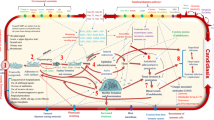
Abstract
Mucormycosis co-infection with COVID-19 patients has a poor prognosis for fatality. However, they do not have any therapeutic choices right now. Galangin is prone to both COVID-19 and Mucormycosis, according to an ongoing study; galangin was investigated as a prospective molecular mechanism against COVID-19 with Mucormycosis co-infection to determine its functional role and underlying mechanisms of action. In SARS-COV-2 and Mucormycosis co-infection, we have conducted a series of computational approaches to identify and describe the beneficial mechanism, and pharmacological targets with therapeutic strategies of galangin against this type of co-infections. COVID-19 and Mucormycosis were characterized by pathological mechanisms, essential signaling pathways, and potential therapeutic intervention. Protein–protein interaction (PPI) was constructed for 57 common gene targets associated with co-infection. The pharmacological options were TNF (− 7.4 kcal/mol), CASP3 (− 7.3 kcal/mol), and MMP9 (− 7.7 kcal/mol) were proposed potential therapeutic targets validated through molecular docking and molecular dynamics simulation studies. Signaling pathways, molecular properties, and upstream pathway activity were also revealed in COVID-19 and Mucormycosis, along with galangin. However, treatment with galangin indicated that it could inhibit several cytokines, including TNF-α, TGF-β1, and IL-6 release which reduces inflammation and tissue injury through IL-17, HIF-1α, TGF-β, and cytokine-mediated signaling pathways. The most significant transcription factor (TF) E2F1 and miRNA hsa-miR-16-5p were identified through gene regulatory network analysis to serve as diagnostic biomarkers. However, galangin may be a possible therapeutic medication for the treatment of COVID-19 and Mucormycosis.











Similar content being viewed by others
Data Availability
The datasets supporting the conclusions of this study are included within the article and supplementary file. This data may be made available from the authors upon reasonable request.
References
Adhikari S, Gautam AR, Paudyal B, Sigdel KR, Basnyat B (2019) Case report: gastric mucormycosis-a rare but important differential diagnosis of upper gastrointestinal bleeding in an area of Helicobacter pylori endemicity. Wellcome Open Res. https://doi.org/10.12688/wellcomeopenres.15026.2
Alekseyev K, Didenko L, Chaudhry B (2021) Rhinocerebral mucormycosis and COVID-19 pneumonia. J Med Cases 12(3):85–89. https://doi.org/10.14740/jmc3637
Ansó E, Zuazo A, Irigoyen M, Urdaci MC, Rouzaut A, Martínez-Irujo JJ (2010) Flavonoids inhibit hypoxia-induced vascular endothelial growth factor expression by a HIF-1 independent mechanism. Biochem Pharmacol 79(11):1600–1609. https://doi.org/10.1016/j.bcp.2010.02.004
Antony FC, Geetha A, Narayanan DC (2013) Effect of galangin on ethanol and cerulein induced inflammatory changes in pancreas: a biochemical study in rat model. Int J Pharm Pharm Sci 9(10):13
Arefin A, Ema TI, Islam T, Hossen MS, Islam T, Al Azad S, Badal MNU, Islam MA, Biswas P, Alam NU (2021) Target specificity of selective bioactive compounds in blocking α-dystroglycan receptor to suppress Lassa virus infection: an in-silico approach. J Biomed Res 35(6):459. https://doi.org/10.7555/JBR.35.20210111
Arunachalam PS, Wimmers F, Mok CKP, Perera RA, Scott M, Hagan T, Pulendran B (2020) Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science 369(6508):1210–1220. https://doi.org/10.1126/science.abc6261
Bera K, Reeda VSJ, Babila PR, Dinesh DC, Hritz J, Karthick T (2021) An in silico molecular dynamics simulation study on the inhibitors of SARS-CoV-2 proteases (3CLpro and PLpro) to combat COVID-19. Mol Simul 47(14):1168–1184. https://doi.org/10.1080/08927022.2021.1957884
Berendsen HJC, Postma JPM, van Gunsteren WF, Hermans J (1981) Interaction models for water in relation to protein hydration. In: Intermolecular forces: Proceedings of the Fourteenth Jerusalem Symposium on quantum chemistry and biochemistry held in Jerusalem, Israel, April 13–16, 1981. Springer Netherlands, pp 331–342
Bhatia M (2022) The rise of mucormycosis in Covid-19 patients in India. Expert Rev Anti Infect Ther 20(2):137–138. https://doi.org/10.1080/14787210.2021.1960822
Biswas S, Haleyurgirisetty M, Lee S, Hewlett I, Devadas K (2019) Development and validation of plasma miRNA biomarker signature panel for the detection of early HIV-1 infection. EBioMedicine 43:307–316. https://doi.org/10.1016/j.ebiom.2019.04.023
Biswas P, Hasan MM, Dey D, dos Santos Costa AC, Polash SA, Bibi S, Ferdous N, Kaium MA, Rahman MH, Jeet FK, Papadakos S, Islam K, Uddin MS (2021) Candidate antiviral drugs for COVID-19 and their environmental implications: a comprehensive analysis. Environ Sci Pollut Res 28(42):59570–59593. https://doi.org/10.1007/S11356-021-16096-3
Biswas P, Hany Rumi O, Ahmed Khan D, Ahmed MN, Nahar N, Jahan R, Hasan Zilani MN, Paul TK, Hasan A, Bondhon TA (2022) Evaluation of melongosides as potential inhibitors of NS2B-NS3 activator-protease of dengue virus (Serotype 2) by using molecular docking and dynamics simulation approach. J Trop Med. https://doi.org/10.1155/2022/7111786
Bjelkmar P, Larsson P, Cuendet MA., Hess B, Lindahl E (2010) Implementation of the CHARMM force field in GROMACS: analysis of protein stability effects from correction maps, virtual interaction sites, and water models. Journal of chemical theory and computation. 6(2):459–466. https://doi.org/10.1021/ct900549r
Bruno M, Dewi IM, Matzaraki V, Ter Horst R, Pekmezovic M, Rösler B, van de Veerdonk FL (2021) Comparative host transcriptome in response to pathogenic fungi identifies common and species-specific transcriptional antifungal host response pathways. Comput Struct Biotechnol J 19:647–663. https://doi.org/10.1016/j.csbj.2020.12.036
Bulat V, Situm M, Azdajic MD, Likic R (2021) Potential role of IL-17 blocking agents in the treatment of severe COVID-19? Br J Clin Pharmacol 87(3):1578. https://doi.org/10.1111/bcp.14437
Cai SQ, Zhang Q, Zhao XH, Shi J (2021) The in vitro anti-inflammatory activities of galangin and quercetin towards the LPS-injured rat intestinal epithelial (IEC-6) cells as affected by heat treatment. Molecules 26(24):7495. https://doi.org/10.3390/molecules26247495
Catanzaro M, Fagiani F, Racchi M, Corsini E, Govoni S, Lanni C (2020) Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct Target Ther 5(1):84. https://doi.org/10.1038/s41392-020-0191-1
Chavda VP, Apostolopoulos V (2021) Mucormycosis—an opportunistic infection in the aged immunocompromised individual: a reason for concern in COVID-19. Maturitas 154:58–61. https://doi.org/10.1016/j.maturitas.2021.07.009
Chen K, Xue R, Geng Y, Zhang S (2022) Galangin inhibited ferroptosis through activation of the PI3K/AKT pathway in vitro and in vivo. FASEB J. https://doi.org/10.1096/FJ.202200935R
Cheng F, Li W, Zhou Y, Shen J, Wu Z, Liu G, Lee P (2012) admetSAR: a comprehensive source and free tool for assessment of chemical ADMET properties. J Chem Inf Model 52(11):3099–3105. https://doi.org/10.1021/ci300367a
Chin CH, Chen SH, Wu HH, Ho CW, Ko MT, Lin CY (2014) cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst Biol. https://doi.org/10.1186/1752-0509-8-S4-S11
Chulada PC, Thompson MB, Mahler JF, Doyle CM, Gaul BW, Lee C, Langenbach R (2000) Genetic disruption of Ptgs-1, as well as of Ptgs-2, reduces intestinal tumorigenesis in Min mice. Can Res 60(17):4705–4708
Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SC, Dannaoui E, Hochhegger B, Chakrabarti A (2019) Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis 19(12):e405–e421. https://doi.org/10.1016/S1473-3099(19)30312-3
Daina A, Michielin O, Zoete V (2017) SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep 7(1):42717. https://doi.org/10.1038/srep42717
Design LIGAND (2014) Pharmacophore and ligand-based design with Biovia Discovery Studio®. BIOVIA. California. www.3ds.com. Accessed 24 Dec 2022
Dey D, Hasan MM, Biswas P, Papadakos SP, Rayan RA, Tasnim S, Kim B (2022) Investigating the anticancer potential of salvicine as a modulator of topoisomerase II and ROS signaling cascade. Front Oncol. https://doi.org/10.3389/fonc.2022.899009
do Monte Junior ES, Dos Santos MEL, Ribeiro IB, de Oliveira Luz G, Baba ER, Hirsch BS, De Moura EGH (2020) Rare and fatal gastrointestinal mucormycosis (Zygomycosis) in a COVID-19 patient: a case report. Clinical Endoscopy 53(6):746–749. https://doi.org/10.5946/ce.2020.180
Dos Santos AR, Fraga-Silva TF, Almeida DDF, Dos Santos RF, Finato AC, Amorim BC, Venturini J (2020) Rhizopus-host interplay of disseminated mucormycosis in immunocompetent mice. Future Microbiol 15(9):739–752. https://doi.org/10.2217/FMB-2019-0246
Eberhardt J, Santos-Martins D, Tillack AF, Forli S (2021) AutoDock Vina 12..0: New docking methods, expanded force field, and python bindings. J Chem Inform Model 61(8):3891–3898. https://doi.org/10.1021/ACS.JCIM.1C00203
Fang L, Liu J, Ju S, Zheng F, Dong W, Shen M (2010) Experimental and theoretical evidence of enhanced ferromagnetism in sonochemical synthesized BiFeO3 nanoparticles. Appl Phys Lett 97(24):242501. https://doi.org/10.1063/1.3525573
Fasolo A, Sessa C (2012) Targeting mTOR pathways in human malignancies. Curr Pharm Des 18(19):2766–2777. https://doi.org/10.2174/138161212800626210
Feuillet V, Canard B, Trautmann A (2021) Combining antivirals and immunomodulators to fight COVID-19. Trends Immunol 42(1):31–44. https://doi.org/10.1016/j.it.2020.11.003
Flament H, Rouland M, Beaudoin L, Toubal A, Bertrand L, Lebourgeois S, Lehuen A (2021) Outcome of SARS-CoV-2 infection is linked to MAIT cell activation and cytotoxicity. Nat Immunol 22(3):322–335. https://doi.org/10.1038/s41590-021-00870-z
Fornes O, Castro-Mondragon JA, Khan A, Van der Lee R, Zhang X, Richmond PA, Mathelier A (2020) JASPAR 2020: update of the open-access database of transcription factor binding profiles. Nucleic Acids Res 48(D1):D87–D92. https://doi.org/10.1093/nar/gkz1001
Geng XQ, Ma A, He JZ, Wang L, Jia YL, Shao GY, Yang BX (2020) Ganoderic acid hinders renal fibrosis via suppressing the TGF-β/Smad and MAPK signaling pathways. Acta Pharmacol Sin 41(5):670–677. https://doi.org/10.1038/s41401-019-0324-7
Green SP, Chuntharapai A, Curnutte JT (1996) Interleukin-8 (IL-8), melanoma growth-stimulatory activity, and neutrophil-activating peptide selectively mediate priming of the neutrophil NADPH oxidase through the type A or type B IL-8 receptor. J Biol Chem 271(41):25400–25405. https://doi.org/10.1074/jbc.271.41.25400
Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-Pdb Viewer: an environment for comparative protein modeling. Electrophoresis 18(15):2714–2723. https://doi.org/10.1002/elps.1150181505
Guihur A, Rebeaud ME, Fauvet B, Tiwari S, Weiss YG, Goloubinoff P (2020) Moderate fever cycles as a potential mechanism to protect the respiratory system in COVID-19 patients. Front Med 7:564170. https://doi.org/10.3389/fmed.2020.564170
Hashem H (2020) In silico approach of some selected honey constituents as SARS-CoV-2 main protease (COVID-19) inhibitors. Eurasian J Med Oncol. https://doi.org/10.26434/chemrxiv.12115359.v2
Hewage SRKM, Piao MJ, Kang KA, Ryu YS, Fernando PMDJ, Oh MC, Hyun JW (2017) Galangin activates the ERK/AKT-driven Nrf2 signaling pathway to increase the level of reduced glutathione in human keratinocytes. Biomol Therap 25(4):427. https://doi.org/10.4062/biomolther.2016.112
Hsu SD, Lin FM, Wu WY, Liang C, Huang WC, Chan WL, Huang HD (2011) miRTarBase: a database curates experimentally validated microRNA–target interactions. Nucleic Acids Res 39(suppl_1):D163–D169. https://doi.org/10.1093/nar/gkq1107
Huang YT, Lee LT, Lee PPH, Lin YS, Lee MT (2005) Targeting of focal adhesion kinase by flavonoids and small-interfering RNAs reduces tumor cell migration ability. Anticancer Res 25(3B):2017–2025
Islam MA, Zilani MNH, Biswas P, Khan DA, Rahman MH, Nahid R, Hasan MN (2022) Evaluation of in vitro and in silico anti-inflammatory potential of some selected medicinal plants of Bangladesh against cyclooxygenase-II enzyme. J Ethnopharmacol 285:114900. https://doi.org/10.1016/j.jep.2021.114900
Ji X, Li Z (2020) Medicinal chemistry strategies toward host targeting antiviral agents. Med Res Rev 40(5):1519–1557. https://doi.org/10.1002/med.21664
Jia HL, He CH, Wang ZY, Xu YF, Yin GQ, Mao LJ, Deng L (2014) MicroRNA expression profile in exosome discriminates extremely severe infections from mild infections for hand, foot and mouth disease. BMC Infect Dis 14:1–9. https://doi.org/10.1186/1471-2334-14-506
Kadam RU, Wilson IA (2017) Structural basis of influenza virus fusion inhibition by the antiviral drug Arbidol. Proc Natl Acad Sci 114(2):206–214. https://doi.org/10.1073/pnas.1617020114
Ketprasit N, Cheng IS, Deutsch F, Tran N, Imwong M, Combes V, Palasuwan D (2020) The characterization of extracellular vesicles-derived microRNAs in Thai malaria patients. Malar J 19(1):1–14. https://doi.org/10.1186/S12936-020-03360-Z
Khokhar M, Tomo S, Purohit P (2021) Micro RNA-based regulation of genomics and transcriptomics of inflammatory cytokines in COVID-19. medRxiv. https://doi.org/10.1101/2021.06.08.21258565
Kim HH, Bae Y, Kim SH (2013) Galangin attenuates mast cell-mediated allergic inflammation. Food Chem Toxicol 57:209–216. https://doi.org/10.1016/j.fct.2013.03.015
Kim S, Thiessen PA, Bolton EE, Chen J, Fu G, Gindulyte A, Bryant SH (2016) PubChem substance and compound databases. Nucleic Acids Res 44(D1):D1202–D1213. https://doi.org/10.1093/nar/gkv951
Kim WR, Park EG, Kang KW, Lee SM, Kim B, Kim HS (2020) Expression analyses of microRNAs in hamster lung tissues infected by SARS-CoV-2. Mol Cells 43(11):953. https://doi.org/10.14348/molcells.2020.0177
Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD (1997) The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275(5303):1132–1136. https://doi.org/10.1126/SCIENCE.275.5303.1132
Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, Ma’ayan A (2016) Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res 44(W1):W90–W97. https://doi.org/10.1093/nar/gkw377
Kumar M, Sarma DK, Shubham S, Kumawat M, Verma V, Singh B, Tiwari RR (2021) Mucormycosis in COVID-19 pandemic: Risk factors and linkages. Curr Res Microb Sci 2:100057. https://doi.org/10.1016/j.crmicr.2021.100057
Laplante M, Sabatini DM (2009) mTOR signaling at a glance. J Cell Sci 122(20):3589–3594. https://doi.org/10.1242/jcs.051011
Leng L, Cao R, Ma J, Mou D, Zhu Y, Li W, Zhong W (2020) Pathological features of COVID-19-associated lung injury: a preliminary proteomics report based on clinical samples. Signal Transduct Target Ther 5(1):240. https://doi.org/10.1038/s41392-020-00355-9
Li C, Yang P, Sun Y, Li T, Wang C, Wang Z, Jiang C (2012) IL-17 response mediates acute lung injury induced by the 2009 pandemic influenza A (H1N1) virus. Cell Res 22(3):528–538. https://doi.org/10.1038/cr.2011.165
Li Y, Wan Y, Yu N, Zhao Y, Li M (2022) Galangin (GLN) promotes temozolomide-induced apoptosis in glioma cells. Biol Bull 49(6):580–587. https://doi.org/10.1134/S1062359022060085
Loganathan T, Ramachandran S, Shankaran P, Nagarajan D (2020) Host transcriptome-guided drug repurposing for COVID-19 treatment: a meta-analysis-based approach. PeerJ 8:e9357. https://doi.org/10.7717/peerj.9357
Ma A, Zhang L, Ye X, Chen J, Yu J, Zhuang L, Yu X (2021) High levels of circulating IL-8 and soluble IL-2R are associated with prolonged illness in patients with severe COVID-19. Front Immunol 12:626235. https://doi.org/10.3389/fimmu.2021.626235
Magro CM, Mulvey JJ, Laurence J, Sanders S, Crowson AN, Grossman M, Harp J, Nuovo G (2021) The differing pathophysiologies that underlie COVID-19-associated perniosis and thrombotic retiform purpura: a case series. Br J Dermatol 184(1):141–150. https://doi.org/10.1111/bjd.19415
Martínez L (2015) Automatic identification of mobile and rigid substructures in molecular dynamics simulations and fractional structural fluctuation analysis. PLoS ONE 10(3):e0119264. https://doi.org/10.1371/journal.pone.0119264
Mason RJ, Broaddus VC, Martin TR, King TE, Schraufnagel D, Murray JF, Nadel JA (2010) Murray and Nadel’s textbook of respiratory medicine E-book: 2-volume set. Elsevier Health Sciences, Berlin
Mendelsohn J, Baselga J (2003) Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol 21(14):2787–2799
Meyer JJM, Afolayan AJ, Taylor MB, Erasmus D (1997) Antiviral activity of galangin isolated from the aerial parts of Helichrysum aureonitens. J Ethnopharmacol 56(2):165–169. https://doi.org/10.1016/S0378-8741(97)01514-6
Mican JAM, Arora N, Burd PR, Metcalfe DD (1992) Passive cutaneous anaphylaxis in mouse skin is associated with local accumulation of interleukin-6 mRNA and immunoreactive interleukin-6 protein. J Allergy Clin Immunol 90(5):815–824. https://doi.org/10.1016/0091-6749(92)90107-D
Niklaus MC (2017) Online SMILES Translator and Structure File Generator. National cancer institute NCI/CADD group (Accessed 12 May 2023).
Nygaard V, Løland A, Holden M, Langaas M, Rue H, Liu F, Smith-Sørensen B (2003) Effects of mRNA amplification on gene expression ratios in cDNA experiments estimated by analysis of variance. BMC Genom 4(1):1–13. https://doi.org/10.1186/1471-2164-4-11
O’Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR (2011) Open babel: an open chemical toolbox. J Cheminform 3(1):1–14. https://doi.org/10.1186/1758-2946-3-33
Oladele, JO, Oyeleke OM, Oladele OT, Olowookere BD, Oso BJ, Oladiji AT (2020) Kolaviron (Kolaflavanone), apigenin, fisetin as potential Coronavirus inhibitors: in silico investigation. https://doi.org/10.21203/rs.3.rs-51350/v1
Papaiahgari S, Zhang Q, Kleeberger SR, Cho HY, Reddy SP (2006) Hyperoxia stimulates an Nrf2-ARE transcriptional response via ROS-EGFR-PI3K-Akt/ERK MAP kinase signaling in pulmonary epithelial cells. Antioxid Redox Signal 8(1–2):43–52. https://doi.org/10.1089/ars.2006.8.43
Park SS, Bae I, Lee YJ (2008) Flavonoids-induced accumulation of hypoxia-inducible factor (HIF)-1α/2α is mediated through chelation of iron. J Cell Biochem 103(6):1989–1998. https://doi.org/10.1002/jcb.21588
Parrinello M, Rahman A (1981) Polymorphic transitions in single crystals: a new molecular dynamics method. J Appl Phys 52(12):7182–7190. https://doi.org/10.1063/1.328693
Pease JE (2011) Targeting chemokine receptors in allergic disease. Biochemical Journal 434(1):11–24. https://doi.org/10.1042/BJ20101132
Pires DE, Blundell TL, Ascher DB (2015) pkCSM: predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J Med Chem 58(9):4066–4072. https://doi.org/10.1021/acs.jmedchem.5b00104
Pongas GN, Lewis RE, Samonis G, Kontoyiannis DP (2009) Voriconazole-associated zygomycosis: a significant consequence of evolving antifungal prophylaxis and immunosuppression practices? Clin Microbiol Infect 15:93–97. https://doi.org/10.1111/j.1469-0691.2009.02988.x
Prasad K, Khatoon F, Rashid S, Ali N, AlAsmari AF, Ahmed MZ, Kumar V (2020) Targeting hub genes and pathways of innate immune response in COVID-19: a network biology perspective. Int J Biol Macromol 163:1–8. https://doi.org/10.1016/j.ijbiomac.2020.06.228
Rahman MS, Zilani MNH, Islam MA, Hasan MM, Islam MM, Yasmin F, Kim B (2021) In vivo Neuropharmacological potential of Gomphandra tetrandra (Wall.) sleumer and in-silico study against β-amyloid precursor protein. Processes 9(8):1449. https://doi.org/10.3390/pr9081449
Ramaiah MJ (2020) mTOR inhibition and p53 activation, microRNAs: The possible therapy against pandemic COVID-19. Gene Rep 20:100765. https://doi.org/10.1016/j.genrep.2020.100765
Riffe T, Acosta E (2021) Data resource profile: COVerAGE-DB: a global demographic database of COVID-19 cases and deaths. Int J Epidemiol 50(2):390–390f. https://doi.org/10.1093/ije/dyab027
Robinson PC, Liew DF, Liew JW, Monaco C, Richards D, Shivakumar S, Feldmann M (2020) The potential for repurposing anti-TNF as a therapy for the treatment of COVID-19. Med 1(1):90–102. https://doi.org/10.1016/j.medj.2020.11.005
Rydenfelt M, Klinger B, Klünemann M, Blüthgen N (2020) SPEED2: inferring upstream pathway activity from differential gene expression. Nucleic Acids Res 48(W1):W307–W312. https://doi.org/10.1093/nar/gkaa236
Sang ER, Tian Y, Miller LC, Sang Y (2021) Epigenetic evolution of ACE2 and IL-6 genes: non-canonical interferon-stimulated genes correlate to COVID-19 susceptibility in vertebrates. Genes 12(2):154. https://doi.org/10.3390/genes12020154
Saraste J, Prydz K (2021) Assembly and cellular exit of coronaviruses: Hijacking an unconventional secretory pathway from the pre-Golgi intermediate compartment via the Golgi ribbon to the extracellular space. Cells 10(3):503. https://doi.org/10.3390/cells10030503
Sarchio SN, Kok LF, O’Sullivan C, Halliday GM, Byrne SN (2012) Dermal mast cells affect the development of sunlight-induced skin tumours. Exp Dermatol 21(4):241–248. https://doi.org/10.1111/j.1600-0625.2012.01438.x
Schmid N, Eichenberger AP, Choutko A, Riniker S, Winger M, Mark AE, Van Gunsteren WF (2011) Definition and testing of the GROMOS force-field versions 54A7 and 54B7. Eur Biophys J 40:843–856. https://doi.org/10.1007/S00249-011-0700-9
Schnitzler P, Neuner A, Nolkemper S, Zundel C, Nowack H, Sensch KH, Reichling J (2010) Antiviral activity and mode of action of propolis extracts and selected compounds. Phytother Res 24(S1):S20–S28. https://doi.org/10.1002/ptr.2868
Schüttelkopf AW, Van Aalten DM (2004) PRODRG: a tool for high-throughput crystallography of protein–ligand complexes. Acta Crystallogr D Biol Crystallogr 60(8):1355–1363. https://doi.org/10.1107/S0907444904011679
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Ideker T (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13(11):2498–2504. https://doi.org/10.1101/gr.1239303
Sharma S, Grover M, Bhargava S, Samdani S, Kataria T (2021) Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J Laryngol Otol 135(5):442–447. https://doi.org/10.1017/S0022215121000992
Singh AK, Singh R, Joshi SR, Misra A (2021) Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in India. Diabetes Metab Syndr: Clin Res Rev 15(4):102146. https://doi.org/10.1016/j.dsx.2021.05.019
Sivakumar AS, Anuradha CV (2011) Effect of galangin supplementation on oxidative damage and inflammatory changes in fructose-fed rat liver. Chem Biol Interact 193(2):141–148. https://doi.org/10.1016/j.cbi.2011.06.003
Sivashanmugam K, Kandasamy M, Subbiah R, Ravikumar V (2021) Repurposing of histone deacetylase inhibitors: A promising strategy to combat pulmonary fibrosis promoted by TGF-β signalling in COVID-19 survivors. Life Sci 266:118883. https://doi.org/10.1016/j.lfs.2020.118883
Sohel M, Hossain M, Hasan M, Haque A, Islam MS, Hossain MS, Emran TB (2021) Management of mental health during COVID 19 pandemic: possible strategies. J Adv Biotechnol Exp Therap 4(3):276–289. https://doi.org/10.5455/jabet.2021.d128
Sun WK, Lu X, Li X, Sun QY, Su X, Song Y, Shi Y (2012) Dectin-1 is inducible and plays a crucial role in Aspergillus-induced innate immune responses in human bronchial epithelial cells. Eur J Clin Microbiol Infect Dis 31:2755–2764. https://doi.org/10.1007/s10096-012-1624-8
Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Mering CV (2019) STRING v11: protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 47(D1):D607–D613. https://doi.org/10.1093/nar/gky1131
Tian W, Chen C, Lei X, Zhao J, Liang J (2018) CASTp 3.0: computed atlas of surface topography of proteins. Nucleic Acids Res 46(W1):W363–W367. https://doi.org/10.1093/nar/gky473
Toh S, Holbrook-Smith D, Stogios PJ, Onopriyenko O, Lumba S, Tsuchiya Y, McCourt P (2015) Structure-function analysis identifies highly sensitive strigolactone receptors in Striga. Science 350(6257):203–207. https://doi.org/10.1126/science.aac9476
Tufan A, Güler AA, Matucci-Cerinic M (2020) COVID-19, immune system response, hyperinflammation and repurposingantirheumatic drugs. Turk J Med Sci 50(9):620–632. https://doi.org/10.3906/sag-2004-168
Vagapova ER, Lebedev TD, Prassolov VS (2021) Viral fibrotic scoring and drug screen based on MAPK activity uncovers EGFR as a key regulator of COVID-19 fibrosis. Sci Rep 11(1):11234. https://doi.org/10.1038/s41598-021-90701-w
Vandenabeele P, Declercq W, Van Herreweghe F, Vanden Berghe T (2010) The role of the kinases RIP1 and RIP3 in TNF-induced necrosis. Sci Signal 3(115):re4. https://doi.org/10.1126/scisignal.3115re4
Verma A, Wüthrich M, Deepe G, Klein B (2015) Adaptive immunity to fungi. Cold Spring Harbor Perspect Med 5(3):a019612. https://doi.org/10.1101/cshperspect.a019612
Walczak H (2011) TNF and ubiquitin at the crossroads of gene activation, cell death, inflammation, and cancer. Immunol Rev 244(1):9–28. https://doi.org/10.1111/j.1600-065X.2011.01066.x
Wang HX, Tang C (2017) Galangin suppresses human laryngeal carcinoma via modulation of caspase-3 and AKT signaling pathways. Oncol Rep 38(2):703–714. https://doi.org/10.3892/or.2017.5767
Wang S, Griffiths G, Midgley CA, Barnett AL, Cooper M, Grabarek J, Fischer PM (2010) Discovery and characterization of 2-anilino-4-(thiazol-5-yl) pyrimidine transcriptional CDK inhibitors as anticancer agents. Chem Biol 17(10):1111–1121. https://doi.org/10.1016/j.chembiol.2010.07.016
Wang D, Zhao J, Li S, Wei J, Nan L, Mallampalli RK, Zhao Y (2018) Phosphorylated E2F1 is stabilized by nuclear USP11 to drive Peg10 gene expression and activate lung epithelial cells. J Mol Cell Biol 10(1):60–73. https://doi.org/10.1093/jmcb/mjx034
Wang B, Ding Y, Zhao P, Li W, Li M, Zhu J, Ye S (2022) Systems pharmacology-based drug discovery and active mechanism of natural products for coronavirus pneumonia (COVID-19): An example using flavonoids. Comput Biol Med 143:105241. https://doi.org/10.1016/j.compbiomed.2022.105241
Watkins TN, Gebremariam T, Swidergall M, Shetty AC, Graf KT, Alqarihi A, Bruno VM (2018) Inhibition of EGFR signaling protects from mucormycosis. Mbio 9(4):e01384-e1418. https://doi.org/10.1128/mbio.01384-18
Werth N, Beerlage C, Rosenberger C, Yazdi AS, Edelmann M, Amr A, Kempf VA (2010) Activation of hypoxia inducible factor 1 is a general phenomenon in infections with human pathogens. PLoS ONE 5(7):e11576. https://doi.org/10.1371/journal.pone.0011576
Woo MS, Park JS, Choi IY, Kim WK, Kim HS (2008) Inhibition of MMP-3 or-9 suppresses lipopolysaccharide-induced expression of proinflammatory cytokines and iNOS in microglia. J Neurochem 106(2):770–780. https://doi.org/10.1111/j.1471-4159.2008.05430.x
World Health Organization (2021) WHO Coronavirus (COVID-19) Dashboard Coronavirus (COVID-19) Dashboard [Internet]. [consultado 25 Sep 2021].
Wurster S, Thielen V, Weis P, Walther P, Elias J, Waaga-Gasser AM, Ullmann AJ (2017) Mucorales spores induce a proinflammatory cytokine response in human mononuclear phagocytes and harbor no rodlet hydrophobins. Virulence 8(8):1708–1718. https://doi.org/10.1080/21505594.2017.1342920
Yang Z, Jiang S, Cheng Y, Li T, Hu W, Ma Z, Yang Y (2017) FOXC1 in cancer development and therapy: deciphering its emerging and divergent roles. Therap Adv Med Oncol 9(12):797–816. https://doi.org/10.1177/1758834017742576
Yang CC, Hsiao LD, Yang CM (2020) Galangin inhibits LPS-induced MMP-9 expression via suppressing protein kinase-dependent AP-1 and FoxO1 activation in rat brain astrocytes. J Inflamm Res. https://doi.org/10.2147/JIR.S276925
Ye EA, Liu L, Jiang Y, Jan J, Gaddipati S, Suvas S, Steinle JJ (2016) miR-15a/16 reduces retinal leukostasis through decreased pro-inflammatory signaling. J Neuroinflamm 13(1):1–9. https://doi.org/10.1186/s12974-016-0771-8
Zahra S (2020) Study of expression changes of CotH, GRP78 and their targetting microRNAs (miR-335–5p, miR-16-5p, miR-93-3p) genes in human macrophages and diabetic mouse exposed to Rhisopus oryzae. Doctoral dissertation, Mazandaran university of medical sciences
Zheng Y, Li R, Liu S (2020) Immunoregulation with mTOR inhibitors to prevent COVID-19 severity: a novel intervention strategy beyond vaccines and specific antiviral medicines. J Med Virol 92(9):1495–1500. https://doi.org/10.1002/jmv.26009
Zhu L, Luo Q, Bi J, Ding J, Ge S, Chen F (2014) Galangin inhibits growth of human head and neck squamous carcinoma cells in vitro and in vivo. Chem Biol Interact 224:149–156. https://doi.org/10.1016/j.cbi.2014.10.027
Zou WW, Xu SP (2018) Galangin inhibits the cell progression and induces cell apoptosis through activating PTEN and Caspase-3 pathways in retinoblastoma. Biomed Pharmacother 97:851–863. https://doi.org/10.1016/j.biopha.2017.09.144
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hasan, M.I., Hossain, M.A., Rahman, M.H. et al. Galangin for COVID-19 and Mucormycosis co-infection: a potential therapeutic strategy of targeting critical host signal pathways triggered by SARS-CoV-2 and Mucormycosis. Netw Model Anal Health Inform Bioinforma 12, 26 (2023). https://doi.org/10.1007/s13721-023-00421-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13721-023-00421-6