were performed in a randomized order as per design of experiment by central composite rotatable design and responses were evaluated for model development. A significant (p = 0.005) two-factor interaction model was optimized. Initial temperature was observed to be insignificant (p = 0.64), while ramp function (p = 0.0043) and salt addition (p = 0.04) were significant. Product of salt addition and ramp was significant (p = 0.004), while product of initial temperature and salt addition was insignificant (p = 0.008). With a desirability function of 0.97, an initial temperature of 50 ºC, 6 ºC rise/min to 180 ºC and 0.5 g salt were optimized. It was found that development and optimization of the analytical methods for rapid trihalomethane detection would improve optimization of the current treatment practices in the country.
Similar content being viewed by others
Introduction
Secure drinking water sources and well-managed water treatment plants are indispensable to provide safe drinking water for human consumption. However, illegal industrial waste disposal practices stressed or poorly maintained distribution systems, aging pipes, absence of or ineffective filtration and disinfection facilities result in the deterioration of piped drinking water quality below acceptable levels, posing serious health risks to the community (Blokker et al. 2016). In addition, lack of proper sanitation and pollution control measures cause an increase in natural organic matter (NOM) in open water channels and distribution network (Sillanpaa et al. 2018; Abdullah 2014).
The intermixing of sewage with drinking water due to cross-connections is leading to chemical and biological/microbial contamination of drinking water, which in turn results in waterborne diseases within communities in Pakistan (Daud et al. 2017; Prest et al. 2016; Nabeela et al. 2014). An average of approximately 95% of drinking water samples were reported to be bacteriologically contaminated (Azizullah et al. 2011; Raza et al. 2017). In Punjab province, Pakistan, 90% of the people suffer from major waterborne diseases, e.g., cholera and diarrhea with cramps (Hannan et al. 2010).
In Pakistan, chlorine is added to the water in the treatment plant to inactivate pathogenic bacteria that have not been removed during the previous treatment steps and it is referred to as primary disinfection. In addition to the primary disinfection, to reduce the risk of a pathogen’s re-growth and to maintain biological stability of the drinking water, residual chlorine of 0.2 to 0.5 mg/L in the distribution network is permitted by the World Health Organization (WHO) (WHO 2011). However, the chlorination of drinking water containing NOM generates chlorinated by-products (CBPs) mostly trihalomethanes (THMs) (Fattahi and Shariati-Rad 2020; Ramavandi et al. 2015; Rezaee et al. 2014; Ibrahim and Abdul Aziz 2014). Poor management practices in Pakistan result in excessive THM production within the distribution networks most of the time. Various studies reported the presence of THM in the distribution networks of different cities in Pakistan (Abbas et al. 2015; Qaiser et al. 2014; Karim et al. 2011 and Amjad et al. (2013). These THMs are reported to be carcinogenic, as reported by several epidemiological studies (Wang et al. 2011; Brown et al. 2011).
The THM formation in the distribution network has been reported to be a function of the quality of water being chlorinated (Bourdon and Linares 2014), especially in relation to the concentration and characteristics of NOM and the chlorination conditions (Ramavandi 2015). In general, treated surface waters have higher THM concentrations than treated ground waters because of the potential high organic matter level caused by vegetation and warm temperatures (Pagano et al. 2014). In addition, treatment processes such as CT (chlorine initial concentration × contact time) may have a pivotal role in THM formation downstream along with other environmental conditions like water pH and temperature (Ramavandi 2015; Navalon et al. 2008; Sadiq and Rodriguez 2004). On the other hand, water distribution networks, due to extended contact time between chlorine residuals and THM precursors in the system, provide suitable environments for THM formation (Al- Omari et al. 2014). Higher chlorine residuals and the presence of NOM may augment the formation of THMs (Singh et al. 2012; Lee et al. 2010).
It has also been reported that THM concentrations in water increase significantly from the distribution network to the consumer’s tap, however, being higher at the extremities of the distribution network corresponding to the longest retention time (Hien et al. 2015). It is further documented that the levels of THMs increased as the distance from the treatment plant increased (Valdivia-Garcia et al. 2016; Le Bel et al. 1997). Since THMs pose a serious health risk to humans, the US Environmental Protection Agency (US-EPA) has regulated the maximum contaminant level (MCL) for THMs of 80 µg/L (Selvam et al. 2018; Fooland 2011; Valdivia-Garcia et al. 2016) which require water utilities to provide drinking water of high quality (Hua et al. 2016). The formation of THM could be used directly to assess the efficiency of chlorination for THM formation and the efficiency of the disinfection process (Fakour and Lo 2018). Nevertheless, monitoring and minimization of THM formation during chlorination of surface water sources, rich in NOM, pose a real challenge for the authorities in Pakistan (Valdivia-Garcia et al. 2016). In addition, THMs in the drinking water supply system should be monitored periodically to minimize or eliminate their presence whenever the concentration approaches threshold levels (Poleneni and Inniss 2013). Thus, the use of modern available tools, like the Response Surface Methodology–Central Composite Design (RSM-CCRD) for GC–MS process variables optimization and modeling, may result in a better understanding of the interactive effect of these factors and in turn a better prediction of the response through the suggested model.
THMs are usually analyzed through various chromatographic techniques such as gas chromatography (GC) with electron capture detection (GC-ECD), GC mass spectrometry (GC–MS), high-performance liquid chromatography with mass spectrometry (HPLC–MS), ultrahigh-performance liquid chromatography with mass spectrometry (Up-LC–MS), ion chromatography (IC) and capillary electrophoresis (Liu et al. 2013a, b). Because of the volatile nature of THMs, GC–MS is preferred over HPLC due to its high sensitivity for volatile organic compounds (VOCs) (Niri et al. 2008), its detection limits in the μg/L range, and its good linearity and reproducibility (Valencia et al. 2013).
In GC–MS analysis, although different sample preparation techniques (e.g., extraction and concentration using salting out effect) and operational parameters (e.g., initial temperature and column separation by applying the temperature gradient, i.e., ramp) were evaluated individually by many researchers (Yuan et al. 2018; Valente et al. 2013), these two categorical sets of parameters have not been optimized together; nor was there any statistical optimization ever applied to model the effect of both on THM detection and quantification. Thus, for simultaneous detection and quantification of THM, the GC–MS process variables need to be optimized.
Therefore, the present study was conducted to (1) optimize and model the GC–MS operating conditions for THM detection through liquid–liquid extraction (LLE) using RSM-CCRD and (2) apply on-site the optimized conditions to determine and quantify THMs in drinking water from Ratta Amral, Rawalpindi. This is the first study optimizing and modeling the GC–MS operating and extraction conditions simultaneously. It is expected that this study will set a baseline for the optimization and modeling of the critical GC–MS operating condition necessary for accurate detection and quantification of THMs in drinking water samples. The results of this study will further be helpful to water treatment authorities, regulatory bodies and stakeholders regarding formulation and implementation of the regulation regarding occurrence of THMs in drinking water distribution networks, and their monitoring and removal strategies. This will raise awareness about the potential presence of THMs and the risk linked to consumption of THM containing water in Rawalpindi and Islambad, Pakistan.
Materials and methods
Sample collection
Ratta Amral, under UC-01, Dhoke Ratta, Rawal Town, Rawalpindi is situated at 33° and 36.29" North and 73° and 2.45" East (Fig. 1). Rawal dam and Khanpur dam supply water to Rawal and Sangjani treatment plants, respectively, where water is treated by coagulation, flocculation, sedimentation, filtration and chlorination before distribution to the densely populated area of Ratta Amral and adjacent areas of Rawalpindi. Water samples were collected in glass bottles (1 L each) from the Water Treatment Plant (WTP) outlet, overhead reservoir (OHR) and from consumer’s taps as per standard methods (APHA 2012). For water sample collection, the faucets were turned on for about 5 min before samples were collected to make sure the water was coming directly from the mains and not from the building’s piping system. On site measurement for residual chlorine was performed immediately using portable multimeter (Spectroquant Picco). For THM extraction, amber glass bottles were used with 0.01 N sodium thiosulfate (Na2S2O3) as a quenching reagent for residual chlorine to prevent additional TTHM formation during transportation. Bottles with Teflon-coated rubber septa were filled to zero head space to prevent TTHM volatilization, sealed and stored at 4 °C. Duplicate samples for THM measurement were collected once a month from each sampling location. Samples were analyzed for THMs within 24 h of collection for chloroform (CF), bromodichloromethane (BDCM), dibromochloromethane (DBCM) and bromoform (BF).
Location of the sampling area of Ratta Amral, Rawalpindi (Courtesy: Google 3D maps). In an effort to assess water quality changes after each treatment operation and evaluate treatment efficacy relevant to the potential cause for high TTHM in the finished water of Ratta Amral DWDN, water samples were collected on a monthly basis for a period of six months. Drinking water samples were collected as per standard methods (APHA 2012) from the water treatment
Design of experiment (DoE) using RSM-CCRD
RSM is useful for scheming experiments, structure models and investigating the effects of independent variables on dependent variables (Ramyadevi et al. 2012). In this study, RSM coupled with CCRD was used to optimize the GC–MS analytical conditions for THM detection and quantification. The optimization technique in RSM includes displayable and interactive three-dimensional (3D) plots and two-dimensional (2D) contour graphs. In addition, a graphical representation of the regression equation is used to visualize the relationship between the response and experimental points of each factor (Younis et al. 2014). RSM-CCRD quantifies the relationship between the controllable input parameters and resultant response surfaces. Despite having other more effective statistical and mathematical tools for process optimization such as the Box–Behnken design (BBD), CCRD was selected because it predicts response based on a small number of experimental data set, in which all parameters vary within a chosen range (Czyrski and Jarzębski 2020; Almeida et al. 2017). Also, the degree of freedom offered by CCRD helps the creation of more reliable models, especially in situations when some experiments can be affected by experimental error (Rakic et al. 2014). The development of statistical models may be valuable for forecasting and understanding the results of experimental factors. The main advantage of RSM-CCRD is its optimization capacity for multiple operational variables simultaneously with a small number of experiments, saving time and labor.
In liquid–liquid extraction GC–MS (LLE–GC–MS), salt is added to enhance the THM extraction from the aqueous phase to the solvent phase as a sample preparation parameter (Budziak and Carasek 2007). The addition of salt increases the ionic strength of the solution, changing the vapor pressure, solubility and surface tension of the analytes, resulting in the change of liquid/vapor equilibrium of the system, therefore making it easy to be extracted and separated from the aqueous phase (Niri et al. 2008). Maximum THM extraction was observed at low concentration of the salt as described by Santos et al. (2013); therefore, low salt concentration between 0.25 and 1 g was selected for optimization studies. Parkinson et al. (2016) described a 10% increase in extraction by adding an additional 10% of salt. As operational parameters, initial temperature and ramp functions were selected (Uppeegadoo et al. 1999). To overcome challenges, the optimization and modeling of the GC–MS process variables are highly required through the modern available modeling and statistical tools like response surface methodology coupled with a central composite rotatable design (RSM-CCRD).
Three independent variables, i.e., initial temperature (temp, ºC), ramp (ºC/min) and salt addition (g), were selected for the optimization process in this study. The variables with the ranges are shown in Table 1, applying RSM-CCRD (Design-Expert: version 9). The selection of these variables with defined experimental ranges was carefully chosen based on a previous screening of these variables determined in earlier preliminary experiments using the classical one-variable-at-a-time approach and from earlier documented literature (Elfghi and Amin. 2013; Niri et al. 2008). But single-factor optimization is time-consuming and laborious. Furthermore, it hardly ever promises the determination of optimal conditions for effective and efficient production of the target product (Shafi et al. 2018). Based on these facts, the lowest, center and the highest levels of selected variables, including axial star points of (− ά and + ά), are shown in Table 1.
The independent variables were used as input variables by RSM-CCRD, which resulted in a composite factorial design with the lowest value (on extreme left) of – α, − 1, 0, + 1 and + α (the highest value on extreme right) (Table 2). The design of experiments (DoE), with twenty experimental runs, was carried out randomly to guarantee the independence of the results, reducing the effects of uncontrolled factors. After the assessment of the responses, the model was predicted, evaluated and analyzed as described by Teglia et al. (2015) and Kohli and Singh (2011).
TTHM extraction
Prepared THM standards were purchased from Supelco in a stock concentration of 5000 µg/mL dilution in methanol as solvent, and a known working solution of fluorobenzene (FB) (2000 µg/mL) as an internal standard was prepared. The USEPA method for THM detection 551.1 via GC–MS was applied using LLE with methyl-ter butyl ether (MtBE) as an extraction solvent. For extraction of THMs, 7 mL of the water sample and various masses of sodium sulfate (anhydrous) were mixed briskly to observe the salting out effect. One mL of the MtBE was vortexed in the same test tube and left undisturbed for 2 min from which 1 µL of organic layer was analyzed through GC–MS. The GC–MS conditions are described elsewhere (Rasheed et al. 2016).
Statistical analysis
Analysis of variance (ANOVA) was applied to observe the significance of interactive effect and strength between each independent variable as determined by the F and p value, respectively. A p value less than 0.05 shows a constructive influence on overall response (i.e., THM extraction and quantification) for each factor (Behin and Farhadian 2016).
Multifactorial optimization
Multifactorial optimization is the arrangement of different optimized parameters, giving a specific reaction concurrently (Ramyadevi et al. 2012). The simultaneous optimization of all responses is only possible by combining input variables into a single-objective function or desirability function, denoted by (D), which basically represents the relationship of all responses that are to be optimized (Ackey and Anagun 2013; Kohli et al. 2011; Mohan et al. 2012). A value of D closer to 1 is considered most appropriate (Hegazy et al. 2013).
Results and discussion
TTHM quantification by GC–MS and optimization of critical variables
Analytical method development and validation procedures are vital in the determination and quantification of any contaminant in water samples. Experimental design was used to find the optimal analytical conditions for the chromatographic separation. For THM extraction, and its subsequent detection from water samples through GC, it is reported to be dependent on salt concentration during LLE, while for individual component separation and detection, it is reported to be column initial temperature and ramp function dependent. Therefore, the optimum performance of GC–MS analytical conditions was optimized using RSM-CCRD and data analysis was performed using Design-Expert (DX version 9). RSM-CCRD establishes the effects of the input variables on the dependent response and their interactions (Karimi et al. 2011). The result would be helpful to optimize extraction and analytical conditions which are required to isolate these by-products from water, thus allowing their detection through GC–MS.
The design matrix consisted of 20 experimental runs incorporating the independent variables (salt concentration, initial temperature and ramp function) and the responses (THMs) as displayed in Table 3. The TTHMs were extracted under various experimental conditions defined by DoE, and THM concentrations were recorded as a response on the extreme right of Table 3. The ANOVA was calculated through CCRD. The suggested two-factor interaction (2FI) model was significant (p = 0.005) at a 95% confidence level with an F value of 5.35 (Table 4). A huge F value, while with a very small p value (p = 0.005), indicated a significance of the derived model as described earlier by Appavoo et al. (2014), Chen et al. (2022) and Liu et al. (2015). Furthermore, the higher the F value, the higher the probability that the variance contributed by the RSM model will be considerably larger than random error (Alman-Abed et al. 2020). The determination coefficient R2 value was 0.85, while adjusted R2 (Adjust. R2) was 0.68, authorizing that the model elaborated the experimental statistics well. Adequate precision (Adeq. Pre.˃ 4) suggests that the model could be applicable for describing the effect of variables on THM detection and quantification. It is evident that the effect of initial temperature was insignificant (p = 0.64), while ramp function (p < 0.0043) and salt addition (p < 0.04 were observed to be significant.
Furthermore, product of initial temperature and ramp (A*B; p = 0.0081), and ramp and salt addition (B*C; p = 0.046) were observed significant. The interactive influences of the studied factors were plotted as 2D and 3D graphs using DX-9. These graphs described the precise elucidation of an interactive effect studying independent variables as suggested by Asadzadeh et al. (2018). The effect of the different variables is discussed one by one below.
Interactive effect of ramp and initial temperature
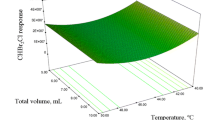
Column temperature was documented as a critical factor for separation of the target analytes by Liu et al. (2015). While observing the interactive effect of initial temperature (X1: A) and ramp function (X2: B) in Fig. 2, where TTHM concentration (µg/L) is shown on z-axis, the color gradient from blue to red showed the increased concentration of TTHM species, and the same color representation is depicted in the 2D contour graph (Fig. 2, X1: A: initial temperature, X2: B: ramp function). When discussing the initial temperature, a reasonable TTHM detection was observed at 45 ºC, while it is apparent that by increasing the ramp from 4*150 (4 degree rise in temperature per minute to 6*180 (6 ºC rise in temperature per minute to 180 ºC), TTHM detection and quantification increased from 300 to 700 µg/L, respectively (Fig. 2). This may be due to an increased temperature which increased the desorption rate of the TTHM species from the column; therefore, reaching the detector resulted in good peak signal production. It is further reported that these compounds spend more time in the mobile phase at higher temperatures, helping them elute faster and reducing band-broadening (Bloomberg and Klee 2001). Allard et al. (2012) also documented an increased response from 160 to 200 ºC for CHBrI2 and CHI3.
Effect of salt addition and ramp function
Shi and Adams (2012) discovered that the use of sodium sulfate improved the sensitivity of THM detection in their study. To enhance the separation of the organic layer from water sample, various salt concentrations were applied. The mutual effect of salt concentration and ramp function (X1: B: ramp, X2: C: salt addition) showed enhanced TTHM separation in the organic layer at higher salt concentration (Fig. 3) which resulted in a significant increase in the peak area of TTHMs, depicting higher concentration of TTHMs in the same water sample than when a small amount of salt was used. Figure 3 shows that the salt addition of 0.5 g was sufficient to cause maximum separation of the organic layer from the solvent layer, while maximum TTHM separation as peaks and their detection was observed at a ramp function of 6*180. The same effect can be observed in a 2D contour graph (Fig. 3: X1: B: ramp, X2: C: salt addition). This may be due to the fact that an increase in salt concentration could enhance the ionic strength of the aqueous phase and thus drive the target analytes into the organic phase (Liu et al. 2013a, b). Therefore, more extraction of TTHMs from the water sample was observed with increasing salt concentration from 0.25 to 0.5 mg/L. According to Santos et al. (2013), the addition of salt resulted in the change of liquid/vapor equilibrium of the system. These results agreed with those already described by Budziak and Carasek (2007).
Modeling THM extraction and separation conditions
The Pareto analysis compares the relative contribution of each studied variable on the response in a graphical form (Asadzadeh et al. 2018). So, to assess the contribution of most significant factors toward effective extraction of TTHMs from water samples, the interactive plot of significant factors was taken into consideration (Table 4). A Pareto chart of effect was plotted for each factor taken as an individual, as well as a mutual interaction, in various combinations, based on percentage share in the overall process (Fig. 4). The bar lengths, in Fig. 4, described the percentage contribution of each variable factor in TTHM extraction and detection by GC–MS as mentioned by Tuncel and Topal (2011). The most significant variable was determined to be the product of A and B (initial temp*ramp), which contributed almost 40% to the process. This may be due to the fact that temperature plays a vital role in the separation of each and every constituent of the mixture by interplaying with the stationary and mobile phase as described by Blumberg and Klee (2001). The analysis indicated that the order of effective variables was B (ramp) > a product of B*C (ramp*salt addition) > C (salt addition) > A (initial temperature) > and a product of A and C (initial temperature*salt addition).
Model development for the THM extraction and detection
Based on the ANOVA for the interactive effect of the studied factors (Table 1), a relationship between the input variables and the resulting response was attained and expressed by a reduced model.
The proposed statistical model provides a critical analysis of individual and simultaneous interactive impacts of the selected independent variables.
Evaluation of the model
The adequacy of the model was verified and validated by correlation between normal plots of residuals (difference between the experimental and the predicted values) as shown in Fig. 5 (x-axis: X1: Externally studentized residuals, y-axis: X2: normal percentage probability) as described by Singh et al. (2012). Graphical data close to a straight line showed that the model sufficiently predicted the effect of the studied factors which is further confirmed by R2 = 0.85 and p ≤ 0.005) (Rezaee et al. 2014) with no serious violation of the assumptions underlying the analyses and confirming residuals independence.
Numerical optimization
The optimum conditions for all the studied factors were optimized by attaining the maximized objective function (D) by numerical optimization technique using RSM-CCRD. For maximum TTHM extraction and its quantification, the initial temperature at 50 ºC, ramp at 6 ºC rise/min to 180 ºC and 0.5 g salt were optimized at D value approximately 0.97 as a multifactorial optimization process (Fig. 6).
Graphical optimization
Graphical optimization was used to trace the optimum levels for maximum response, which could be visualized in the form of a graph as shown in Fig. 7; x-axis: A: initial temperature, y-axis: B: ramp). The maximum predicted value is indicated by the surface confined in the smallest eclipse in the yellow contour known as the overlay plots (Trinh and Kang 2010). These overlay plots allow a visual selection of the optimum conditions according to a certain criterion which is in full agreement with the set limits in this yellow region (Almeida et al. 2017).
The CCRD determined and modeled the TTHM extraction and quantification process efficiently. It is a technical and economic method to gain the maximum amount of information within a small period of time and with few experiments. As a conclusion, significant variables affecting the extraction efficiency were determined, including the amount of salt for solvent separation in the LLE extraction process as a sample preparation step. Oven temperature and ramp function were optimized as GC–MS operational conditions for maximum THM detection and quantification. The optimum experimental conditions obtained from this statistical evaluation included a 0.5 g salt during the LLE process resulted in the maximum TTHM extraction at an initial temperature of 50 ºC, ramp at 6 ºC rise/min to 180 ºC at D = 0.97.
These proposed optimized conditions were applied in the second phase of the experiment to determine and quantify TTHMs from water samples of a local populated area, and good agreement was observed between modeled and actual data.
Application of optimized parameters on real water samples for the determination of THMs in the water distribution network of Ratta Amral, Rawalpindi
An assessment of the influent and effluent water in WTP can determine the relationships between WTP performance, water quality and DBP formation (Cook and Drikas 2011). As discussed earlier, the constitution of various THM compounds in chlorinated water depends on the chlorine dose, the nature and concentration of organic precursors, pH, temperature, presence of iodide ions and residual chlorine concentration (Hamed et al. 2017; Ramavandi et al.2015). After optimizing the analytical conditions for the THM extractions and analysis, drinking water samples for TTHMs from Ratta Amral, Rawalpindi, were analyzed. Residual chlorine, including free and total chlorine, were also analyzed to determine their effect on THM formation in the area (Table 5). The proposed optimized conditions were applied to determine and quantify TTHMs from water samples of a local populated area, and good agreement was observed between the modeled and actual data.
THMs were observed in all sampling stations ranging from 248 to 305 µg/L as shown in Fig. 8 far above the WHO permissible limit of 80 µg/L. These results were in accordance with those reported earlier by Amjad et al. (2013). Meanwhile, the free chlorine concentration at the outlet of the water treatment plant was 0.85 mg/L which reduced by more than a factor of 3 to 0.25 mg/L, i.e., 0.25 mg/L, when water reached the OHR. After that, no free chlorine was detected at any sampling point. The presence of TTHMs in samples from the WTP could be attributed to the presence of organic matter compounds, which are difficult to remove using conventional water treatment technologies and the continuous presence of residual chorine in the distribution network. These results were found in accordance with Toroz and Uyak (2005), who observed seasonal changes in the concentration of THM in a distribution system. According to El Shafey et al. (2000), the formation of THMs in the treatment plant only represented about 45% of the THMs found at the end of the pipelines. The provision of adequate residual chlorine to protect the drinking water from bacterial contamination/re-growth is recommended by USEPA (2006). But this practice could lead to additional THMs in drinking water under improper organic removal (Chaudhary et al. 2008). CF was found to have the highest concentration (approximately 90% of total THM) among the THMs, followed by BDCM and DBCM with BFM having concentrations below the detection limit (Fig. 8). This THM species distribution may be attributed to the fact that bromine–carbon bonds are more tolerant to dissociation, compared to chlorine, as a result of lower dissociation energies (Abusallout et al. 2017). These results were in accordance with Clayton et al. (2019), Hua et al. (2016) and Elsheikh and Basiouny (2011). This high concentration of TTHMs is of significant concern, requiring the use of an alternative water source or additional treatment such as activated carbon to remove DBPs prior to water distribution.
On the other hand, it was further observed that with increasing distance from the WTP, the formation of THMs increased. Chang et al. (2010) reported most of the THM formation occurred during the initial contact hours of treatment. However, the results contradicted Kolla (2004) and Chang et al. (2001) who observed no noteworthy rise in THM concentration beyond 48 h of disinfection. In a study by Le Bel et al. (1997), levels of THMs increased as the distance from the treatment plant increased. Generally, under favorable environmental conditions, the presence of NOM and sufficient residual chlorine in the distribution network, THMs continue to be formed (Sun et al. 2009).
Conclusion
The proposed optimized conditions were applied to determine and quantify TTHMs from water samples of a local populated area, and good agreement was observed in proposed and actual data. The effect of key parameters (initial temperature, ramp function and salt addition) on TTHM extraction and quantification through GC–MS was optimized using RSM-CCRD. This investigation shows that RSM-CCRD is a suitable method to optimize the operating conditions in order to maximize the TTHM response. The use of RSM-CCRD in optimizing the separation, as well as operational conditions, turned out to be a significant innovation.
The main conclusions drawn from this work are:
-
A ramp function and salt addition were observed to be significant with a p value of 0.004 and 0.04, respectively. On the other hand, a product of initial temperature and ramp (A*B; p = 0.008) and ramp and salt addition (B*C; p = 0.04) were observed to be significant as two-factor interactions.
-
An initial temperature of 50 ºC, ramp at 6 ºC rise/min to 180 ºC and 0.5 g salt resulted in the maximum TTHM extraction at D = 0.97.
-
TTHMs were observed in all sampling stations ranging from 248 to 305 µg/L, which were far above the USEPA allowable limit of 80 µg/L. This high TTHM concentration could be linked to the presence of organic matter and residual chlorine in the distribution network.
-
CF had the highest concentration (approximately 90% of total THM) among the THMs, followed by BDCM, while DBCM and BFM concentrations were below the detection limit.
-
The free chlorine concentration exiting the water treatment plant was found to be approximately 0.85 mg/L, which reduced to more than one third of 0.85 mg/L, i.e., 0.25 mg/L, when water reached the OHR. After this point, no free chlorine was detected at any sampling point.
-
Despite the significant findings of the study, sometimes it is not easy to achieve the adjusted values of the chosen factors in the RSM-CCRD design. There is also a predictable inability of the tool to estimate individual interaction terms.
Based upon the above conclusions, the following recommendations are made:
-
Continuous monitoring of THM species should be carried out to check the stability of the system.
-
The entry of the total organic carbon causing THM formation in the system could be minimized by taking control measures.
-
Additional treatment options like granular activated carbon adsorption may be used before the water reached the end user.
References
Abbas S, Hashmi I, Saif Ur Rehman M, Qazi IA, Awan MA, Nasir H (2015) Monitoring of chlorination disinfection by-products and their associated health risks in drinking water of Pakistan. J Water Health 13(1):270–284
Abdullah AM (2014) Assessment of potential risks from trihalomethanes in water supply at Alexandria Governorate. J Pollut Eff Contrl 2(2):119
Abusallout I, Rahman S, Hua G (2017) Effect of temperature and pH on dehalogenation of total organic chlorine, bromine and iodine in drinking water. Chemosphere 187:11–18. https://doi.org/10.1016/j.chemosphere.2017.07.149
Akçay H, Anagun AS (2013) Multi response optimization application on a manufacturing factory. Comput Math with Appl 18(3):531–538
Allard S, Charrois JW, Joll CA, Heitz A (2012) Simultaneous analysis of 10 trihalomethanes at nanogram per liter levels in water using solid-phase microextraction and gas chromatography mass-spectrometry. J Chromatogr A 1238:15–21
Alman-Abad ZS, Pirkharrati H, Asadzadeh F (2020) Application of response surface methodology for optimization of zinc elimination from a polluted soil using tartaric acid. Adsorp Sci Tech 38(3–4):79–93
Almeida D, Silva R, Luna J, Rufino R, Santos V, Sarubbo L (2017) Response surface methodology for optimizing the production of bio surfactant by Candida tropicalis on industrial waste substrates. Front Micro. https://doi.org/10.3389/fmicb.2017.00157
Al-Omari A, Muhammetoglu A, Karadirek E, Jiries A, Batarseh M, Topkaya B, Soyupak S (2014) A review on formation and decay kinetics of trihalomethanes in water of different qualities. Clean-Soil Air Water 42(12):1687–1700
Amjad H, Hashmi I, Rehman MS, Awan MA, Ghaffar S, Khan Z (2013) Cancer and non-cancer risk assessment of trihalomethanes in urban drinking water supplies of Pakistan. Ecotox Environ Safe 91:25–31
APHA (2012) Standard methods for the examination of water and wastewater. 22nded. Washington, American Public Health Association. 1360. ISBN978-087553-013-0
Appovoo IA, Hu JO, Huang Y, Li SMY, Ong SL (2014) Response surface modeling of carbamazepine (CBZ) removal by graphene-P25 nano composites/UVA process using central composite design. Water Res 57:270–279
Asadzadeh F, Maleki-Kaklar M, Soiltanalinejad N (2018) Central composite design optimization of zinc removal from contaminated soil, using citric acid as biodegradable chelant. Sci Rep 8:2633. https://doi.org/10.1038/s41598-018-20942-9
Azizullah A, Khattak MNK, Richter P, Hader DP (2011) Water pollution in Pakistan and its impact on public health—a review. Environ Int 37:479–497
Behin J, Farhadian N (2016) Response surface methodology for ozonation of trifluralin using advanced oxidation processes in an airlift photoreactor. Appl Water Sci. https://doi.org/10.1007/s13201-016-0443-y
Blokker EJM, Furnass WR, Machell J, Mounce SR, Schaap PJ, Boxall JB (2016) Relating water quality and age in drinking water distribution systems using self-organising maps. Environments 3:10. https://doi.org/10.3390/environments3020010
Blumberg LM, Klee MS (2001) Elution parameters in constant-pressure, single-ramp temperature-programmed gas chromatography. J Chromatogr A 918(1):113–20
Bourdon JH, Linares FM (2014) Trihalomethanes in comerio drinking water and their reduction by nano-structured materials. Soft NanoscI Lett 4:31–41.https://doi.org/10.4236/snl.2014.42005
Brown D, Bridgeman J, West JR (2011) Predicting chlorine decay and THM formation in water supply systems. Rev Environ Sci Bio-Technol 10(1):79–99
Budziak D, Carasek E (2007) Determination of trihalomethanes in drinking water from three different water sources in Florianopolis-Brazil using purge and trap and gas chromatography. J Braz Chem Soc 18(4):741–747
Chang EE, Lin YP, Chiang PC (2001) Effect of bromide on formation of THMs and HAA. Chemosphere 43:1029–1034
Chang E, Guo HC Li IS, Chiang PC, Huang CP (2010) Modeling the formation and assessing the risk of disinfection by-products in water distribution systems. J Environ Sci Health A. Toxic and Hazardous Substance and Environ Engineering. 45(10):1185–94
Chen X, Huang G, An C, Feng R, Wu Y, Huang C (2022) Super wetting polyether sulfone membrane functionalized with ZrO2 nanoparticles for polycyclic aromatic hydrocarbon removal. J Mater Sci Techno l98:14–25
Choudhury S, Champagne P, McLellan PJ (2008) Factors influencing formation of trihalomethanes in drinking water: results from multivariate statistical investigation of the Ontario drinking water surveillance program database. Wat Qual Res J 43(2–3):189–199
Clayton GE, Thorn R, Reynolds DM (2019) Comparison of trihalomethane formation using chlorine-based disinfectants within a model system; applications within point-of-use drinking water treatment. Front Env Sci 7:35
Cook D, Drikas M (2011) Impact of water quality and treatment on disinfection by-product formation. 74th Annual water industry engineers and operators’ conference bendigo exhibition centre 6 to 8 September.
Czyrski A, Jarzębski H (2020) Response surface methodology as a useful tool for evaluation of the recovery of the fluoroquinolones from plasma—the study on applicability of box-behnken design, central composite design and doehlert design. Processes 8(4):473
Daud MK, Nafees M, Ali S, Rizwan M, Bajwa RA, Shakoor, MB, Chatha SAH (2017) Farah drinking water quality status and contamination in Pakistan. Hindawi Bio Med Res Intl. 7908183
Elfghi FM, Amin NAS (2013) Optimization of hydrodesulfurization activity in the hydro treating process: canonical analysis and the combined application of factorial design and response surface methodology. React Kinet Mech Catal 108(2):371–390
El-Shafy M, Abd N, Grue A (2000) THM formation in water supply in south bohemia. Czech Republic Water Res 34(13):3453–3459
Elsheikh MA, Basiouny M E (2011). Deposition and formation of THMs in water supply system. Sust Environ Res. 21(2)
Fakour H, Lo SL (2018) Formation of trihalomethanes as disinfection by products in herbal spa pools. Sci Rep 8:5709
Fattahi F, Shariati-Rad M (2020) A cotton pad-based sensor for the detection and determination of trihalomethanes in water by the colorimetric method. Food Anal Methods 12(13):1779–1785
Fooland M, Ramavandi B, Zandi K, Ardestani M (2011) Investigation of trihalomethanes formation potential in Karoon River water. Iran Environ Monit Assess 178:63–71
Hamed Y, Redhaounia B, Ben Saad A, Hadji R, Zahri F, El-Hidouri B (2017) Groundwater inrush caused by the fault reactivation and the climate impact in the mining Gafsa basin (south western Tunisia). J Tethys 5(2):154–164
Hannan A, Shan S, Arshad U (2010) Bacteriological analysis of drinking water from 100 families of Lahore by membrane filtration technique and chromagar. Biomedica 26:152–156
Hegazy AK, Abdel-Ghani NT, El-Chaghaby GA (2013) Adsorption of phenol onto activated carbon from Rhazyastricta: determination of the optimal experimental parameters using factorial design. Appl Water Sci 4:273–281
Hien VTD, Tin NT, Ngoc HT, Thanh BX, Tuc DQ, Dan NP (2015) Investigation of trihalomethanes contamination in surface water treatment plants and water supply network in an Giang-mekong delta province of Viet Nam. J Wat Sustain 5(3):85–94
Hua B, Mu R, Shi H, Inniss E, Yang J (2016) Water quality in selected small drinking water systems of Missouri rural communities. Beverages MDPI 2:10. https://doi.org/10.3390/beverages2020010
Ibrahim N, Abdul-Aziz H (2014) Trends on natural organic matter in drinking water sources and its treatment. Int J Sci Res Env 2(3):94–106
Karim Z, Kamal T, Mumtaz M (2011) Health risk assessment of trihalomethanes from tap water in Karachi. Pak J Chem Soc Pak 33(2):215–219
Karimi P, Abdollahi H, Aslan N, Noaparast M, Shafaei SZ (2011) Application of response recovery in cyanidation process. Min Proc Ext Met Rev 32:1–16
Kohli A, Singh H (2011) Optimization of processing parameters in induction hardening using response surface methodology. Sadhana 36(2):141–152
Le Bel GL, Benoit FM, Williams DT (1997) A one-year survey of halogenated disinfection by-products in the distribution system of treatment plants using three different disinfection process. Chemosphere 34(11):2301–2317
Lee J, Ha KW, Zoh KD (2010) Characteristics of trihalomethane (THM) production and associated health risk assessment in swimming pool waters treated with different disinfection methods. Sci Total Environ 407:1990–1997
Liu X, Wei X, Zheng W, Jiang S, Templeton MR, He G, Qu W (2013) An optimized analytical method for the simultaneous detection of iodoform, iodoacetic acid, and other trihalomethanes and haloacetic acids in drinking water. PloS One 8(4):e60858
Liu X, Wei X, Zheng W, Jiang S, Templeton MR, He G, Qu W (2013b) An optimized analytical method for the simultaneous detection of iodoform, iodoacetic acid and other trihalomethanes and haloacetic acids in drinking water. PLoS One 8(4):e60858. https://doi.org/10.1371/journal.pone.0060858
Liu J, Zhao W, Zhang Li SA, Zhang Y, Liu S (2015) Determination of volatile compounds in foxtail millet sake using headspace solid-phase microextraction and gas chromatography-mass spectrometry. J Chem. https://doi.org/10.1155/2015/239016
Mohan N, Kannan GK, Upendra S, Kumar NS (2012) Studies of benzene adsorption using response surface methodology. Chem Eng 19:257–265
Nabeela F, Azizullah A, Bibi R, Uzma S, Murad W, Shakir SK, Ullah W, Qasim M, Haider DP (2014) Microbial contamination of drinking water in Pakistan—a review. Environ Sci Pollut Res 21:13929–13942. https://doi.org/10.1007/s11356-014-3348-z
Navalon S, Alvaro MH, Garcia H (2008) Carbohydrates as trihalomethanes precursors. Influence of pH and the presence of Cl (−) and Br(−) on trihalomethane formation potential. Water Res 42:3990–4000
Niri VH, Bragg L, Pawliszyn J (2008) Fast analysis of volatile organic compounds and disinfection by-products in drinking water using solid-phase microextraction–gas chromatography/time-of-flight mass spectrometry. J Chromatogr A 1201(2):222–227
Pagano T, Bida M, Kenny JE (2014) Trends in levels of allochthonous dissolved organic carbon in natural water: a review of potential mechanisms under a changing climate. Water 6(10):2862–2897. https://doi.org/10.3390/w6102862
Parkinson DR, Barter D, Gaultois R (2016) Comparison of spot and time weighted averaging (TWA) Sampling with SPME-GC/MS Methods for Trihalomethane (THM) Analysis. Separations 3(1):5
Poleneni SR, Inniss EC (2013) Small water distribution system operations and disinfection by product fate. J Wat Res and Prot 5(8A):35–41. https://doi.org/10.4236/jwarp.2013.58A005
Prest EI, Frederik FH, Loosdrecht MC, Van M, Johannes S, Vrouwenvelder JS (2016) Biological stability of drinking water: controlling factors, methods and challenges. Front Microbiol. https://doi.org/10.3389/fmicb.2016.00045
Qaiser S, Hashmi I, Nasir H (2014) Chlorination at treatment plant and drinking water quality: a case study of different sectors of Islamabad. Pakistan Arab J Sci Eng 39(7):5665–5675
Rakic T, Kasagic-Vujanovic I, Jovanovic M, Jancic-Stojanovic B, Ivanovic D (2014) Comparison of full factorial design, central composite design, and box-behnken design in chromatographic method development for the determination of fluconazole and its impurities. Anal Lett 47(8):1334–1347
Ramavandi B, Farjadfard S, Ardjmand M, Dobaradaran S (2015) Effect of water quality and operational parameters on trihalomethanes formation potential in Dez River water. Iran Wat Res Industry 11:1–12
Ramyadevi D, Subathira A, Saravanan S (2012) Use of response surface methodology to evaluate the extraction of protein from shrimp waste by aqueous two-phase system (polyethylene glycol and ammonium citrate). Int J Environ Sci Dev 6(4):1012–1018
Rasheed S, Campos LC, Kim JK, Zhou Q, Hashmi I (2016) Optimization of total trihalomethanes’ (TTHMs) and their precursors’ removal by granulated activated carbon (GAC) and sand dual media by response surface methodology (RSM). Water Sci Tech-W Sup 6(3):783–793
Raza M, Hussain F, Lee JY, Shakoor MB, Kwona KD (2017) Groundwater status in Pakistan: a review of contamination, health risks, and potential needs. Crit Rev Env Sci Tec 47(18):1713–1762
Rezaee R, Maleki A, Jafari A, Mazloomi S, Zandsalimi Y, Mahvi AH (2014) Application of response surface methodology for optimization of natural organic matter degradation by UV/H2O2 advanced oxidation process. J Environ Health Sci 12:67–78
Sadiq R, Rodriguez MJ (2004) Disinfection by-products (DBPs) in drinking water and predictive models for their occurrence: a review. Sci Total Environ 321:21–46
Santos dos, Silveirados, MS Eduardo C (2013) Development of a simple analytical method for determining trihalomethanes in beer using a headspace solid-phase micro-extraction technique. Quím Nova 36 (7) Sao Paulo
Selvam R, Muniraj S, Duraisamy T, Muthunarayanan V (2018) Identification of disinfection by-products (DBPs) halo phenols in drinking water. Appl. Water Sci 8:13
Shafi J, Sun Z, Ji M, Gu Z, Ahmad W (2018) ANN and RSM based modelling for optimization of cell dry mass of Bacillus sp. strain B67 and its antifungal activity against Botrytis cinerea. Biotechnol Biotechnol Equip 32(1): 58–68
Shi H, Adams C (2012) Occurrence and formation of trihalomethanes in marine aquaria studied using solid-phase microextraction gas chromatography-mass spectrometry. Water Environ Res 84(3):202–208
Sillanpaa M, Ncibi MC, Matilainen A, Vepsalainen M (2018) Removal of natural organic matter in drinking water treatment by coagulation: a comprehensive review. Chemosphere 190:54–71. https://doi.org/10.1016/j.chemosphere.2017.09.113
Singh UP, Rai P, Pandey P, Sinha S (2012) Modeling and optimization of trihalomethanes formation potential of surface water (a drinking water source) using Box-Behnken design. Environ Sci Pollut Res 19(1):113–127
Sun YX, Qian-Yuan W, Hong-Ying H, Jie T (2009) Effects of operating conditions on THMs and HAAs formation during wastewater chlorination. J Hazard Mater 1681290–5: https://doi.org/10.1016/j.jhazmat.2009.03.013
Teglia CM, De MM, Zan MM, Camara MS (2015) Multiple responses optimization in the development of a headspace gas chromatography method for the determination of residual solvents in pharmaceuticals. J Pharm Anal 5(5):296–306. https://doi.org/10.1016/j.jpha.2015.02.004
Toroz I, Yuak V (2005) Seasonal variations of trihalomethanes (THMs) in water distribution networks of Istanbul City. Desalination 176(1–3):127–141
Trinh TK, Kang LS (2010) Application of response surface method as an experimental design to optimize coagulation tests. Environ Eng Res 15(2):63–70
Tuncel SG, Topal T (2011) Multifactorial optimization approach for determination of polycyclic aromatic hydrocarbons in sea sediments of Turkish Mediterranean Coast. Am J Analyt Chem (AJAC) 2:783–794
Uppeegadoo A, Yive NCK, Gopaul K (1999) Determination of trihalomethanes in drinking water in southern Mauritius. Univ Maurit Res J 3:109–114
USEPA (2006) Decision documents for atrazine. Environmental Protection Agency, U.S
Valdivia-Garcia M, Weir P, Frogbrook Z, Graham DW, Werner D (2016) Climatic, geographic and operational determinants of trihalomethanes (THMs) in drinking water systems. Sci Rep. https://doi.org/10.1038/srep35027
Valencia S, Marín J, Restrepo G (2013) Method for trihalomethane analysis in drinking water by solid-phase microextraction with gas chromatography and mass spectrometry detection. Water Sci Technol Water Supply 13(2):499–506
Valente IM, Gonçalves LM, Rodrigues JA (2013) Another glimpse over the salting-out assisted liquid–liquid extraction in acetonitrile/water mixtures. J Chromatogr A 1308:58–62
W.H.O (2011) Guidelines for drinking—water quality, 4th edn. World Health Organization, Geneva, pp 1–541
Wang Q, Gao B, Wang Y, Yang Z, Xu W, Yue Q (2011) Effect of pH on humic acid removal performance in coagulation–ultrafiltration process and the subsequent effects on chlorine decay. Sep Purif 80:549–555
Younis SA, El-Azab WI, El-GendyNS Aziz SQ, Moustafa YM, Abdul Aziz H, Abu Amr SS (2014) Application of response surface methodology to enhance phenol removal from refinery wastewater by microwave process. Int J Microw Sci Technol. https://doi.org/10.1155/2014/639457
Yuan Y, Wang Y, Yang M, Xu Y, Chen W, Zou X, Zheng B (2018) Application of response surface methodology to vortex-assisted dispersive liquid–liquid extraction for the determination of nicotine and cotinine in urine by gas chromatography–tandem mass spectrometry. J Sep Sci 41(10):2261–2268
Acknowledgements
The authors are thankful to the International Research Support Initiative Program under Higher Education Commission-Pakistan (IRSIP-HEC-PAK), NUST R&D, for financial assistance; and CEGE-UCL, UK, for technical assistance.
Author information
Authors and Affiliations
Contributions
SR—developed the research, prepared the manuscript; Dr. IH—researcher supervisor from Pakistan, reviewed and edited the manuscript; Dr. QZ—supported laboratorial analysis at UCL, reviewed the manuscript; Dr. JKK—provided the training on Central composite rotatable design (CCRD) and reviewed the manuscript; Dr. LCC—researcher co-supervisor from UCL, reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no objections in the submission of this article and have no conflict of interest involved in publication of this data.
Additional information
Editorial responsibility: Samareh Mirkia.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rasheed, S., Hashmi, I., Zhou, Q. et al. Central composite rotatable design for optimization of trihalomethane extraction and detection through gas chromatography: a case study. Int. J. Environ. Sci. Technol. 20, 1185–1198 (2023). https://doi.org/10.1007/s13762-022-04070-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-022-04070-6