Abstract
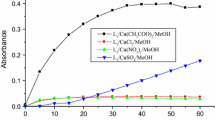
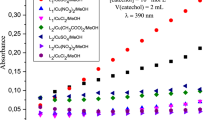
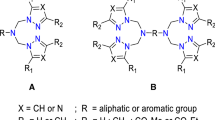
In situ complexes, arising from six ligands based on pyrazol (L1-L6): N,N-bis((3,5-dimethyl-1H-pyrazol-1-yl)methyl)-4-fluoroaniline (L1); 5-chloro-N-((3,5-dimethyl-1H-pyrazol-1-yl)methyl)pyridin-2-amine (L2); N-((3,5-dimethyl-1H-pyrazol-1-yl)methyl)-N-phenylaniline (L3); N-((1H-pyrazol-1-yl) methyl)-N-phenylbenzenamine (L4); N,N-bis((1H-pyrazol-1-yl)methyl)-4-fluoroaniline (L5); and N-((3,5-dimethyl-1H-pyrazol-1-yl)methyl)aniline (L6), were reported and examined, in combination with different metallic salts, for their catecholase and phenoxazinone synthase activities at ambient conditions. We highlight the utility of spectroscopic and electrochemical methods, for studying the catalytic activity of biomimetic complexes and understanding the catalytic mechanism of substrate oxidation; the electrochemical oxidation of catechol has been successfully performed by cyclic voltammetry at room temperature and electrochemical cell with three electrodes. The role of metallic salt and the ligand structure of these complexes on their catecholase and phenoxazinone synthase activity have been examined. The metallic salt Cu(CH3COO)2 appears a better candidate to produce the best model of the two studied enzymes in neutral mediums.













Similar content being viewed by others
References
A. Aljawish, I. Chevalot, J. Jasniewski, J. Scher, L. Muniglia, J. Mol. Catal. B Enzym. 112, 25–39 (2015). https://doi.org/10.1016/j.molcatb.2014.10.014
J. Xu, S. Strandman, J.X.X. Zhu, J. Barralet, M. Cerruti, Biomaterials 37, 395–404 (2015). https://doi.org/10.1016/j.biomaterials.2014.10.024
C. Cougnon, E. Lebegue, G. Pognon, J. Power Sources 274, 551–559 (2015). https://doi.org/10.1016/j.jpowsour.2014.10.091
B.P. Lee, A. Meng-HsienLin, S. Narkar, R. Konst, Wilharm. Sens. Actuators B 206, 456–462 (2015). https://doi.org/10.1016/j.snb.2014.09.089
M. Muslim, A. Ali, M. Ahmad, A. Alarifi, M. Afzal, N. Sepa, N. Dege, J. Mol. Liq. 363, 119767 (2022). https://doi.org/10.1016/j.molliq.2022.119767
Y. Thio, J.J. Vittal, Inorg. Chim. Acta 526, 120502 (2021). https://doi.org/10.1016/j.ica.2021.120502
N. Bandopadhyay, K. Paramanik, P.K. Mudi, G. Sarkar, M. Kotakonda, M. Shit, B. Biswas, H.S. Das, Polyhedron 218, 115783 (2022). https://doi.org/10.1016/j.poly.2022.115783
S. Roy, T. Dutta, M.G.B. Drew, S. Chattopadhyay, Chattopadhyay. Polyhedron 178, 114311 (2020). https://doi.org/10.1016/j.poly.2019.114311
M.I. Ayad, Arab. J. Chem. 9, S1297–S1306 (2016). https://doi.org/10.1016/j.arabjc.2012.02.007
S. Bittner, Amino Acids 30, 205–224 (2006). https://doi.org/10.1007/s00726-005-0298-2
A.M. Mayer, E. Hareli, Phytochem. 18, 193–215 (1979). https://doi.org/10.1016/0031-9422(79)80057-6
F. Taranto, A. Pasqualone, G. Mangini, P. Tripodi, M.M. Miazzi, S. Pavan, C. Montemurro, Int. J. Mol. Sci. 18, 377 (2017). https://doi.org/10.3390/ijms18020377
A. Rompel, H. Fischer, K. Dirk Meiwes, R.D. Büldt-Karentzopoulos, F. Tuczek, B. Herbert Witzel, JBIC 4(1), 56–63 (1999). https://doi.org/10.1007/s007750050289
Y. Jiang, J. Fu, G. Zauberman, Y. Fuchs, J. Sci Food. Agric. 79, 950–954 (1999)
C. Eicken, B. Krebs, J.C. Sacchettini, Curr. Opin. Struct. Biol. 9, 677–683 (1999). https://doi.org/10.1016/S0959-440X(99)00029-9
C.E. Barry III., P.G. Nayar, T.P. Begley, J. Am. Chem. Soc. 110, 3333–3334 (1988). https://doi.org/10.1021/ja00218a072
R.B. Womer, Eur. J. Cancer 33, 2230–2236 (1997)
S. Faber, JAMA 198, 826–836 (1966). https://doi.org/10.1001/jama.1966.03110210076025
E. Katz, H. Weissbach, J. Biol. Chem. 237, 666–675 (1963)
C.E. Barry III., P.G. Nayar, T.P. Begley, Biochem. 28, 6323–6333 (1989). https://doi.org/10.1021/bi00441a026
J.C. Freeman, P.G. Nayar, T.P. Begley, J.J. Villafranca, Biochem. 32, 4826–4830 (1993). https://doi.org/10.1021/bi00069a018
N.P. Jayaweera, A.E. Hall, A.A. Wilson, M.E. Konkle, K.A. Wheeler, Semeniuc. Inorg. Chim. Acta 506, 119507 (2020). https://doi.org/10.1016/j.ica.2020.119507
N. Boussalah, R. Touzani, I. Bouabdallah, S. El Kadiri, S. Ghalem, J. Mol. Catal. A: Chem. 306, 113–117 (2009). https://doi.org/10.1016/j.molcata.2009.02.031
I. Bouabdallah, R. Touzani, I. Zidane, A. Ramdani, J. Iran. Chem. Soc. 4(3), 299–303 (2007). https://doi.org/10.1007/BF03245978
A. Mouadili, A. Attayibat, S. El Kadiri, S. Radi, R. Touzani, Appl. Catal. A-Gen. 454, 93–99 (2013). https://doi.org/10.1016/j.apcata.2013.01.011
H. allali, Y. kaddouri, El. Yousfi, M. El Kodadi, R. Touzani, J. Appl. Sci. Envir. Stud. 2(1) (2019) 13-29
A. Jana, P. Brandão, H. Jana, A.D. Jana, G. Mondal, P. Bera, A. Santra, A.K. Mahapatra, P. Bera, J. Coord. Chem. Rev. 72(16), 2636–2653 (2019). https://doi.org/10.1080/00958972.2019.1658192
E.C. Constable, P.J. Steel, J. Coord. Chem. Rev. 93, 205–223 (1989). https://doi.org/10.1016/0010-8545(89)80016-5
D.A. House, P.J. Steel, A.A. Watson, J. Chem. Soc. Chem Communicat (1987). https://doi.org/10.1039/c39870001575
W.L. Driessen, R.A.G. de Graaff, W.G.R. Wiesmeijer, Acta. Cryst. C43, 2319–2321 (1987). https://doi.org/10.1107/S0108270187087912
S. Trofimenko, Chem. Rev. 93, 943–980 (1993). https://doi.org/10.1021/cr00019a006
I. Bertini, G. Lanini, C. Luchinat, C. Haas, W. Maret, M. Zeppezauer, Eur. Biophys. J. 14, 431–439 (1987). https://doi.org/10.1007/BF00254867
L.-J. Huang, S.-C. Kuo, K. Jih-Pyang Wang, H.N. Ishii, J. Chem. Pharm. Bull. 42(10), 2036–2041 (1994). https://doi.org/10.1248/cpb.42.2036
Y. Kaddouri, F. Abrigach, N. Mechbal, Y. Karzazi, M. El Kodadi, A. Aouniti, R. Touzani, Mater. Today: Proceed. 13, 956–963 (2019). https://doi.org/10.1016/j.matpr.2019.04.060
A. Boulouiz, I. Hajji, Y. Kaddouri, K. Zaidi, R. Touzani, B. Hammouti, Mater. Today: Proceed. 31, S190–S196 (2020). https://doi.org/10.1016/j.matpr.2020.08.273
S. Shin, S. Nayab, H. Lee, Polyhedron 141, 309–321 (2017). https://doi.org/10.1016/j.poly.2017.12.021
N. Bouroumane, M. El Kodadi, R. Touzani, M. El Boutaybi, A. Oussaid, B. Hammouti, A.B.D. Nandiyanto, Arab. J. Sci. Eng. 47, 269–279 (2021). https://doi.org/10.1007/s13369-021-05343-x
F. Abrigach, Y. Karzazi, R. Benabbes, M. El Youbi, M. Khoutoul, N. Taibi, N. Karzazi, N. Benchat, M. Bouakka, E. Saalaoui, R. Touzani, Med. Chem. Res. 26, 1784–1795 (2017). https://doi.org/10.1007/s00044-017-1888-8
E.I. Solomon, U.M. Sudaram, T.E. Machonkin, Chem. Rev. 96, 2563–2605 (1996). https://doi.org/10.1021/cr950046o
A. Mouadili, S. Chtita, A. El Ouafi, M. Bouachrine, A. Zarrouk, R. Touzani, J. Mater. Environ. Sci. 7(1), 210–221 (2016)
M.A. Motin, M.A. Uddin, P.K. Dhar, M.A.H. Mia, M.A. Hashem, Port. Electrochim. Acta 35(2), 103–116 (2017). https://doi.org/10.4152/pea.201702103
S. Shahrokhian, A. Hamzehloei, Electrochem. Commun. 5(8), 706–710 (2003). https://doi.org/10.1016/S1388-2481(03)00170-X
D. Nematollahi, M. Rafiee, L. Fotouhi, J. Iran. Chem. Soc. 6(3), 448–476 (2009). https://doi.org/10.1007/BF03246523
P.J. O’Brien, D. Herschlag, Catalytic promiscuity and the evolution of new enzymatic activities. Chem. Biol. 6(4), R91–R1055 (1999)
A. Ercan, H.I. Park, Li-June Ming, A “Moonlighting” Dizinc Aminopeptidase from Streptomyces griseus: Mechanisms for Peptide Hydrolysis and the 4_1010-Fold Acceleration of the Alternative Phosphodiester Hydrolysis. Biochem. 45, 13779–13793 (2006). https://doi.org/10.1021/bi061086x
L. Cariati, M. Oliverio, G. Mutti, S. Bonacci, T. Knaus, P. Costanzo, A. Procopio, Hydrolases-mediated transformation of oleuropein into demethyloleuropein. Bioorg. Chem. 84, 384–388 (2019). https://doi.org/10.1016/j.bioorg.2018.12.005
M.R. Malachowski, B. Dorsey, J.G. Sackett, R.S. Kelly, A.L. Ferko, R.N. Hardin, Effect of ligand donors on the catalytic properties of metal-complexes - copper(ii) complexes as catalysts for the oxidation of 3,5-di-tert-butylcatechol. Inorg. Chim. Acta 249(1996), 85–92 (1996). https://doi.org/10.1016/0020-1693(96)05026-8
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Boutaybi, M.E., Mouadili, A., Oussaid, A. et al. Spectroscopic and electrochemical study of biomimetic catecholase and phenoxazinone synthase activities of in situ complexes bearing pyrazolic ligands. J IRAN CHEM SOC 20, 961–976 (2023). https://doi.org/10.1007/s13738-022-02729-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-022-02729-y