Abstract
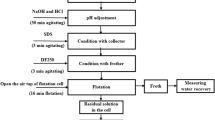
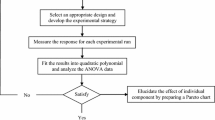
Discharge of heavy metals into the environment by wastewater from production and application processes is nowadays getting more and more serious due to the rapid development of such processes. Produced water during oil production and wastewater form metal plating and ore dressing are few examples to mention. In this research, removal of chromium ions from aqueous solutions has been studied by foam fractionation process using cetyl trimethyl ammonium bromide as surfactant. Effect of processing time, gas flow rate, initial surfactant concentration, and initial pH on removal efficiency has been investigated. Preliminary screening tests were performed, and initial surfactant concentration and processing time were recognized as the variables with a significant effect on removal efficiency. Response surface methodology based on central composite design as a design of experiment method was employed to design experiments to study the effect of important variables more precisely. Five levels for each significant variable were considered and design of experiment. An accurate correlative model is developed for the estimation of removal efficiency under different operating conditions in foam fractionation process with a maximum relative error of 1.674%. Four sets of validation tests were also conducted, and the results of validation tests were shown to be in accordance with those obtained on tests dictated by design of experiment. Optimum operating conditions were found to be with an initial surfactant concentration of 175 ppm and 45 min of processing time that yielded 97.74% removal of Cr(VI) from a solution of 10 ppm initial concentration.





Similar content being viewed by others
References
Amooey AA, Ghasemi S, Mirsoleimani-azizi SM, Gholaminezhad Z, Chaichi MJ (2014) Removal of Diazinon from aqueous solution by electrocoagulation process using aluminum electrodes. Korean J Chem Eng 31:1016–1020
Arulmozhi M, Begum KMS, Anantharaman N (2007) Foam separation of copper(II) from aqueous solutions. J Environ Res Dev 2:217–225
Brito F, Ascanio J, Mateo S, Hernández C, Araujo L, Gili P, Martín-Zarza P, Domínguez S, Mederos A (1997) Equilibria of chromate(VI) species in acid medium and ab initio studies of these species. Polyhedron 16:3835–3846
Cheballah K, Sahmoune A, Messaoudi K, Drouiche N, Lounici H (2015) Simultaneous removal of hexavalent chromium and COD from industrial wastewater by bipolar electrocoagulation. Chem Eng Process 96:94–99
Chen H-R, Chen C-C, Reddy AS, Chen C-Y, Li WR, Tseng M-J, Liu H-T, Pan W, Maity JP, Atla SB (2011) Removal of mercury by foam fractionation using surfactin, a biosurfactant. Int J Mol Sci 12:8245–8258
Cheng Q, Wang C, Doudrick K, Chan CK (2015) Hexavalent chromium removal using metal oxide photocatalysts. Appl Catal B 176:740–748
Czitrom V (1999) One-factor-at-a-time versus designed experiments. Am Stat 53:126–131
Dekhil A, Hannachi Y, Ghorbel A, Boubaker T (2011) Removal of lead and cadmium ions from aqueous solutions using dried marine green macroalga (Caulerpa racemosa). Int J Environ Res 5:725–732
Gerken BM, Nicolai A, Linke D, Zorn H, Berger RG, Parlar H (2006) Effective enrichment and recovery of laccase C using continuous foam fractionation. Sep Purif Technol 49:291–294
Ghosh R, Sahu A, Pushpavanam S (2019) Removal of trace hexavalent chromium from aqueous solutions by ion foam fractionation. J Hazard Mater 367:589–598
Heidmann I, Calmano W (2008) Removal of Cr(VI) from model wastewaters by electrocoagulation with Fe electrodes. Sep Purif Technol 61:15–21
Jiang C, Wu Z, Li R, Liu Q (2011) Technology of protein separation from whey wastewater by two-stage foam separation. Biochem Eng J 55:43–48
Kinoshita T, Ishigaki Y, Shibata N, Yamaguchi K, Akita S, Kitagawa S, Kondou H, Nii S (2011) Selective recovery of gallium with continuous counter-current foam separation and its application to leaching solution of zinc refinery residues. Sep Purif Technol 78:181–188
Kongsricharoern N, Polprasert C (1996) Chromium removal by a bipolar electro-chemical precipitation process. Water Sci Technol 34:109–116
Lazarova V, Levine B, Sack J, Cirelli G, Jeffrey P, Muntau H, Salgot M, Brissaud F (2001) Role of water reuse for enhancing integrated water management in Europe and Mediterranean countries. Water Sci Technol 43:25–33
Liu Y, Wu Z, Zhao B, Li L, Li R (2013) Enhancing defoaming using the foam breaker with perforated plates for promoting the application of foam fractionation. Sep Purif Technol 120:12–19
Lu K, Zhang X-L, Zhao Y-L, Wu Z-L (2010) Removal of color from textile dyeing wastewater by foam separation. J Hazard Mater 182:928–932
Lu J, Li Y, Zhang S, Sun Y (2015) Removal of trace Cd2+ from aqueous solution by foam fractionation. J Hazard Mater 286:466–473
Mahmoud MR, Lazaridis NK (2015) Simultaneous removal of nickel (II) and chromium (VI) from aqueous solutions and simulated wastewaters by foam separation. Sep Sci Technol 50:1421–1432
Matsuoka K, Miura H, Karima S, Taketaka C, Ouno S, Moroi Y (2018) Removal of alkali metal ions from aqueous solution by foam separation method. J Mol Liq 263:89–95
Mella B, Glanert AC, Gutterres M (2015) Removal of chromium from tanning wastewater and its reuse. Process Saf Environ Prot 95:195–201
Merz J, Burghoff B, Zorn H, Schembecker G (2011) Continuous foam fractionation: performance as a function of operating variables. Sep Purif Technol 82:10–18
Miller JN, Miller JC (2005) Statistics and chemometrics for analytical chemistry. Pearson Education, London
Mirsoleimani-azizi SM, Amooey AA, Ghasemi S, Salkhordeh-panbechouleh S (2015) Modeling the removal of endosulfan from aqueous solution by electrocoagulation process using artificial neural network (ANN). Ind Eng Chem Res 54:9844–9849
Mirsoleimani-azizi SM, Setoodeh P, Zeinali S, Rahimpour MR (2018) Tetracycline antibiotic removal from aqueous solutions by MOF-5: adsorption isotherm, kinetic and thermodynamic studies. J Environ Chem Eng 6:6118–6130
Mishra S, Bharagava RN (2016) Toxic and genotoxic effects of hexavalent chromium in environment and its bioremediation strategies. J Environ Sci Health Part C 34:1–32
Montgomery DC (2017) Design and analysis of experiments. Wiley, Hoboken
Moussavi M, Javidnejad M (2007) Separation of Hg(II) by foam fractionation in the acidic range: effect of complexation. J Hazard Mater 144:187–193
Pagana A, Sklari S, Kikkinides E, Zaspalis V (2008) Microporous ceramic membrane technology for the removal of arsenic and chromium ions from contaminated water. Microporous Mesoporous Mater 110:150–156
Peykar S, Anvaripour B, Motavassel M, Jadidi N (2013) Mercury removal from wastewater by batch foam fractionation. Int Res J Appl Basic Sci 4:3198–3203
Qu Y-H, Zeng G-M, Huang J-H, Xu K, Fang Y-Y, Li X, Liu H-L (2008) Recovery of surfactant SDS and Cd2+ from permeate in MEUF using a continuous foam fractionator. J Hazard Mater 155:32–38
Qu Y, Zhang X, Xu J, Zhang W, Guo Y (2014) Removal of hexavalent chromium from wastewater using magnetotactic bacteria. Sep Purif Technol 136:10–17
Rengaraj S, Yeon K-H, Moon S-H (2001) Removal of chromium from water and wastewater by ion exchange resins. J Hazard Mater 87:273–287
Roosta M, Ghaedi M, Daneshfar A, Sahraei R, Asghari A (2014) Optimization of the ultrasonic assisted removal of methylene blue by gold nanoparticles loaded on activated carbon using experimental design methodology. Ultrason Sonochem 21:242–252
Sereshti H, Farahani MV, Baghdadi M (2016) Trace determination of chromium (VI) in environmental water samples using innovative thermally reduced graphene (TRG) modified SiO2 adsorbent for solid phase extraction and UV–vis spectrophotometry. Talanta 146:662–669
Shadmehr J, Mirsoleimani-azizi S, Zeinali S, Setoodeh P (2018) Electrocoagulation process for propiconazole elimination from wastewater: experimental design for correlative modeling and optimization. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-018-1891-8
Shah M, Pathak K (2010) Development and statistical optimization of solid lipid nanoparticles of simvastatin by using 23 full-factorial design. AAPS PharmSciTech 11:489–496
Stevenson P (2007) Hydrodynamic theory of rising foam. Miner Eng 20:282–289
Tadakamalla K, Marathe K (2011) Hydrodynamic study and optimization strategy for the surfactant recovery from aqueous solutions. Desalination 266:98–107
Venkateswaran P, Palanivelu K (2004) Solvent extraction of hexavalent chromium with tetrabutyl ammonium bromide from aqueous solution. Sep Purif Technol 40:279–284
Yan J, Wu Z, Zhao Y, Jiang C (2011) Separation of tea saponin by two-stage foam fractionation. Sep Purif Technol 80:300–305
Yang Q-W, Wu Z-L, Zhao Y-L, Wang Y, Li R (2011) Enhancing foam drainage using foam fractionation column with spiral internal for separation of sodium dodecyl sulfate. J Hazard Mater 192:1900–1904
Yuan X, Meng Y, Zeng G, Fang Y, Shi J (2008) Evaluation of tea-derived biosurfactant on removing heavy metal ions from dilute wastewater by ion flotation. Colloids Surf A 317:256–261
Acknowledgements
The authors wish to thank all who assisted in conducting this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: J Aravind.
Rights and permissions
About this article
Cite this article
Piri, S., Mehranbod, N., Moussavi, M. et al. Application of response surface method for removal of Cr(VI) from aqueous solutions using foam fractionation process. Int. J. Environ. Sci. Technol. 17, 321–332 (2020). https://doi.org/10.1007/s13762-019-02349-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-019-02349-9