Abstract
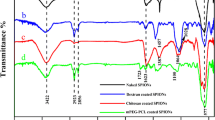
This study aims to fabricate and formulate a new magnetic resonance imaging (MRI) contrast agent based on a dextran–spermine nanoparticulate system loaded with super paramagnetic iron oxide nanoparticles (SPION). SPION-loaded spermine–dextran nanoparticles were prepared according to a procedure based on the ionic gelation of dextran–spermine with sodium tripolyphosphate (TPP) anions. The effects of process parameters such as pH, concentration of spermine dextran, TPP to dextran–spermine and SPION to dextran–spermine weight ratios, and TPP addition rate were fully investigated to find the optimized formulation through the response surface methodology. At the optimum condition, 75% of the magnetic iron oxide nanoparticles added to the polymeric solution were entrapped in dextran–spermine nanoparticles. Samples were investigated by transmission electron microscopy. The mean particle size of the nanoparticles determined by particle size analyzer was found to be 65 nm at the optimum condition with zeta potential of +90 mV. The SPION-loaded dextran–spermine nanoparticle formulation has the same superparamagnetic properties as SPIONs and at same iron concentration the saturation magnetization (Ms) of the SPION-loaded dextran–spermine nanoparticles was larger than SPIONs. In vitro MRI was performed with gradient echo and spin-echo sequences at 1.5 T. By increasing of iron concentration, the T 2 relaxation times were reduced. Thus, indicating that the saturation magnetization and r 2 and \( r_{2}^{*} \) relaxivities were enhanced, and the contrast effects were improved in comparison to commercial SPIONs.







Similar content being viewed by others
References
Chandrasekharan P, Maity D, Chang Tong Y, Kai Hsiang C, Ding J, Si Shen F (2010) Superparamagnetic iron oxide-loaded poly (lactic acid)–d-α-tocopherol polyethylene glycol 1000 succinate copolymer nanoparticles as MRI contrast agent. Biomaterials 31:5588–5597
Arruebo M, Fernández Pacheco R, Ricardo Ibarra M, Santamaría J (2007) Magnetic nanoparticles for drug delivery. Nano Today 3:23–32
Hamoudeh M, Fessi H (2006) Preparation, characterization and surface study of poly-epsilon caprolactone magnetic microparticles. J Colloid Interface Sci 300:584–590
Neves AA, Brindle KM (2006) Assessing responses to cancer therapy using molecular imaging. Biochim Biophys Acta 1766:242–261
Bulte JWM, Kraitchman DL (2004) Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed 17:484–499
Moore A, Marecos E, Bogdanov A, Weissleder R (2000) Tumoral distribution of long-circulating dextran-coated iron oxide nanoparticles in a rodent model. Radiology 214:568–574
Himmelreich U, Hoehn M (2008) Stem cell labeling for magnetic resonance imaging. Minim Invasive Ther 17:132–142
Modo M, Cash D, Mellodew K, Williams SC, Fraser SE, Meade TJ, Price J, Hodegs H (2002) Tracking transplanted stem cell migration using bifunctional contrast agent-enhanced magnetic resonance imaging. Neuroimage 17:803–811
Kustermann E, Himmelreich U, Kandal K, Geelen T, Ketkar A, Wiedermann D, Strecker C, Esser J, Arnhold S, Hoehn M (2008) Efficient stem cell labeling for MRI studies. Contrast Media Mol Imaging 3:27–37
Weissleder R, Bogdanov A, Neuwelt EA, Papisov M (1995) Long-circulating iron oxides for MR imaging. Adv Drug Deliv Rev 16:321–334
Liu H, Li J (2010) Preparation and characterization of poly (PEGMA) modified superparamagnetic nanogels used as potential MRI contrast agents. Iran Polym J 17(9):721–727
Briley-Saebo K, Bjørnerud A, Grant D, Ahlstrom H, Berg T, Kindberg GM (2004) Hepatic cellular distribution and degradation of iron oxide nanoparticles following single intravenous injection in rats: implications for magnetic resonance imaging. Cell Tissue Res 316:315–323
Chertok B, David AE, Yang VC (2008) Delivery of functional proteins to brain tumor using MRI-monitored magnetically targeted nanoparticles. J Control Release 132:e61–e62
Dias AMGC, Hussain A, Marcos AS, Roque ACA (2011) A biotechnological perspective on the application of iron oxide magnetic colloids modified with polysaccharides. Biotechnol Adv 29:142–155
Villa C, Erratico S, Razini P, Fiori F, Rustichelli F, Torrente Y, Belicchi M (2010) Stem cell tracking by nanotechnologies. Int J Mol Sci 11:1070–1081
Bulte JW, Arbab AS, Douglas T, Frank JA (2004) Preparation of magnetically labeled cells for cell tracking by magnetic resonance imaging. Method Enzymol 386:275–299
Lewin M, Carlesso N, Tung CH, Tang XW, Cory D, Scadden DT, Weissleder R (2000) Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat Biotechnol 18:404–410
Boyer C, Whittaker MR, Bulmus V, Liu J, Davis TP (2010) The design and utility of polymer stabilized iron oxide nanoparticles for nanomedicine applications. NPG Asia Mater 2:23–30
Kim D, Hong KS, Song J (2007) The present status of cell tracking methods in animal models using magnetic resonance imaging technology. Mol Cells 23:132–137
Kaim AH, Wischer T, O’Reilly T, Jundt G, Fröhlich J, Schulthess GK, Allegrini PR (2002) MR imaging with ultrasmall superparamagnetic iron oxide particles in experimental soft-tissue infections in rats. Radiology 225:808–814
Jung CW, Jacobs P (1995) Physical and chemical properties of superparamagnetic iron oxide MR contrast agents: ferumoxides, ferumoxtran, ferumoxsil. Magn Reson Imaging 13:661–674
Mody VV, Nounou MI, Bikram M (2009) Novel nanomedicine-based MRI contrast agents for gynecological malignancies. Adv Drug Deliv Rev 61:795–807
Strable E, Bulte JM, Moskowitz B, Vivekanandan K, Allen M, Douglas T (2001) Synthesis and characterization of soluble iron oxide-dendrimer composites. Chem Mater 13:2201–2209
Kohler N, Sun C, Fichtenholtz A, Gunn J, Fang C, Zhang MQ (2006) Methotrexate-immobilized poly (ethylene glycol) magnetic nanoparticles for MR imaging and drug delivery. Small 2:785–792
Liu HL, Ko SP, Wu JH, Jung MH, Min JH, Lee JH, An DH, Kim YK (2007) One-pot polyol synthesis of mono size PVP-coated sub-5 nm Fe3O4 nanoparticles for biomedical applications. J Magn Magn Mater 310:815–817
Lee HY, Lee SH, Xu C, Xie J, Lee JH, Wu B, Koh AL, Wang X, Sinclair R, Wang SX, Nishimura DG, Biswal S, Sun S, Cho SH, Chen X (2008) Synthesis and characterization of PVP-coated large core iron oxide nanoparticles as an MRI contrast agent. Nanotechnology 19:1–6
Arsalani N, Fattahi H, Nazarpoor M (2010) Synthesis and characterization of PVP-functionalized superparamagnetic Fe3O4 nanoparticles as an MRI contrast agent. eXPRESS Polym Lett 6:329–338
Wang Y, Woon NY, Chen Y, Shuter B, Yi J, Ding J, Wang SC, Sishen F (2008) Formulation of superparamagnetic iron oxides by nanoparticles of biodegradable polymers for magnetic resonance imaging. Adv Funct Mater 18:308–318
Pich A, Bhattacharya S, Ghosh A, Adler A (2005) Composite magnetic particles: 2. Encapsulation of iron oxide by surfactant-free emulsion polymerization. Polymer 46:4596–4603
Zheng W, Gao F, Gu H (2005) Magnetic polymer nanospheres with high and uniform magnetite content. J Magn Magn Mater 288:403–410
Ngaboni Okassa L, Marchais H, Douziech Eyrolles H, Cohen S, Souce M, Dubois P, Chourpa R (2005) Development and characterization of sub-micron poly (d, l-lactide-co-glycolide) particles loaded with magnetite/maghemite nanoparticles. Int J Pharm 302:187–196
Joumaa N, Toussay P, Lansalot M, Elaissari A (2008) Surface modification of iron oxide nanoparticles by a phosphate-based macromonomer and further encapsulation into submicrometer polystyrene particles by miniemulsion polymerization. J Polym Sci Part A 46:327–340
Lee SJ, Jeong JR, Shina SC, Kim JC, Changa YH, Chang YM, Kim JD (2004) Nanoparticles of magnetic ferric oxides encapsulated with poly(d, l lactide-co-glycolide) and their applications to magnetic resonance imaging contrast agent. J Magn Magn Mater 272:2432–2433
Sundar S, Kundu J, Kundu SC (2010) Biopolymeric nanoparticles. Sci Technol Adv Mat 11:014104
Yang J, Gunn J, Dave SR, Zang M, Wang YA, Gao X (2008) Ultrasensitive detection and molecular imaging with magnetic nanoparticles. Analyst 133:154–160
Azzam T, Eliyahu H, Shapira L, Linial M, Barenholz Y, Domb AJ (2002) Polysaccharide–oligoamine based conjugates for gene delivery. J Med Chem 45:1817–1824
Abedini F, Ismail M, Hosseinkhani H, Azmi T, Omarb A, PeiPei C, Ismail N, Farber I, Domb AJ (2010) Toxicity evaluation of dextran–spermine polycation as a tool for gene therapy in vitro. J Cell Anim Biol 4:170–176
Liu F, Huang L (2002) Development of non-viral vectors for systemic gene delivery. J Control Release 78:259–266
Hosseinkhani H, Azzam T, Kobayashi H, Hiraoka Y, Shimokawa H, Domb AJ, Tabata Y (2006) Combination of 3-D tissue engineered scaffold and non-viral gene enhance in vitro DNA expression of mesenchymal stem cells. Biomaterials 27:4269–4278
Hosseinkhani H, Inatsugu Y, Hiraoka Y, Inoue S, Shimokawa H, Tabata Y (2005) Impregnation of plasmid DNA into three-dimensional scaffolds and medium perfusion enhance in vitro DNA expression of mesenchymal stem cells. Tissue Eng 11:1459–1475
Hosseinkhani H, Inatsugu Y, Inoue S, Hiraoka Y, Tabata Y (2005) Perfusion culture enhances the osteogenic differentiation of rat mesenchymal stem cells in collagen sponge rein forced with poly (glycolic acid) fiber. Tissue Eng 11:1476–1488
Hosseinkhani H, Yamamoto M, Inatsugu Y, Hiraoka Y, Inoue S, Shimokawa H, Tabata Y (2006) Enhanced ectopic bone formation using a combination of plasmid DNA impregnation into 3-D scaffold and bioreactor perfusion culture. Biomaterials 27:1387–1398
Ahmad A, Mukherjee P, Senapati S, Mandal D, Islam Khan M, Kumar R, Sastry M (2003) Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloid Surf B 28:313–318
Calvo P, Remunan Lopez C, Vila Jata JL, Alonso MJ (1997) Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J Appl Polym Sci 63:125–132
Katas H, Oya Alpar H (2006) Development and characterization of chitosan nanoparticles for SIRNA delivery. J Control Release 115:216–225
Hasanzadeh- Kafshgari M, Khorram M, Khodadoost M, Khavari S (2011) Reinforcement of chitosan nanoparticles obtained by an ionic cross-linking process. Iran Polym J 20(5):445–456
Lin KF, Hsu CY, Huang TS, Chiu WY, Lee YH, Young TH (2005) A novel method to prepare chitosan/montmorillonite nanocomposites. J Appl Polym Sci 98(5):2042–2047
Gan Q, Wang T, Cochrane T, Mccarron P (2005) Modulation of surface charge, particle size and morphological properties of chitosan–TPP nanoparticles intended for gen delivery. Colloid Surf B 44:65–73
Kavaz D, Çirak T, Özturk E, Bayram C, Denkba EB (2008) Preparation of magnetic chitosan nanoparticle for biomedical diverse applications. NATO Sci Peace Secur 2:313–320
Hashemi-Najafabadi S, Vasheghani-Farahani E, Shojaosadati SA, Rasaee MJ, Moin M, Pourpak Z (2006) Factorial design optimization of red blood cell PEGylation with a low molecular weight polymer. Iran Polym J 15(8):675–683
Bagheri-Khoulenjani S, Etrati-Khosroshahi M, Mirzadeh H (2010) Particle size and distribution modelling of nanohydroxyapatite-in-gelatin nanocomposite microspheres by surface response method. Iran Polym J 19(10):743–755
Bhumkar DR, Pokharkar VB (2006) Studies on effect of pH on cross-linking of chitosan with sodium tripolyphosphate: a technical note. AAPS PharmSciTechnol 7:e1–e6
Win KY, Feng SS (2005) Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials 26:2713–2722
Lin CR, Chiang RK, Wang JS, Sung TW (2006) Magnetic properties of mono disperse iron oxide nanoparticles. J Appl Phys 99:710–713
Ai H, Flask C, Weinberg B, Shuai X, Pagel MD, Farrell D, Duerk J, Gao J (2005) Magnetite-loaded polymeric micelles as ultrasensitive magnetic-resonance probes. Adv Mater 17:1949–1952
Lu J, Ma S, Sun J, Xia C, Liu C, Wang Z, Zhao X, Gao F, Gong Q, Song B, Shuai X, Ai H, Gu Z (2009) Manganese ferrite nanoparticle micellar nanocomposites as MRI contrast agent for liver imaging. Biomaterials 30:2919–2928
Nasongkla N, Bey E, Ren JM, Ai H, Khemtong C, Guthi JS, Chin SF, Sherry D, Boothman DA, Gao J (2006) Multifunctional polymeric micelles as cancer-targeted, MRI-ultrasensitive drug delivery systems. Nano Lett 6:2427–2430
Kim EH, Lee HS, Kwak BK, Kim BK (2005) Synthesis of ferrofluid with magnetic nanoparticles by sonochemical method for MRI contrast agent. J Magn Magn Mater 289:328–330
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohammad-Taheri, M., Vasheghani-Farahani, E., Hosseinkhani, H. et al. Fabrication and characterization of a new MRI contrast agent based on a magnetic dextran–spermine nanoparticle system. Iran Polym J 21, 239–251 (2012). https://doi.org/10.1007/s13726-012-0027-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-012-0027-0