Abstract
Sorafenib (Nexavar®, BAY 43-9006) is a novel multikinase inhibitor that has been recently approved for the treatment of advanced hepatocellular carcinoma (HCC). The increased use of sorafenib has been accompanied by an increase in the number of reports on the adverse effects of this drug. Generally, the adverse effects of sorafenib are well tolerated, and the most common drug-related toxicities are hand-foot skin reaction, diarrhea, fatigue, rash, and hypertension. Here, we report the case of a 54-year-old man who developed small bowel perforation 2 months after receiving sorafenib for advanced HCC. The exact pathogenesis of the perforation is unclear. The cause of small bowel perforation in this patient was most likely metastatic tumor necrosis due to sorafenib. From this case, we can infer that treatment with antiangiogenic multikinase inhibitors may be associated with gastrointestinal (GI) perforations. Physicians should keep the possibility of this rare but potentially serious GI complication related to sorafenib treatment in mind.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sorafenib (Nexavar®, BAY 43-9006) is an oral multikinase inhibitor that targets vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and Raf receptor tyrosine kinase signaling; it is the first and only approved drug that prolongs median survival and time to progression by nearly 3 months in patients with advanced hepatocellular carcinoma (HCC) [1]. Patients receiving sorafenib have been reported to develop adverse effects that are gastrointestinal (GI), dermatologic, and constitutional in nature and generally well tolerated. However, a few recent studies have reported the potential risk of GI perforation in patients receiving sorafenib; this has increased awareness about this adverse effect of sorafenib [2–5].
Bowel perforation related to antiangiogenic chemotherapeutic agents has been previously reported, particularly for the VEGF receptor (VEGFR) inhibitor bevacizumab; the action mechanisms of bevacizumab are similar to those of sorafenib [6]. Previous studies have reported that the incidence of GI perforation due to bevacizumab is 0.3–5.4 % [7, 8]. A meta-analysis showed that the mortality among patients with bevacizumab-associated GI perforation was 21.7 % [8].
GI perforation is a rare but serious and potentially life-threatening complication of sorafenib therapy. Immediate diagnosis followed by surgical exploration is required. Here, we report, for the first time, a case of small bowel (SB) perforation caused by metastatic tumor necrosis after sorafenib therapy for HCC.
Case report
A 54-year-old man with a history of recurrent HCC with metastasis to the lung was transferred to our hospital for chemotherapy. One year before admission, he was diagnosed with HCC and portal vein invasion and underwent left liver lobectomy. At admission, the patient was in good health (Eastern Cooperative Oncology Group performance status 0) and had a history of hepatitis B virus-related liver cirrhosis (Child–Pugh class A). He was administered sorafenib alone (400 mg twice daily). The chemotherapy was tolerated for 1 month although the patient developed grade 1 hand-foot skin reaction, fatigue, and hypertension. Two months after the start of sorafenib treatment, he presented to the emergency department with acute onset of abdominal pain and distension. Physical examination revealed diffuse abdominal tenderness. His blood pressure was 100/70 mmHg, and his body temperature was 38 °C. Laboratory studies showed leukocytosis (1,100 cells/μL, 78 % neutrophils) and elevated serum level of C-reactive protein (8.76 mg/dL; normal level less than 0.5 mg/dL). Computed tomography revealed pneumoperitoneum, dilated SB loop, and fluid accumulation, which were suggestive of bowel perforation. He underwent emergent surgical exploration, which revealed a 0.5-cm-wide distinct perforation of the SB with ulceration. The perforation was detected on the jejunum 50 cm from the ligament of Treitz. Wedge resection of the perforated bowel was performed.
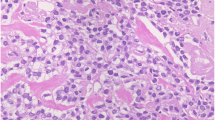
On the basis of the macroscopic pathological findings, we resected a 3-cm-long jejunal segment. The mucosa showed ulceration in a 0.5-cm-wide area communicating with the perforation (Fig. 1). On histopathologic examination, a neoplastic infiltration involving the full thickness of the bowel wall was noted at the site of perforation. Tumor cells had invaded the subserosa (Fig. 2). The patient did not develop any postoperative complications and was discharged on the 12th postoperative day in a stable condition. Sorafenib treatment was discontinued because of radiological and clinical progression of the disease. The patient died on the 34th postoperative day because of septic pneumonia.
Discussion
To the best of our knowledge, this is the first report on SB perforation after sorafenib administration for advanced HCC. Since no other relevant causes were detected in this case, the SB perforation was deemed to have a causal relation with the administered treatment.
Sorafenib acts by inhibiting the serine/threonine kinase Raf-1 and the receptor tyrosine kinase activity of VEGFRs 1, 2, and 3 and PDGF receptor β (PDGFR-β) [9]. The Raf/MEK/ERK pathway plays a major role in tumor cell proliferation and apoptosis, and the VEGFR/PDGFR signaling pathway is involved in angiogenesis and metastasis [9]. VEGF is considered one of the most important mediators of angiogenesis and is a new emerging target for cancer treatment. The blocking of this pathway may cause an array of adverse effects due to the impairment of the angiogenic process. Hence, anti-VEGF/VEGFR agents have overlapping toxicities [7, 9]. Bevacizumab is a humanized monoclonal antibody against VEGFR. The association between bevacizumab therapy and GI perforation has been clearly demonstrated; a recent review of literature revealed that the reported incidence of bevacizumab-associated GI perforation is in the range of 0.3–5.4 % [7, 8]. On the other hand, the incidence of GI perforation with sorafenib has not been clearly established, since only a few cases have been reported [2–5]. Since GI perforations can have potentially severe outcomes, it would be useful to identify risk factors for predicting patients likely to develop GI perforation and elucidate the underlying biological mechanisms.
The mode of spread in HCC is direct invasion from a contiguous HCC (66.7 %), hematogenous metastasis (16.7 %), and peritoneal seeding (0.4 %) [10]. The major mode of metastasis is direct invasion to the contiguous GI tract via adhesion to the serosal side by a bulky, exophytic tumor. Hematogenous spread of HCC to the GI tract may be caused by tumor thrombi disseminated by the hepatofugal portal blood flow via the portal system to the GI tract [10]. In the present case, the presumed mode of metastasis to the SB was hematogenous spread because the metastatic site was not adjacent to the HCC and the lymph nodes were not involved.
Several mechanisms for bowel perforations associated with bevacizumab treatment have been proposed. However, it remains to be clarified whether the same risk factors and mechanisms are involved in sorafenib-related perforation, although both agents appear to inhibit the VEGF signal pathway. The pathophysiologic mechanism of bowel perforation associated with antiangiogenic agents could be multifactorial. Firstly, antiangiogenic drugs have been postulated to promote tumor regression and necrosis by stabilizing the tumor vasculature and decreasing capillary permeability and interstitial pressure [11]. Tumor invasion of the entire bowel wall with subsequent necrosis can predispose patients to bowel perforation [11]. Secondly, antiangiogenic drugs might impair blood flow in the mesenteric vasculature through thrombosis or vasoconstriction, which is mediated by the inhibition of VEGF-mediated nitric oxide release [12]. Antiangiogenic drugs might also promote arterial thromboembolic disorders, including cholesterol emboli syndrome [13]. Lastly, antiangiogenic drugs may lead to poor wound healing, which can subsequently lead to perforations [14].
In the present case, factors that may have contributed to the perforation include the effect of sorafenib and bowel metastasis itself. Large tumors can outgrow their blood supply, resulting in necrosis and possible perforation [15]. Thus, SB perforation as a direct consequence of the changes in the tumor after sorafenib appears rather unlikely. None of the initial imaging studies found any evidence of bowel metastasis. There was also a time delay between the bowel perforation and the initiation of sorafenib treatment. Notably, our patient showed evidence of bowel metastasis in the resected segment involving the full thickness of the bowel wall. With the progression of disease, the tumor may have become more susceptible to the effects of sorafenib, eventually resulting in perforation at the sites of bowel metastasis [11]. The mechanism responsible for bowel perforation was, therefore, thought to be metastatic tumor necrosis caused by sorafenib.
Risk factors for GI perforations associated with antiangiogenic agents targeting VEGF are also poorly understood. Proposed predisposing factors for GI perforation are peptic ulcer disease, ileus, bowel metastasis, diverticulitis, prior radiation exposure, and colon surgery 2 months prior to initiation of chemotherapy [6, 7, 14]. Our patient had one identifiable risk factor, namely, bowel metastasis, although it was not initially detected. Patients with bowel metastasis receiving sorafenib may be at increased risk of GI perforation and should be carefully monitored for this complication. In addition, since the risk factors for GI perforation have not been fully established, even patients receiving sorafenib but not having any apparent risk factors should be closely monitored so that signs of bowel perforation are detected as early as possible.
Our review of the cases of bowel perforation due to sorafenib therapy (Table 1) indicated that the large bowel is the most common location of perforation. The risk of large bowel perforation appears to be high when sorafenib is used to treat renal cell carcinoma and melanoma. Although this is the first report of the complication in an HCC patient, higher rates of perforation can be expected with increased use in such patients. The potential risk factors identified in our review were prior radiotherapy and bowel metastasis, which were similar to the findings of previous studies [6, 7, 14]. We speculate that diverticulitis, prior radiotherapy, prior bowel surgery, and bowel obstruction may be other risk factors of perforation. Further investigations are necessary to identify patient groups at an increased risk for developing bowel perforation under sorafenib therapy. Among the previously reported cases, one was managed nonoperatively, which conflicts with the traditional view that GI perforation requires surgical exploration. Patients undergoing surgery during treatment with sorafenib could be at increased risk for wound healing complications following the operation. Scappaticci et al. [16] reported an increased rate of wound healing complications (13 %) in patients who underwent major surgery during treatment with bevacizumab. Therefore, caution is required when performing primary anastomosis in a patient with an antiangiogenic agent-related perforation. Since sorafenib therapy is commonly accompanied by diarrhea and vague GI symptoms, a high level of clinical suspicion may be required to quickly diagnose GI perforation [17]. These circumstances call for better reporting of potential toxicities, improved understanding of underlying mechanisms, more accurate prediction of high-risk patients, and the development of methods to mitigate toxicity.
In conclusion, sorafenib, which has been approved for the treatment of advanced HCC, might cause an unexpected serious GI perforation. Additional data are required to adequately assess the risk and pathogenesis of GI perforation associated with sorafenib.
References
Llovet JM, Ricci S, Mazzaferro V et al (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359(4):378–390. doi:10.1056/NEJMoa0708857
Eng FC, Easson AM, Szentgyorgyi E et al (2009) Sorafenib and surgical complications: a case report of adverse reaction to sorafenib during treatment for renal cell carcinoma. Eur J Surg Oncol 35(2):219–221. doi:10.1016/j.ejso.2007.09.009
Peters NA, Richel DJ, Verhoeff JJ et al (2008) Bowel perforation after radiotherapy in a patient receiving sorafenib. J Clin Oncol 26(14):2405–2406. doi:10.1200/JCO.2007.15.8451
Frieling T, Heise J, Wassilew SW (2009) Multiple colon ulcerations, perforation and death during treatment of malignant melanoma with sorafenib. Dtsch Med Wochenschr 134 (28–29):1464–1466, e1–2. doi:10.1055/s-0029-1225311
Walraven M, Witteveen PO, Lolkema MP et al (2011) Antiangiogenic tyrosine kinase inhibition related gastrointestinal perforations: a case report and literature review. Angiogenesis 14(2):135–141. doi:10.1007/s10456-010-9197-6
Hurwitz H, Fehrenbacher L, Novotny W et al (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350(23):2335–2342. doi:10.1056/NEJMoa032691350/23/2335
Hapani S, Chu D, Wu S (2009) Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: a meta-analysis. Lancet Oncol 10(6):559–568. doi:10.1016/S1470-2045(09)70112-3
Han ES, Monk BJ (2007) What is the risk of bowel perforation associated with bevacizumab therapy in ovarian cancer? Gynecol Oncol 105(1):3–6. doi:10.1016/j.ygyno.2007.01.038
Wilhelm SM, Carter C, Tang L et al (2004) BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 64(19):7099–7109. doi:10.1158/0008-5472.CAN-04-1443
Park MS, Kim KW, Yu JS et al (2002) Radiologic findings of gastrointestinal tract involvement in hepatocellular carcinoma. J Comput Assist Tomogr 26(1):95–101 pii: 0004728-200201000-00014
Gray J, Murren J, Sharma A et al (2007) Perforated viscus in a patient with non-small cell lung cancer receiving bevacizumab. J Thorac Oncol 2(6):571–573. doi:10.1097/JTO.0b013e31805fea51
Abbrederis K, Kremer M, Schuhmacher C (2008) Ischemic anastomotic bowel perforation during treatment with bevacizumab 10 months after surgery. Chirurg 79(4):351–355. doi:10.1007/s00104-007-1339-z
Mir O, Mouthon L, Alexandre J et al (2007) Bevacizumab-induced cardiovascular events: a consequence of cholesterol emboli syndrome? J Natl Cancer Inst 99(1):85–86. doi:10.1093/jnci/djk011
Saif MW, Elfiky A, Salem RR (2007) Gastrointestinal perforation due to bevacizumab in colorectal cancer. Ann Surg Oncol 14(6):1860–1869. doi:10.1245/s10434-006-9337-9
Zech CJ, Bilzer M, Mueller-Lisse UG et al (2006) Perforation of the colon: a rare complication of hepatocellular carcinoma. Acta Radiol 47:538–542
Scappaticci FA, Fehrenbacher L, Cartwright T et al (2005) Surgical wound healing complications in metastatic colorectal cancer patients treated with bevacizumab. J Surg Oncol 91(3):173–180. doi:10.1002/jso.20301
Motzer RJ, Hutson TE, Tomczak P et al (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356(2):115–124. doi:10.1056/NEJMoa065044
Acknowledgment
This article was supported by Wonkwang university research fund 2012.
Conflict of interest
The authors have no conflicts of interest relevant to this article to disclose.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Choi, JH., Kim, T.H., Choi, K.H. et al. Small intestinal perforation caused by metastatic tumor necrosis after sorafenib (Nexavar®) therapy for advanced hepatocellular carcinoma. Int Canc Conf J 1, 155–158 (2012). https://doi.org/10.1007/s13691-012-0030-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13691-012-0030-5