Abstract
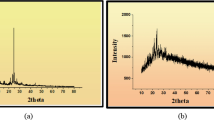
Mixed ligand metal complexes of Co(II), Fe(III), Cu(II), Zn(II), Cd(II) and Hg(II) metal ions with azo coumarin and thiosemicarbazone derivative have been synthesized. Synthesized ligands were supported by spectroscopic techniques such as IR, 1HNMR, 13CNMR and mass analysis while the structure and probable geometry of metal chelates were explored by FTIR, elemental analysis, molar conductance, mass spectrum, thermogravimetry (TG), and electronic spectra (UV). From thermal studies, activation energy and kinetic parameters (ΔG, ΔH, ΔS) of the complexes were also reported. Synthesized ligands and their metal complexes were studied for antimicrobial potential against various bacterial and fungal pathogens. Further, the antioxidant potential screening was done using 1,1-diphenyl-2-picrylhydrazyl. Synthesized metal complexes displayed modest to excellent antimicrobial potential. The topoisomerase II receptor protein was used for molecular docking of the extraordinary antimicrobial Co and Zn-complex.






Similar content being viewed by others
References
J. Sahoo, S.K. Paidesetty, J. Taibah Univ. Med. Sci. 12, 115 (2017). https://doi.org/10.1016/j.jtumed.2016.10.004
J. Sahoo, S. Kumar Mekap, P. Sudhir Kumar, J. Taibah, Univ. Sci. 9, 187 (2015). https://doi.org/10.1016/j.jtusci.2014.08.001
C. Ranjan Sahoo, J. Sahoo, M. Mahapatra, D. Lenka, P. Kumar Sahu, B. Dehury, R. Nath Padhy, S. Kumar Paidesetty, Arab. J. Chem. 14, 102922 (2021). https://doi.org/10.1016/j.arabjc.2020.102922
B. Lake, Coumarin Metabolism 37, 423 (1999). https://doi.org/10.1016/S0278-6915(99)00010-1
A. Rawat, A. Vijaya Bhaskar Reddy, Eur. J. Med. Chem. Reports. 5, 100038 (2022). https://doi.org/10.1016/j.ejmcr.2022.100038
I. Kostova, I. Manolov, M. Karaivanova, Arch. Pharm. (Weinheim). 334, 157 (2001). https://doi.org/10.1002/1521-4184(200105)334:5%3c157::AID-ARDP157%3e3.0.CO;2-S
H.-L. Qin, Z.-W. Zhang, L. Ravindar, K.P. Rakesh, Eur. J. Med. Chem. 207, 112832 (2020). https://doi.org/10.1016/j.ejmech.2020.112832
M. Gökalp, B. Dede, T. Tilki, Ç. Karabacak Atay, J. Mol. Struct. 1212, 128140 (2020). https://doi.org/10.1016/j.molstruc.2020.128140
C.T. Keerthi Kumar, J. Keshavayya, T.N. Rajesh, S.K. Peethambar, A.R. Shoukat Ali, Org. Chem. Int. 2013, 1 (2013). https://doi.org/10.1155/2013/370626
B.N. Ravi, J. Keshavayya, N.M. Mallikarjuna, V. Kumar, F.N. Zahara, Chem. Data Collect. 33, 100686 (2021). https://doi.org/10.1016/j.cdc.2021.100686
M. Pervaiz, S. Sadiq, A. Sadiq, U. Younas, A. Ashraf, Z. Saeed, M. Zuber, A. Adnan, Coord. Chem. Rev. 447, 214128 (2021). https://doi.org/10.1016/j.ccr.2021.214128
H. Kargar, V. Torabi, A. Akbari, R. Behjatmanesh-Ardakani, A. Sahraei, J. Mol. Struct. 1205, 127642 (2020). https://doi.org/10.1016/j.molstruc.2019.127642
M. Mesbah, T. Douadi, F. Sahli, S. Issaadi, S. Boukazoula, S. Chafaa, J. Mol. Struct. 1151, 41 (2018). https://doi.org/10.1016/j.molstruc.2017.08.098
E. Pahlavani, H. Kargar, N.S. Rad, J. Res. Med. Sci. (2015). https://doi.org/10.17795/zjrms1010
P. Sathyadevi, P. Krishnamoorthy, E. Jayanthi, R.R. Butorac, A.H. Cowley, N. Dharmaraj, Inorganica Chim. Acta. 384, 83 (2012). https://doi.org/10.1016/j.ica.2011.11.033
A.A. Ardakani, H. Kargar, N. Feizi, M.N. Tahir, J. Iran. Chem. Soc. 15, 1495 (2018). https://doi.org/10.1007/s13738-018-1347-6
H. Kargar, A. Adabi Ardakani, K.S. Munawar, M. Ashfaq, M.N. Tahir, J. Iran. Chem. Soc. 18, 2493 (2021). https://doi.org/10.1007/s13738-021-02207-x
P.P. Netalkar, S.P. Netalkar, V.K. Revankar, Polyhedron 100, 215 (2015). https://doi.org/10.1016/j.poly.2015.07.075
N.C. Kasuga, K. Sekino, M. Ishikawa, A. Honda, M. Yokoyama, S. Nakano, N. Shimada, C. Koumo, K. Nomiya, J. Inorg. Biochem. 96, 298 (2003). https://doi.org/10.1016/S0162-0134(03)00156-9
N.M. El Metwally, R. Arafa, U. El-Ayaan, J. Therm. Anal. Calorim. 115, 2357 (2014). https://doi.org/10.1007/s10973-013-3065-8
N. Bharti, K. Husain, M. Gonzalez Garza, D.E. Cruz-Vega, J. Castro-Garza, B.D. Mata-Cardenas, F. Naqvi, A. Azam, Bioorg. Med. Chem. Lett. 12, 3475 (2002). https://doi.org/10.1016/S0960-894X(02)00703-5
M.S. Refat, I.M. El-Deen, M.S. El-Garib, W. Abd El-Fattah, Russ. J. Gen. Chem. 85, 692 (2015). https://doi.org/10.1134/S1070363215030299
J.P. Scovill, D.L. Klayman, C.F. Franchino, J. Med. Chem. 25, 1261 (1982). https://doi.org/10.1021/jm00352a036
H. Kaur, B. Narasimhan, Curr. Top. Med. Chem. 18, 3 (2018). https://doi.org/10.2174/1568026618666180206093107
F.G. Parsa, M.A.H. Feizi, R. Safaralizadeh, S.A. Hosseini-Yazdi, M. Mahdavi, J. Biol. Inorg. Chem. 25, 383 (2020). https://doi.org/10.1007/s00775-020-01769-0
M. Kheirkhahi, B. Shaabani, H. Samadi Kafil, J. Iran. Chem. Soc. 18, 3429 (2021). https://doi.org/10.1007/s13738-021-02281-1
I. Wiegand, K. Hilpert, R.E.W. Hancock, Nat. Protoc. 3, 163 (2008). https://doi.org/10.1038/nprot.2007.521
B. Vivekanand, K. Mahendra Raj, B.H.M. Mruthyunjayaswamy, J. Mol. Struct. 1079, 214 (2015). https://doi.org/10.1016/j.molstruc.2014.08.033
G.M. Morris, R. Huey, W. Lindstrom, M.F. Sanner, R.K. Belew, D.S. Goodsell, A.J. Olson, J. Comput. Chem. 30, 2785 (2009). https://doi.org/10.1002/jcc.21256
A. Hussain, M.A. Rather, M.S. Dar, N.A. Dangroo, M.A. Aga, A. Qayum, A.M. Shah, Z. Ahmad, M.J. Dar, Q.P. Hassan, Microbiol. Res. 207, 196 (2018). https://doi.org/10.1016/j.micres.2017.12.004
D. Steverding, P. Evans, L. Msika, B. Riley, J. Wallington, S. Schelenz, Med. Mycol. 50, 333 (2012). https://doi.org/10.3109/13693786.2011.609186
B.D. Bax, P.F. Chan, D.S. Eggleston, A. Fosberry, D.R. Gentry, F. Gorrec, I. Giordano, M.M. Hann, A. Hennessy, M. Hibbs, J. Huang, E. Jones, J. Jones, K.K. Brown, C.J. Lewis, E.W. May, M.R. Saunders, O. Singh, C.E. Spitzfaden, C. Shen, A. Shillings, A.J. Theobald, A. Wohlkonig, N.D. Pearson, M.N. Gwynn, Nature 466, 935 (2010). https://doi.org/10.1038/nature09197
A. Rai, T.K. Gupta, S. Kini, A. Kunwar, A. Surolia, D. Panda, Biochem. Pharmacol. 86, 378 (2013). https://doi.org/10.1016/j.bcp.2013.05.024
E.F. Pettersen, T.D. Goddard, C.C. Huang, G.S. Couch, D.M. Greenblatt, E.C. Meng, T.E. Ferrin, J. Comput. Chem. 25, 1605 (2004). https://doi.org/10.1002/jcc.20084
R.M. Mahfouz, K.A. Al-Farhan, G.Y. Hassen, A.I. Al-Wassil, S.M. Alshehri, A.A. Al-Wallan, Synth. React. Inorg. Met. Chem. 32, 489 (2002). https://doi.org/10.1081/SIM-120003791
P. Jayaseelan, E. Akila, M. Usha Rani, R. Rajavel, J. Saudi Chem. Soc. 20, 625 (2016). https://doi.org/10.1016/j.jscs.2013.07.001
K.B. Gudasi, M.S. Patil, R.S. Vadavi, Eur. J. Med. Chem. 43, 2436 (2008). https://doi.org/10.1016/j.ejmech.2008.01.028
M.S. Atanassova, G.D. Dimitrov, Spectrochim Acta Part A Mol. Biomol. Spectrosc. 59, 1655 (2003). https://doi.org/10.1016/S1386-1425(02)00397-9
A. Karaküçük-İyidoğan, D. Taşdemir, E.E. Oruç-Emre, J. Balzarini, Eur. J. Med. Chem. 46, 5616 (2011). https://doi.org/10.1016/j.ejmech.2011.09.031
M.B. Ferrari, F. Bisceglie, E. Leporati, G. Pelosi, P. Tarasconi, Bull. Chem. Soc. Jpn 75, 781 (2002). https://doi.org/10.1246/bcsj.75.781
S.A. Abdel-Latif, A.A. Mohamed, J. Mol. Struct. 1134, 307 (2017). https://doi.org/10.1016/j.molstruc.2016.12.068
S. Indira, G. Vinoth, M. Bharathi, K. Shanmuga Bharathi, J. Mol. Struct. 1198, 126886 (2019). https://doi.org/10.1016/j.molstruc.2019.126886
A. Çapan, S. Uruş, M. Sönmez, J. Saudi Chem. Soc. 22, 757 (2018). https://doi.org/10.1016/j.jscs.2017.12.007
R.S. Sreepriya, S.S. Kumar, V. Sadasivan, S. Biju, S.S. Meena, J. Mol. Struct. 1201, 127110 (2020). https://doi.org/10.1016/j.molstruc.2019.127110
P. Mendu, J. Pragathi, B. Anupama, C.G. Kumari, E-J. Chem. 9, 2145 (2012). https://doi.org/10.1155/2012/839789
R. Takjoo, R. Centore, L. Rhyman, P. Ramasami, J. Coord. Chem. 65, 1569 (2012). https://doi.org/10.1080/00958972.2012.675058
A.F. Elhusseiny, A. Eldissouky, A.M. Al-Hamza, H.H.A.M. Hassan, J. Mol. Struct. 1100, 530 (2015). https://doi.org/10.1016/j.molstruc.2015.07.049
M.A. Farrukh, K.M. Butt, K.-K. Chong, W.S. Chang, J. Saudi Chem. Soc. 23, 561 (2019). https://doi.org/10.1016/j.jscs.2018.10.002
A. Meena, R. Sharma, V. Sukhadia, Curr. Phys. Chem. 10, 213 (2020). https://doi.org/10.2174/1877946810666200116091321
S.S. Borhade, P.T. Tryambake, Synth. Asian J. Chem. 33, 885 (2021). https://doi.org/10.14233/ajchem.2021.23110
S.S. Borhade, P.T. Tryambake, Asian J. Chem. 34, 2041 (2022). https://doi.org/10.14233/ajchem.2022.23728
Acknowledgements
The authors would like to express their gratitude to the SAIF at Punjab University Chandigarh for providing the spectral analysis facility.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Borhade, S.S., Tryambake, P.T. Synthesis, characterization, antimicrobial activity and molecular docking study of transition metal complexes based on azo coumarin and thiosemicarbazone derivative. J IRAN CHEM SOC 21, 719–730 (2024). https://doi.org/10.1007/s13738-023-02953-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-023-02953-0