Abstract
Thromboembolism because of coronavirus infection (COVID-19) resulted in increasing the number of sudden deaths after recovery. So, patients were switched to edoxaban tosylate monohydrate (EDTM) therapy after low molecular weight heparin to shorten the hospitalization period. In response to increase the demand for EDTM, we developed a home-made screen-printed electrode to determine EDTM in its pure form and pharmaceutical dosage form. Optimized conditions were achieved with a screen-printed electrode utilizing EDTM-Phosphotungstic acid (EDTM-PTA) as an ion pair complex and a plasticizer dibutyl phthalate with a Nernstian slope of 58.78 ± 0.1 mV decade−1 at the concentrations ranging from 5.0 × 10–6 to 1.0 × 10–2 M EDTM (R2 = 0.9985). The detection limit was 4.3 × 10–6 M. Our method was proven to be eco-friendly after assessment by Eco-Scale and Complex-GAPI. The results obtained were validated according to ICH guidelines and were found to agree with those gained from the reference method.
Similar content being viewed by others
Introduction
The pandemic caused by coronavirus (COVID-19) has changed the world over the last 2 years. One of the major problems encountered after the recovery from respiratory symptoms is pulmonary embolism. Asymptomatic young COVID-19 cases ending with sudden death because of acute pulmonary embolism have been reported. Even in physically active cases [1]. Treatment guidelines recommend that all patients after starting low molecular weight heparin as anticoagulant therapy were switched to edoxaban tosylate monohydrate (EDTM) therapy. This decreases the average hospitalization time and decreases the load on the health care system [2].
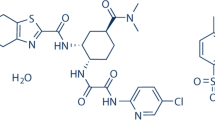
EDTM is a non-vitamin K antagonist, one of the direct oral anticoagulants (DOACs) class of drugs, it is a rapidly acting oral drug and selective factor Xa inhibitor. It inhibits thrombin-induced platelet aggregation and prothrombinase activity. Inhibition of factor Xa in the coagulation cascade decreases thrombus formation by decreasing thrombin generation. Hence, it is now used to manage pulmonary embolism associated with COVID-19. EDTM is chemically known as N'-(5- chloropyridin-2-yl)-N-[(1S,2R,4S)-4- (dimethylcarbamoyl)- 2- [(5-methyl-6,7-dihydro4H- [1, 3] thiazolo [5,4-c] pyridine-2-carbonyl) amino] cyclohexyl] oxamide Fig. 1. FDA approved EDTM in 2015 [3, 4].
Several instrumental techniques have been reported to determine EDTM. Among these techniques are direct UV spectrophotometric methods [5, 6], but they are not selective. HPLC [7,8,9,10,11], UPLC-MS/MS [12,13,14,15], LC–MS/MS techniques [16]. Although LC–MS/MS and UPLC are highly sensitive and selective they are complicated, time-consuming, unavailable in most quality control laboratories, especially in developing countries, and more expensive sample preparation than that of the suggested potentiometric method. Compared to the other techniques, the use of screen-printed electrodes is widely used in the field of pharmaceutical analyses due to their high selectivity, sensitivity, low cost of measurement, and the ability to analyze small volumes of samples in a short time. The work’s goal is to develop and optimize disposable homemade screen-printed electrodes (SPEs) for pharmaceutical analysis. The method is rapid, simple, precise, sensitive, and accurate to determine EDTM in pure and pharmaceutical dosage form.
Materials
-
EDTM drug substance was supplied kindly from Rameda pharmaceutical company, as the tablet formulation was not available in the Egyptian market; tablet containing 60 mg Edoxaban were prepared in-house.
-
Dibutyl phthalate (DBP), Polyvinyl chloride (PVC) of high molecular weight tetrahydrofuran (THF) and hydrochloric acid (HCl) were from Sigma-Aldrich, USA.
-
Graphite powder (particle size less than 50 μm) from Merck, Darmstadt, German.
-
Ascorbic acid, mannitol, Mg- stearate, microcrystalline cellulose, calcium, magnesium, sodium, potassium, and ammonium chloride were tested as interferants; El-Nasr Pharmaceutical Chemicals Company, Cairo, Egypt.
-
Glycine, Alanine, Arginine, cysteine, phenylalanine, and isoleucine amino acids; Piochem, Cairo, Egypt.
-
Sodium tetraphenylborate (Na-TPB) was obtained from fluka.
-
Phosphotungstic acid (PTA) was from S D Fine-Chem Limited, India.
-
All the reagents used in the experiment were of analytical grade, and the water was double distilled throughout.
Reagents
-
Solutions of 10–4 M ascorbic acid, glycine, alanine, arginine, cysteine, phenylalanine, isoleucine, calcium chloride, magnesium chloride, sodium chloride, potassium chloride, and mannitol Mg-stearate, microcrystalline cellulose sodium bicarbonate all were prepared by dissolving an appropriate amount of the reagent into the double-distilled water.
-
1 M NaOH and 1 M HCl were prepared and used to adjust the pH of the medium
Apparatus
All potentiometric measurements were performed using 46-Range Digital Multimeter (Radioshack, China) with PC interface and AgCl reference electrode. The cell assembly for the potentiometric measurements was as follows: Ag—AgCl—3 M KCl—Sample—SPE. The pH measurements were done by HI 2211 PH/ORP HANNA instruments Meter, stirring by MSH-20D Wisd laboratory instruments and vacuum oven (Memmert, Germany). Elemental analysis of C, N, and H was determined utilizing elementar CHNS analyze, model Vario ELIII).
EDTM, PTA, EDTM-PTA functional groups were characterized utilizing a Fourier transform infrared spectrometer (FTIR) (PerkinElmer Spectrum, ATR Sample base plate Diamond, part number is L1600235 and serial number is 49,754).
Fabrication of screen-printed electrodes (SPEs)
The disposable screen-printed strips (3 × 25 mm) were fabricated manually utilizing a homemade polyester screen. The electrodes were printed in an array of ten electrodes on a 200-µm-thick flexible PVC support. First, a conductive carbon track was printed with homemade ink (graphite/PVC ink) and cured for 2 h at 60 °C. The sensing membrane cocktail containing (15 mg of EDTM-PTA, 200 µg of plasticizer, and 100 mg PVC) in 3 mL tetrahydrofuran (THF) was then printed on the graphite track. The electrodes were dried in a vacuum oven at 40 °C for two hours. Finally, the electrodes were covered with an insulating tape leaving a square area (3.0 × 3.0 mm) as a sensing and conductive part at the two terminals as shown in Fig. 2.
Preparation of stock standard solution
Stock standard solution (1.0 × 10–2 M of EDTM) was prepared into a 50-ml measuring flask using 0.1 M HCl as a solvent, the solution was sonicated for 15 min. Other working solutions (1.0 × 10–3 to 1.0 × 10–6 M) were prepared by dilution with double distilled water.
Preparation of ion-pair complex (IP)
The ion-pairs (IP) were prepared by adding 25 mL of 1.0 × 10–2 M solution of PTA, Na-TPB, or Ammonium reineckate to 25 mL of 1.0 × 10–2 M of EDTM solution. Before filtering through filter paper, each solution was stirred for 10 min. The precipitate was washed several times with double distilled water and dried at room temperature for 2 days.
Construction of calibration graph
After 10 min of preconditioning in 10–3 M EDTM solutions, all fabricated electrodes were directly used in potentiometric measurements. The developed sensors were calibrated by immersing the SPE in various EDTM solutions at 25 °C covering the concentration range from 5.0 × 10–6 to 1.0 × 10–2 M. Potential readings were taken and plotted on a logarithmic scale against drug concentration [17]. All measurements were taken, while the vessel was constantly stirred.
Results and discussion
Characterization of ion exchanger
Elemental analysis
PTA was examined as an ion-pairing agent for the preparation of ion complexes with EDTM. Upon the addition of PTA solution to EDTM solution, an insoluble complex of EDTM-PTA has been formed. The elemental analysis of the formed complex showed that EDTM reacts with PTA in a ratio of 1:1, respectively, as shown in Table 1.
Infrared spectrum analysis
Using Fourier transformed infrared (FTIR) spectroscopy PTA, EDTM, and PTA-EDTM were characterized. As shown in Figure 3a, PTA shows four characteristic bands for (W–Oe–W), (W–Oc–W), (W–O), and (P–O) stretching vibrations at 746.85, 887.27, 972.30, and 1073.49 cm−1 where Oe and Oc refer to the edge and corner oxygen’s, respectively. A broad and strong band present at 3449.33 cm−1 refers to (O–H) stretching vibrations of the hydroxyl groups of PTA.
EDTM spectrum Fig. 3b. shows the characterized peak at 3343.34 cm−1 belongs to the N=H stretch of the amide groups. A broad peak at 3208.93 cm−1 due to stretching of the hydrogen bond of the N–H group. A sharp peak A strong and sharp peak at 1552.11 cm−1 due to C=N and C=C.
PTA-EDTM spectrum Fig. 3c. shows the characteristic peaks of PTA that appear beside the characteristic peaks of EDTM which confirm complex formation.
Effect of membrane composition
Since factors such as sensitivity, linearity, and detection limit for certain electrodes are based on the electrode composition. Screen printing was prepared to contain different types of ion pairs and the influence of the nature of the ion pairs in the SPE composition was investigated. The ion pair is essential for establishing thermodynamic ion exchange equilibrium at the membrane/ solution interface. As a result, it must be stable over a wide pH range, practically insoluble in water, and hydrophobic. Simultaneously, the plasticizer should be capable of dissolving the ion exchanger, water-insoluble and nonvolatile. In this study, screen-printed electrodes SPE with variable membrane compositions were prepared using EDTM-TPB, EDTM-PTA, EDTM-TPB/PTA as ion pair, and DBP as a plasticizer (Table 1). For each membrane composition, a calibration curve was constructed, and the slope was obtained.
As shown in Fig. 4, all electrodes cover the concentration range from 5.0 × 10–6 to 1.0 × 10–2 M. The electrode (EDTM-PTA) has the highest Nernstian slope of 58.78 ± 1 mV decade−1 (r = 0.9985).
Electrode performance
Presence of interference
The potentiometric selectivity coefficient (\({logK}_{i,j}^{pot}\)) is the electrode’s ability to respond only to the target ion I in the presence of other ions j in the sample matrix. The selectivity of the electrode for EDTM was studied using the separate solutions method [18]. The selectivity coefficient was calculated for two types of interferences:
-
Tablet excipients including magnesium stearate, mannitol, and microcrystalline cellulose.
-
Magnesium stearate is water-insoluble so dispersed using ultrasonic before measuring.
-
interference from common inorganic cations and amino acids including Na+1, K+1, Ca+2, Mg+2, NH4+1, Glycine, L-isoleucine, cysteine, L-arginine HCl, D-alanine, DL-phenylalanine, and ascorbic acid.
Using the following equation, the selectivity coefficient was calculated:
In this equation:1- The electromotive force value (Ei and Ej) of the electrode in a pure solution of the drug (primary) (EDTM; i) and the interfering ion (j), respectively, of equal concentration (1.0 × 10–2 M). 2-Zi and Zj are the charges on the pure solution of the drug and interfering ions (secondary), respectively.3-S is the slope of the calibration curve for the primary ion.
The small values of \({logK}_{i,j}^{pot}\) as shown in Table 2 reflect the high selectivity of the proposed sensors to EDTM over the interfering substances.
The influence of pH
The electrode potential is significantly affected by the pH of the sample solution. As a result, potentiometric measurements should be carried out within/or at a pH where the potentiometric response is pH independent. The influence of pH on the electrode’s response was investigated by taking the potential readings for a 1.0 × 10–3 M solution of EDTM at various pH values. pH values were varied by adding very small volumes of 1 M NaOH solution to 50 mL of the EDTM solution and plotting E (mV) versus pH values. The plot of E (mV) versus pH Fig. 5 demonstrates that the electrode’s response was pH independent throughout the pH range (1.3–3.7).
The dynamic response time and potential stability
The response time of the electrode is an important factor that characterizes SPE. It is the time required to reach a stable potential value (limited ∆E/∆t) after successive immersing in a series of solutions with tenfold concentration differences. The dynamic response time of the electrode under investigation was determined for the concentration range from 1.0 × 10–6 to 1.0 × 10–3 M. The potential reading was monitored at each concentration level for 2 min. Figure 6 shows a plot of the potential changes against time for the electrode under investigation. The proposed electrode has an extremely fast response time of 5–10 s. The stability of the electrode was studied by immersion of the electrode in 1.0 × 10–3 M EDTM and the potential was monitored over 30 min. The potential was highly stable with a change of less than 0.5 mV. The electrode’s high stability can be attributed to the efficient connection between the conductive carbon track and the sensing layer which was achieved by using the same binder for both layers. Figure 7 shows how such a configuration reduces water uptake by the membrane while increasing sensor stability.
Reproducibility and hysteresis
Electrode hysteresis also known as electrode memory is observed when the potential of the electrode recorded in a definite concentration of an analyte is changed after being exposed to a different concentration of the same analyte [19]. The electrode potential was measured in 1.0 × 10–4 M and 1.0 × 10–3 M while exchanged repeatedly. When the electrode was moved between different concentrations of EDTM solutions, it displayed a very stable and fast potential response.
As illustrated in Fig. 8, the electrode retained its initial potential value measured in 1.0 × 10–3 M EDTM (261.6 ± 2.6 mV) after exposing to 1.0 × 10–4 M EDTM. The results indicate that the electrode is appropriate for flow injection measurements where the electrode can be exposed to variety of concentrations in a short period of time.
Electrode lifetime
The lifetime of the investigated electrode was monitored over 20 days by measuring its potential in 10–3, 10–4, and 10–5 M standard drug solution each day. It was found that the electrode is stable for 14 days.
Calibration plots
The results shown in Fig. 2 and Table 3 showed that the proposed electrode (EDTM-PTA) can be used to successfully determine the EDTM potentiometrically with a linear response in the concentration ranging from 1.0 × 10–2 to 5.0 × 10–6 M with a slope value of 59.25 mV decade−1
Analytical application
The proposed electrode was used successfully for the assay of EDTM in its pharmaceutical dosage form by applying a standard addition method. An electrode was immersed in a sample of 50 mL with an unknown concentration, and the equilibrium potential of E1 was recorded using the standard addition method. The equilibrium potential of E2 was then determined by adding a known volume of 1.0 × 10–2 M standard solution of EDTM into the testing sample. The concentration of the testing sample can be calculated using the change of Δ (E2−E1). Table 4. The obtained results were statistically compared with the official method using a F-ratio and student's t test at the 95%confidence level with 2 degrees of freedom. Also, Table 5 shows that there was no statistically significant difference between the reference and suggested methods.
Assessment of method greenness
Analytical methods use a lot of chemicals that may generate toxic residues in the environment. Herein, green analytical chemistry was first presented in 2000 to remove or reduce the hazardous effect on the environment [20]. Various assessment tools were introduced to evaluate the greenness of the analytical method depending on wastes, energy consumption, and solvents used per study. Each tool has a unique assessment protocol advantages and disadvantages, so in this study, we applied two assessment tools in green assessment the analytical eco-scale assessment (ESA) [21] and the newest tool in green assessment called complex green analytical procedure index (Complex-GAPI).
ESA has the advantage of providing a quantitative evaluation of the analytical methods by considering all of the reagents used, rather than just the most hazardous ones, as other matrices do. It is based on penalty points subtracted from a 100 mark a total eco scale score if more than 75 represents excellent greenness, more than 50 represents acceptable greenness and less than 50 represents inadequate greenness Table 6 represents the scores for the suggested method. Our method achieved 52 penalty points, which demonstrated its acceptable greenness. It has several disadvantages including insufficient information about the causes of the analytical procedure’s environmental impact and no data about the structure of the hazards is found. Our method is regarded as an acceptable greenness.
Complex-GAPI is the most recent advanced technique or assessment of green chemistry. It is the developed version of the GAPI assessment. It represents a comprehension evaluation of the whole analytical process from sample collection to final analysis including transport, preservation, storage, and sample preparation. The scale is based on utilizing 5 pentagrams, and the additional hexagonal part is the pre-analysis step. Green color represents an eco-friendly step yellow color represents a medium environmental impact and red color represents a hazardous environmental impact of this step [22]. There is no need for a purification step, which corresponds to the white region in the hexagonal shape. This technique has a lot of advantages as the software is available that will facilitate the use of such a tool, it is simple, friendly use, and includes all the factors that characterize the analytical protocol as well as the pre-analysis process (conditions, techniques, and reagents). Our method is eco-friendly according to the application of two approaches: Eco-Scale and Complex-GAPI, as presented in Table 6.
Conclusion
For the assay of EDTM, a simple, reproducible, and affordable screen-printed electrode is provided. EDTM-PTA ion pair and DBP plasticizer exhibited the highest level of sensitivity among the tested ion pairs. The electrode showed a Nernstian slope of 59.25 mV decade−1 over a broad range of concentration from 5.0 × 10–6 to 1 × 10–2 M (R2 = 0.9981). The electrode works with pH levels ranging from 1.3 to 3.7. Our method was utilized to assay EDTM accurately and precisely in both pure form and pharmaceutical dosage form. Unlike chromatographic and spectrophotometric approaches, the suggested method does not require expensive solvents or time-consuming calculations.
References
V. Polat, G.İ Bostancı, Sudden death due to acute pulmonary embolism in a young woman with COVID-19. J. Thromb. Thrombolysis 50(1), 239–241 (2020)
V. Langella, R. Bottino, A. Asti, G. Maresca, G. di Palma, D. Pomponi et al., Edoxaban for the treatment of pulmonary embolism in hospitalized COVID-19 patients. Expert Rev. Clin. Pharmacol. 14(10), 1289–1294 (2021)
C. Rognoni, M. Marchetti, S. Quaglini, N.L. Liberato, Edoxaban versus warfarin for stroke prevention in non-valvular atrial fibrillation: a cost-effectiveness analysis. J. Thromb. Thrombolysis 39(2), 54–149 (2015)
M.S.M.H.O.T.H.L.L.C.M.J. Bathala, Pharmacokinetics, biotransformation, and mass balance of edoxaban, a selective, direct factor Xa inhibitor, in humans. Drug Metab. Dispos. 40(12), 2250–2255 (2012)
G.G. Kalyankar, P.H. Vansiya, K.B. Bodiwala, S.R. Lodha, P.B. Prajapati, K.M. Ranch, Development and validation of spectrophotometric method for the estimation of edoxaban tosylate monohydrate in its synthetic mixture. Am. J. PharmTech Res. 8(2), 296 (2018)
P. Ravisankar, D. Srikanth, C.V. Reddy, P.R. Rao, P.S. Babu, Development and validation of UV spectrophotometric method for the determination of edoxaban tosylate monohydrate in pharmaceutical dosage form. Indian J. Res. Pharm. Biotechnol. 6(2), 73–78 (2018)
R.R. Kadiri, R. Peraman, S.R. Dakinedi, Stability indicating RP-HPLC method for quantification of edoxaban tosylate. Int. J. Res. Pharm. Sci. 9(2), 314–320 (2018)
P.S. Reddy, V.S.K. Jagarlapudi, C.B. Sekharan, Determination of edoxaban in bulk and in tablet dosage form by stability indicating high-performance liquid chromatography. Pharm. Sci. 22(1), 35–41 (2016)
P. Shah, S. Saroj, S. Rajput, Stability indicating assay method development and validation of edoxabantosylate monohydrate a comprehensive study involving impurities isolation identification. Int. J. Pharm. Sci. Res. (2019). https://doi.org/10.13040/IJPSR.0975-8232.10
F. Gouveia, J. Bicker, J. Santos, M. Rocha, G. Alves, A. Falcão et al., Development, validation and application of a new HPLC-DAD method for simultaneous quantification of apixaban, dabigatran, edoxaban and rivaroxaban in human plasma. J. Pharm. Biomed. Anal. 20, 181 (2020)
S.E. Younis, S.A. El-Nahass, M.A.W. Elkhatib, S.A. Soliman, R.M. Youssef, Gradient HPLC-DAD method for quantification of novel oral anticoagulant “edoxaban” in plasma: Its selective determination in presence of sixteen co-administered drugs. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 1, 1160 (2020)
W.L. Zhang, D. Lou, D.T. Zhang, Y. Zhang, H.J. Huang, Determination of rivaroxaban, apixaban and edoxaban in rat plasma by UPLC–MS/MS method. J. thrombo. thrombolysis 42, 205–211 (2016)
Lindahl S, Dyrkorn R, Spigset O, Hegstad S (2018) Quantification of apixaban, dabigatran, edoxaban, and rivaroxaban in human serum by UHPLC-MS/MS-method development, validation, and application. Available from: www.drug-monitoring
M.H.J. Wiesen, C. Blaich, T. Streichert, G. Michels, C. Müller, Paramagnetic micro-particles as a tool for rapid quantification of apixaban, dabigatran, edoxaban and rivaroxaban in human plasma by UHPLC-MS/MS. Clin. Chem. Lab Med. 55(9), 1349–1359 (2017)
R. Siriez, L. Alpan, K. Elasaad, P. Devel, J. Laloy, J.M. Dogné et al., Importance of measuring pharmacologically active metabolites of edoxaban: development and validation of an ultra-high-performance liquid chromatography coupled with a tandem mass spectrometry method. J. Thromb. Thrombolysis 49(3), 395–403 (2020)
K. Hanada, S.I. Matsumoto, S. Shibata, H. Matsubara, Y. Tsukimura, H. Takahashi, A quantitative LC/MSMS method for determination of edoxaban, a Xa inhibitor and its pharmacokinetic application in patients after total knee arthroplasty. Biomed. Chromatogr. 32(6), e4213 (2018)
R.P. Buck, E. Lindneri, Recomendation for nomenclature of ion-selective electrode. J. Buffle. 66(12), 1991–1993 (1994)
K. Srinivasan, G.A. Rechnitz, Selectivity studies on liquid membrane, ion-selective electrodes. Anal. Chem. 41(10), 1203–1208 (1969)
E.M. Hussien, A.R. Derar, Selective determination of diclofenac and clomiphene with a single planar solid-state potentiometric ion selective electrode. J. Electrochem. Soc. 166(10), B780–B786 (2019)
S. Armenta, S. Garrigues, M. de la Guardia, Green analytical chemistry. TrAC—Trends Anal. Chem. 27(6), 497–511 (2008)
A. Gałuszka, Z.M. Migaszewski, P. Konieczka, J. Namieśnik, Analytical eco-scale for assessing the greenness of analytical procedures. TrAC—Trends Anal. Chem. 37, 61–72 (2012)
J. Płotka-Wasylka, W. Wojnowski, Complementary green analytical procedure index (ComplexGAPI) and software. Green Chem. 23(21), 8657–8665 (2021)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rizk, M., Abou El-Alamin, M.M., Abd Elkhalek, O. et al. Validated and selective potentiometric analysis of anti-coagulant edoxaban via a screen-printed electrode: green assessment by Eco-Scale and Complex-GAPI. J IRAN CHEM SOC 20, 2319–2327 (2023). https://doi.org/10.1007/s13738-023-02842-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-023-02842-6