Abstract
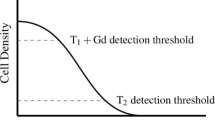
Glioblastoma is known to be among one of the deadliest brain tumors in the world today. There have been major improvements in the detection of cancerous cells in the twenty-first century. However, the threshold of detection of these cancerous cells varies in different scanning techniques such as magnetic resonance imaging (MRI) and computed tomography (CT). The growth of these tumors and different treatments have been modeled to assist medical experts in better predictions of the related tumor growth and in the selection of more accurate treatments. In clinical terms the tumor consisted of two parts known as the visible part, which is the part of the tumor that is above the threshold of the detecting device and the invisible part, which is below the detecting threshold. In this study, the common reaction–diffusion model of tumor growth is used to simulate the growth of the glioblastoma tumor. Also resection and radiotherapy have been modeled as methods to prevent the growth of the tumor. The results demonstrate that although the selected treatments were effective in reducing the number of cancerous cells to under the threshold of detection, they did not eliminate all cancerous cells and if no further treatments were applied, the cancerous cells would spread and become malignant again. Although previous studies have suggested that the ratio of proliferation to diffusion could describe the malignancy of the tumor, this study in addition shows the importance of each of the coefficients regarding the malignancy of the tumor.









Similar content being viewed by others
References
Swanson KR, Bridge C, Murray J, Alvord EC (2003) Virtual and real brain tumors: using mathematical modeling to quantify glioma growth and invasion. J Neurol Sci 216(1):1–10
Swanson KR, Alvord EC Jr, Murray J (2002) Quantifying efficacy of chemotherapy of brain tumors with homogeneous and heterogeneous drug delivery. Acta Biotheor 50(4):223–237
Gevertz JL, Torquato S (2006) Modeling the effects of vasculature evolution on early brain tumor growth. J Theor Biol 243(4):517–531
Harpold HL, Alvord EC, Swanson KR (2007) The evolution of mathematical modeling of glioma proliferation and invasion. J Neuropathol Exp Neurol 66(1):1–9
Eikenberry SE, Sankar T, Preul M, Kostelich E, Thalhauser C, Kuang Y (2009) Virtual glioblastoma: growth, migration and treatment in a three-dimensional mathematical model. Cell Prolif 42(4):511–528
Iwamoto F, Abrey L, Beal K, Gutin P, Rosenblum M, Reuter V, DeAngelis L, Lassman A (2009) Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology 73(15):1200–1206
Mahmoudi K, Hadjipanayis CG (2014) The application of magnetic nanoparticles for the treatment of brain tumors. Front Chem 2
Hogea C, Davatzikos C, Biros G (2008) An image-driven parameter estimation problem for a reaction–diffusion glioma growth model with mass effects. J Math Biol 56(6):793–825
Meghdadi N, Soltani M, Niroomand-Oscuii H, Ghalichi F (2016) Image based modeling of tumor growth. Australas Phys Eng Sci Med 39(3):601–613
Fung LK, Shin M, Tyler B, Brem H, Saltzman WM (1996) Chemotherapeutic drugs released from polymers: distribution of 1, 3-bis (2-chloroethyl)-l-nitrosourea in the rat brain. Pharmaceut Res 13(5):671–682
Swanson K, Harpold H, Peacock D, Rockne R, Pennington C, Kilbride L, Grant R, Wardlaw J, Alvord E (2008) Velocity of radial expansion of contrast-enhancing gliomas and the effectiveness of radiotherapy in individual patients: a proof of principle. Clin Oncol 20(4):301–308
Wang CH, Rockhill JK, Mrugala M, Peacock DL, Lai A, Jusenius K, Wardlaw JM, Cloughesy T, Spence AM, Rockne R (2009) Prognostic significance of growth kinetics in newly diagnosed glioblastomas revealed by combining serial imaging with a novel biomathematical model. Cancer Res 69(23):9133–9140
Murray JD (2001) Mathematical biology. II Spatial models and biomedical applications {Interdisciplinary Applied Mathematics V. 18}. Springer, New York Incorporated
Murray JD (2002) Mathematical biology I: an introduction, vol 17 of interdisciplinary applied mathematics. Springer, New York, NY
Swanson K, Rostomily R, Alvord E (2008) A mathematical modelling tool for predicting survival of individual patients following resection of glioblastoma: a proof of principle. Br J Cancer 98(1):113–119
Mandonnet E, Broët P, Swanson K, Carpentier A, Delattre J, Capelle L (2002) Linear growth of mean tumor diameter in low grade gliomas. Neurology 58(Suppl 3):A13
Rockne R, Rockhill J, Mrugala M, Spence A, Kalet I, Hendrickson K, Lai A, Cloughesy T, Alvord E Jr, Swanson K (2010) Predicting the efficacy of radiotherapy in individual glioblastoma patients in vivo: a mathematical modeling approach. Phys Med Biol 55(12):3271
Swanson KR, Rockne RC, Claridge J, Chaplain MA, Alvord EC, Anderson AR (2011) Quantifying the role of angiogenesis in malignant progression of gliomas: in silico modeling integrates imaging and histology. Cancer Res 71(24):7366–7375
Rockne R, Alvord E Jr, Rockhill J, Swanson K (2009) A mathematical model for brain tumor response to radiation therapy. J Math Biol 58(4–5):561–578
Hall EJ, Giaccia AJ (2006) Radiobiology for the radiologist. Lippincott Williams & Wilkins
Bashir R, Hochberg F, Oot R (1988) Regrowth patterns of glioblastoma multiforme related to planning of interstitial brachytherapy radiation fields. Neurosurgery 23(1):27–30
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All of the authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Moshtaghi-Kashanian, N., Niroomand-Oscuii, H. & Meghdadi, N. Simulating glioblastoma growth consisting both visible and invisible parts of the tumor using a diffusion–reaction model followed by resection and radiotherapy. Acta Neurol Belg 120, 629–637 (2020). https://doi.org/10.1007/s13760-018-0952-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-018-0952-6