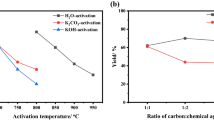
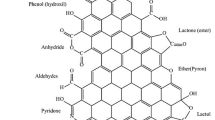
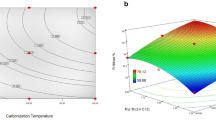
Abstract
Highly porous adsorbent materials were developed from industrial waste lignin for denitrification of surface and groundwater. The effect of microwave treatment and zinc ion impregnation on denitrification capacities of the material was investigated. The denitrification efficiencies of prepared materials were investigated by performing batch and continuous column adsorption experiments. The effect of different experimental parameters, including initial nitrate ion concentration, solution pH, and contact time, was also investigated. Adsorption kinetic follows the pseudo-second-order kinetic mechanism. Adsorption isotherm follows the monomolecular adsorption and experimental data best fitted into the Langmuir isotherm model. Continuous column denitrification experiments were performed with granulated adsorbent at different experimental conditions to understand its applicability in the household water treatment unit. Thomas model breakthrough time and the nitrate removal efficiency increase with increasing bed depth, whereas it declines with flow rate and nitrate concentration. The economic viability and environmental friendliness were understood by conducting adsorption–desorption experiments with loaded materials. The performance of adsorbent did not alter significantly even after four consecutive adsorption–desorption cycles.
Graphic abstract











Similar content being viewed by others
Abbreviations
- Qe:
-
Equilibrium adsorption (mg/g)
- Ci:
-
Initial concentration of nitrate (mg/l)
- Ce:
-
Equilibrium concentration of nitrate (mg/l)
- V:
-
Sample volume (l)
- M:
-
Adsorbent weight (g)
- qt:
-
Amount of nitrate adsorbed per mass unit of adsorbent at time t.
- qe:
-
Quantity of nitrate adsorbed per adsorbed mass unit at equilibrium (mg/gm)
- k1:
-
Rate constant for pseudo-first-order
- k2:
-
Rate constant pseudo-second-order kinetic model
- N:
-
Number of data points
- qeexp :
-
Nitrate adsorbed per mass unit of adsorbent at the equilibrium from laboratory experiments
- qecal :
-
Nitrate adsorbed per mass unit of adsorbent at the equilibrium from applied model
- K:
-
Langmuir constant related to free energy
- Kf:
-
Freundlich constant related to intensity of adsorption
- Xm:
-
Maximum adsorption capacity (mg/g)
- 1/n:
-
Dimensionless heterogeneity factor connected to nature of adsorbent
- CO:
-
Nitrate concentration of feed water (mg/l)
- Q:
-
Adsorption capacity of MLAC (mg/g)
- x/m:
-
Mass of adsorbate per unit mass of adsorbent in column bed (g)
- tB:
-
Time for breakthrough
- CB:
-
Amount of nitrate adsorbed just before breakthrough point (mg/l)
- Qo:
-
Adsorption capacity (mg/g)
- Kth:
-
Thomas model rate constant (ml/mg min)
- V:
-
Effluent volume (ml)
- m:
-
Adsorbent mass in grams used in column
- f:
-
Flow rate (ml/min)
- Ct:
-
Effluent nitrate concentration at any time
- C0/Ct:
-
Ratio of outlet and influent nitrate concentration
References
Bedmohata MA, Choudhari AR, Singh AP, Choudhari MD (2015) Adsorption capacity of activated carbon prepared by chemical activation of lignin for the removal of methylene blue dye. Int J Adv Res Chem Sci 2(8):1–13
Bekele W, Faye G, Fernandez N (2014) Removal of nitrate ion from aqueous solution by modified Ethiopian bentonite clay. Int J res pharm chem 4(1):192–201
Bensalah N, Nicola R, Abdel-Wahab A (2014) Nitrate removal from water using UVM/S2O42− advanced reduction process. Int J Environ Sci Technol 11:1733–1742
Bhatnagar A, Ji M, Choi YN et al (2008) Removal of Nitrate from water by adsorption onto zinc chloride treated activated carbon. Sep sci technol 43(4):886–907
Biswas S, Mishra U (2015) Continuous fixed-bed column study and adsorption modeling: removal of lead ion from aqueous solution by charcoal originated from chemical carbonization of rubber wood sawdust. J Chem. https://doi.org/10.1155/2015/907379
Cheenmatchaya A, Kungwankunakorn S (2014) Preparation of activated carbon derived from rice husk by simple carbonization and chemical activation for using as gasoline adsorbent. Int J Environ sci Develop 5(2):171–175. https://doi.org/10.7763/IJESD.2014.V5.472
Chintala R, Mollinedo J, Schumacher TE et al (2013) Nitrate sorption and desorption in biochars from fast pyrolysis. Microporous Mesoporous Mater 179:250–257
Francisco R, Maria J, Fernandez T (2019) Effective catalytic removal of nitrates from drinking water: an unresolved problem. J cleaner prod 270:398–408
Ghanbari F, Moradi M, Mohseni-Bandpei A et al (2014) Simultaneous application of iron and aluminum anodes for nitrate removal: a comprehensive parametric study. Int J Environ Sci Technol 11:1653–1660
Hekmatzadeh AA, Karimi- Jashni A, Talebbeydokhti N, Kløve B (2013) Adsorption kinetics of nitrate ions on ion exchange resin. Desalination 326:125–134
Indian standard for drinking water (IS: 10500:2012)
Jahangiri-rad M, Jamshidi A, Mohammad R, Nabizadeh R (2014) Adsorption performance of packed bed column for nitrate removal using PAN-oxime-nano Fe2O3. J Environ Health Sci Eng 12:90–94. https://doi.org/10.1186/2052-336X-12-90
Khandare H (2013) Scenario of nitrate contamination in groundwater: its causes and prevention. Int J ChemTech Res 5(4):1921–1926
Lacasa E, Canizares P, Saez C et al (2011) Removal of nitrates from groundwater by electrocoagulation. Chem Eng J 17(3):1012–1017
Langmuir I (1916) The constitution and fundamental properties of solids and liquids Part I. Solids J Am Chem Soc 38:2221–2295
Loganathan P, Vigneswaran S, Kandasamy J (2013) Enhanced removal of nitrate from water using surface modification of adsorbents: a review. J Environ Manage 131:363–374
Malakootian M, Yousefi N, Fatehizadeh A (2011) Survey efficiency of electrocoagulation on nitrate removal from aqueous solution. Int J Environ Sci Technol 8:107–114
Maldhure AV, Ekhe JD (2011a) Preparation and characterizations of microwave assisted activated carbons from industrial waste lignin for Cu (II) sorption. Chem Eng J 168:1103–1111
Maldhure AV, Ekhe JD (2011b) Microwave treated activated carbon from industrial waste lignin for endosulfan adsorption. J chem technol biotechnol 86(8):1074–1080
Mishra PC, Patel RK (2009) Use of agricultural waste for the removal of nitrate nitrogen from aqueous medium. J Environ Manage 90:519–522
Munoth P, Tiwari K, Goyal, R (2015) Fluoride and nitrate groundwater contamination in Rajasthan, India: a review. Hydro–2015 international, 20th International conference on hydraulics, water resources and river engineering. IIT Roorkee, India.
Namashivayam C, Sangeeta D (2005) Removal and recovery of nitrate from water by Zncl2 treated coconut core pitch as an agricultural waste. Indian J Chem Technol 12:513–521
Nur T, Shim WG, Loganathan P et al (2015) Nitrate removal using purolite A520E ion exchange resin: batch and fixed-bed column adsorption modelling. Int J Environ Sci Technol 12:1311–1320
Ouardi ME, Qourzal S, Alahiane S, Assabbane A, Douch J (2015) Effective removal of nitrates ions from aqueous solution using new clay as potential low-cost adsorbent. J Encapsul Adsorpt Sci 5:178–190
Öztürk N, Bektas TE (2004) Nitrate removal from aqueous solution by adsorption onto various materials. J Hazard Mater B 112:155–162
Pandian G, Ramakrishanan K, Subramanyan V (2013) Application of isotherm, kinetic and thermodynamic models for the adsorption of nitrate ions on grapheme from aqueous solution. J Taiwan Inst Chem Eng 44(5):808–814
Pirsaheb M, Khosravi T, Sharafi K, Mouradi M (2016) Comparing operational cost and performance evaluation of electrodialysis and reverse osmosis systems in nitrate removal from drinking water in Golshahr. Mashhad, Desalin water treat 57(12):5391–5397
Samatya S, Kabay N, Yuksel U, Arda M, Yuksel M (2006) Removal of nitrate from aqueous solution by nitrate selective ion exchange resins. React Funct Polym 66:1206–1214
Shakya AK, Ghosh PK (2019) Arsenic, iron and nitrate removal from groundwater by mixed bacterial culture and fate of arsenic-laden biosolids. Int J Environ Sci Technol 16:5901–5916
Shekarriz M, Ramezani Z, Elhami F (2017) Preparation and characterization of ZSM5-supported nano-zero-valent iron and its potential application in nitrate remediation from aqueous solution. Int J Environ Sci Technol 14:1081–1090
Suleiman A, Gupta AK, Basheer AB (2009) A fixed bed sorption system for defluoridation of ground water. J Urban Environ Eng 3(1):17–22
Sunitha V (2013) Nitrates in groundwater: health hazards and remedial measures. Indian J Adv chem sci 1(3):164–170
Wang XM, Wang JL (2013) Nitrate removal from groundwater using solid-phase denitrification process without inoculating with external microorganisms. Int J Environ Sci Technol 10:955–960
Xing X, Baoyu G, Xin T et al (2013) Nitrate adsorption by stratified wheat straw resin in lab-scale columns. Chem Eng J 226:1–6
Yagub MT, Sen TK, Afroz S, Ang HM (2015) Fixed-bed dynamic column adsorption study of methylene blue (MB) onto Pine cone. Desalin Water Treat 55:1026–1039. https://doi.org/10.1080/19443994.2014.924034
Yuan J, Amano Y, Machida M (2020) Study on the characteristics of nitrogen-doped activated carbon fibers to remove nitrate ions by multi-factor analysis. Int J Environ Sci Technol 17:2563–2570
Acknowledgement
The authors wish to thank all who assisted in conducting this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they no conflict of interest.
Additional information
Editorial responsibility: Hari Pant.
Rights and permissions
About this article
Cite this article
Maldhure, A., Waghela, A., Nagarnaik, P. et al. Microwave treated activated carbon from industrial waste lignin for denitrification of surface water. Int. J. Environ. Sci. Technol. 19, 4987–4996 (2022). https://doi.org/10.1007/s13762-021-03361-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-021-03361-8