Abstract
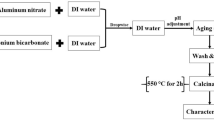
Nano-γ-Al2O3 adsorbent was synthesized by the novel sol–gel method. The adsorbent was characterized by transmission electron microscope, Fourier transform infrared and X-ray powder diffraction. The effects of several variables such as, adsorbent weight, pH and contact time on adsorption of chromium (Cr6+), nickel (Ni2+), cadmium (Cd2+) and lead (Pb2+) ions were studied in batch experiments. The results showed that the synthesized nano-γ-Al2O3 had a good capacity to adsorb Cr and Pb. The kinetic data were described with pseudo-first- and pseudo-second-order models. Three isotherm models, namely Freundlich, Langmuir and Tempkin, were used for analysis of equilibrium data, and results showed that Langmuir and Freundlich models were suitable for describing the equilibrium data of Cr6+, Cd2+, Ni2+ and Pb2+. Using Langmuir isotherm, the maximum sorption capacities of Cr6+, Pb2+, Cd2+ and Ni2+ were estimated to be 13.3, 6, 1.1 and 0.33 (mg/g) at 25 °C, respectively. The sorption capacity did not change remarkably after reuse of sorbent for sorption–desorption cycle. The selectivity order of Cr6+, Pb2+, Cd2+ and Ni2+ sorption onto the adsorbent was Cr6+ > Pb2+ > Cd2+ > Ni2+.








Similar content being viewed by others
References
Ahmedna M, Marshall WE, Husseiny AA, Rao RM, Goktepe I (2004) The use of nutshell carbons in drinking water filters for removal of trace metal. Water Res 38(4):1062–1068
Ahn CK, Park D, Woo SH, Park JM (2009) Removal of cationic heavy metal from aqueous solution by activated carbon impregnated with anionic surfactants. J Hazard Mater 164:1130–1136
Ai ZH, Chen Y, Zhang LZ, Qiu JR (2008) Efficient removal of Cr(VI) from aqueous solution with Fe@Fe2O3 core–shell nanowires. J Environ Sci Technol 42:6955–6960
Aksu Z (2002) Determination of the equilibrium, kinetic and thermodynamic parameters of the batch biosorption of nickel(II) ions onto Chlorella vulgaris. Prog Biochem. 38(1):89–99
Asencios YJO, S-Kou MR (2012) Synthesis of high surface γ- Al2O3 from aluminum scrap and its use for the adsorption of metals: Pb(II), Cd(II) and Zn(II). Appl Surf Sci 258:10002–10011
Aydin FA, Soylak M (2010) Separation, pre concentration and inductively coupled plasma-mass spectrometric (ICP-MS) determination of thorium (IV), titanium (IV), iron (III), lead (II) and chromium (III) on 2-nitroso-1-naphthol Impregnated MCI GEL CHP20P resin. J Hazard Mater 173:669–674
Aziz HA, Adlan MN, Ariffin KS (2008) Heavy metals (Cd, Pb, Zn, Ni, Cu and Cr(III)) removal from water in Malaysia: post treatment by high quality limestone. Bioresour Technol 99:1578–1583
Baybaş D, Ulusoy U (2011) The use of polyacrylamide-aluminosilicate composites for thorium adsorption. J Appl Clay Sci 5:138–146
Chandramouli V, Anthonysamy S, Vasudeva Rao PR, Divakar R (1996) PVA aided microwave synthesis: a novel route for the production of nanocrystalline thoria powder. J Nucl Mater 231:213–220
Debnah S, Ghosh UC (2009) Nano structured hydrous titanium (IV) oxide: synthesis, characterization and Ni (II) adsorption behavior. J Chem Eng 152:480–491
El-Kamash AM (2008) Evaluation of zeolite A for the sorptive removal of Cs+ and Sr2+ ions from aqueous solutions using batch and fixed bed column operations. J Hazard Mater 151:432–445
Frost RL, Scholz R, Lopes A, Xi Y, Gobac ZI (2013) LFC. Horta, Raman and infrared spectroscopic characterization of the phosphate mineral paravauxite Fe2+Al2(PO4)2(OH)2_8H2O. Spectrochim Acta A 116:491–496
Goncharuk VV, Kornilovich BY, Pavlenko VM, Babak MI, Pshinko GN, Pysmennyi BV, Kovalchuk IA, Safronova VG (2001) Uranium compounds purification from water and wastewater. J Water Chem Technol 23:44–50
Gurgel LVA, Melo JCPD, De Lena JC, Gil LF (2009) Adsorption of chromium (VI) ion from aqueous solution by succinylated mercerized cellulose functionalized with quaternary ammonium groups. J Bioresour Technol 100(13):3214–3220
Hikmet S, Turan U (2014) Removal of heavy metal ions from aqueous medium using Kuluncak(Malatya) vermiculites and effect of precipitation on removal. J Appl Clay Sci 95:1–8
Ibrahim DM, Abu-Ayana YM (2009) Preparation of nano alumina via resin synthesis. Mater Chem Phys 113(2–3):579–586
Kaltchev M, Tysoe WT (1999) An infrared spectroscopic investigation of thin alumina films: measurement of acid sites and surface reactivity. J Surf Sci 430:29–36
Kornilovich BY, Kovalchuk IA, Pshinko GN, Tsapyuk EA, Krivoruchko AP (2000) Water purification of uranium by the method of ultrafiltration. J Water Chem Technol 22:43–47
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. Part I. Solids. J Am Chem Soc 38(11):2221–2295
Ma MG, Zhu JF (2009) A facile solvo thermal route to synthesis of γ-alumina with bundle-like and flower-like morphologies. Mater Lett 63:881–883
Mahapatra A, Mishra BG, Hota G (2013) Electrospun Fe2O3–Al2O3 nanocomposite fibers as efficient adsorbent for removal of heavy metal ions from aqueous solution. J Hazard Mater 258–259:116–123
Mohamed Mahmoud E, Mohamed Abdelwahab S, Fathallah ME (2013) Design of novel nano-sorbents based on nano-magnetic iron oxide–bound-nano-silicon oxide–immobilized-triethylenetetramine for implementation in water treatment of heavy metals. Chem Eng J 223:318–327
Mallakpoura Sh, Baratia A (2011) Efficient preparation of hybrid nanocomposite coatings based on poly (vinyl alcohol) and silane coupling agent modified TiO2 nanoparticles. Prog Org Coat 71:391–398
Neghlani PK, Rafizadeh M, Taromi FA (2011) Preparation of aminated-polyacrilonitril nano fiber membranes for the adsorption of metal ions: comparison with microfiber. J Hazard Mater 186:182–189
Nero MD, Galindo C, Barillon RI, Halter E, Made B (2010) Surface reactivity of α-Al2O3 and mechanisms of phosphate sorption: in situ ATR–FTIR spectroscopy and potential studies. J Colloid Interface Sci 342:437–444
Ngomsik AF, Bee A, Draye M, Cote G, Cabuil V (2005) Magnetic nano- and microparticles for metal removal and environmental applications. J Comput Ren Chem 8(6–7):963–970
Nilchi A, Saberi R, Azizpour H, Moradi M, Zarghami R, Naushad M (2012) Adsorption of caesium from aqueous solution using cerium molybdate–pan composite. Chem Ecol 28(2):169–185
Okada AK, Otsuka N, Somiya S (1991) Review of mullite synthesis routes in Japan. Am Ceram Soc Bull 70:1633–1640
Padmaja P, Anilkumar GM, Mukindan P, Aruldhas G, Warrier KGK (2001) Characterization of stoichiometric sol–gel mullite by Fourier transform infrared spectroscopy. Int J Inorg Mater 3:693–698
Parida KM, Pradhan AC, Das J, Sahu N (2009) Synthesis and characterization of nanosized porous gamma-alumina by control precipitation method. J Mater Chem Phys 113:244–248
Prakash A, McCormick AV, Zachariah MR (2004) Aero-sol-gel synthesis of nano porous iron-oxide particles: a potential oxidizer for nano energetic materials. J Chem Mater 16:1466–1471
Rahmani A, Mousavi HZ, Fazli M (2010) Effect of nanostructure alumina on adsorption of heavy metals. J Desalin 253:94–100
Resterna AB, Cierpiszewski R, Prochaska K (2010) Kinetic and equilibrium studies of the removal of cadmium ions from acidic chloride solutions by hydrophobic pyridinecarboxamide extractants. J Hazard Mater 179:828–833
Rodriguez M, Sifontes AB, Mendez FJ, Diaz Y, Izalesb EC, Brito JL (2013) Template synthesis and characterization of mesoporous γ-Al2O3 hollow nano rods using Stevia rebaudiana leaf aqueous extract. J Ceram Int 39:4499–4506
Sen TK, Sarzali MV (2008) Removal of cadmium metal ion (Cd2+) from its aqueous solution by aluminium oxide (Al2O3): a kinetic and equilibrium study. Chem Eng J 142:256–262
Sharma P, Tamar R (2008) Synthesis and application of an analogue of desolate for the removal of Uranium (VI), Thorium(IV), and europium(III) from aqueous waste. Micropor Mesopor Mater 116:641–652
Shiri-Yekta Z, Yaftian MR, Nilchi A (2013) Silica nanoparticles modified with a Schiff base ligand: an efficient adsorbent for Th(IV), U(VI) and Eu(III) ions. Korean J Chem Eng 30(8):1644–1651
Shoushtari AM, Zargaran M, Abdouss M (2006) Preparation and characterization of high efficiency ionexchange cross linked acrylic fibers. J Appl Polym Sci 101:2202–2209
Shu-Huei H, Jao-Jia H (2007) Adsorption behavior of heavy metal ions by carbon nanotube qrown on microsized Al2O3 particles. J Univ Sci Technol B 14(1):77–84
Soylak M, Erdogan ND (2006) Copper (II)–rubeanic acid co precipitation system for separation–preconcentration of trace metal ions in environmental samples for their flame atomic absorption spectrometric determinations. J Hazard Mater 137:1035–1041
Sung Lee J, Soo Kim H, Su Lee J, Park NK, Lee TJ, Kang M (2012) Synthesis of a-Al2O3 at mild temperatures by controlling aluminum precursor, pH, and ethylene diamine chelating additive. Ceram Int 38:6685–6691
Uluozlu OD, Tuzen M, Mendil D, Soylak M (2010) Co-precipitation of trace elements with Ni2+/2-nitroso-1-naphthol-4-sulfonic acid and their determination by flame atomic absorption spectrometry. J Hazard Mater 176:032–1037
Veriansyah B, Susanti RF, Nugroho A, Min BK, Kim J (2011) Continuous synthesis of high-surface-area aluminum hydroxide methoxide nano- and microparticles in supercritical methanol and their conversion into γ-Al2O3. Mater Lett 65:772–774
Wijnja H, Schulthess CP (1999) ATR–FTIR and DRIFT spectroscopy of carbonate species at the aged γ-Al2O3: water interface. Spectrochim Acta A 55:861–872
Wu Sh, Li F, Wang H, Fu L, Zhang B, Li G (2010a) Effects of poly (vinyl alcohol) (PVA) content on preparation of novel thiol-functionalized mesoporous PVA/SiO2 composite nanofiber membranes and their application for adsorption of heavy metal ions from aqueous solution. Polymer 51:6203–6211
Wu Sh, Li F, Xu R, Wei Sh, Wang H (2010b) Preparation of poly (vinyl alcohol)/silica composite nanofibers membrane functionalized with mercapto groups by electrospinning. Mater Lett 64:1295–1298
Xiadong X, Qin W, Jian Y, Liangguo Y, Rui F, Guodong C, Bin D, He L (2012) Highly efficient removal of heavy metal ions by amine-functionalized mesoporousFe3O4 nanoparticles. Chem Eng J 184:132–140
Yoo YS, Park KY, Jung KY, Cho SB (2009) Preparation of α-alumina nanoparticles via vapor-phase hydrolysis of AlCl3. Mater Lett 63:1844–1846
Zhang J, Shi F, Lin J, Wei SY, Chen D, Gao JM, Huang Z, Ding XX, Tang C (2008) Nanoparticles assembly of boehmite nanofibers without surfactant. Mater Res Bull 43:1709–1715
Zhou SX, Antonietti M, Niederberger M (2007) Low-temperature synthesis of γ-alumina nanocrystals from aluminum acetylacetonate in non aqueous media. Small 3(5):763–767
Zhou LM, Wang YP, Liu ZR, Huang QW (2009) Characteristics of equilibrium, kinetics studies for adsorption of Hg(II), Cu(II), and Ni(II) ions by thiourea-modified magnetic chitosan microspheres. J Hazard Mater 161:995–1002
Acknowledgments
The authors wish to extend their sincere gratitude to all who supported this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shokati Poursani, A., Nilchi, A., Hassani, A.H. et al. A novel method for synthesis of nano-γ-Al2O3: study of adsorption behavior of chromium, nickel, cadmium and lead ions. Int. J. Environ. Sci. Technol. 12, 2003–2014 (2015). https://doi.org/10.1007/s13762-014-0740-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-014-0740-7