Abstract
Syndromic obesity refers to obesity occurring with additional clinical findings, such as intellectual disability/developmental delay, dysmorphic features, and congenital malformations.
Purpose of Review
To present a narrative review regarding the genetic etiology, clinical description, and molecular diagnosis of syndromic obesity, which is a rare condition with high phenotypic variability and genetic heterogeneity. The following syndromes are presented in this review: Prader-Willi, Bardet-Biedl, Pseudohypoparathyroidism, Alström, Smith-Magenis, Cohen, Temple, 1p36 deletion, 16p11.2 microdeletion, Kleefstra, SIM1-related, Börjeson-Forssman-Lehmann, WAGRO, Carpenter, MORM, and MYT1L-related syndromes.
Recent Findings
There are three main groups of mechanisms for syndromic obesity: imprinting, transcriptional activity regulation, and cellular cilia function. For molecular diagnostic, methods of genome-wide investigation should be prioritized over sequencing of panels of syndromic obesity genes. In addition, we present novel syndromic conditions that need further delineation, but evidences suggest they have a higher frequency of obesity.
Summary
The etiology of syndromic obesity tends to be linked to disrupted neurodevelopment (central) and is associated with a diversity of genes and biological pathways. In the genetic investigation of individuals with syndromic obesity, the possibility that the etiology of the syndromic condition is independent of obesity should be considered. The accurate genetic diagnosis impacts medical management, treatment, and prognosis, and allows proper genetic counseling.



Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10. https://doi.org/10.1016/j.metabol.2018.09.005.
Agha M, Agha R. The rising prevalence of obesity: part a: impact on public health. Int J Surg Oncol. 2017;2:17. https://doi.org/10.1097/IJ9.0000000000000017.
WHO Technical Counsultation. Obesity: preventing and managing the global epidemic. WHO Consult 2000. 2000;0512–3054 (Print).
Weir CB, Jan A. BMI classification percentile and cut off points [internet]. StatPearls. 2022:31082114. https://www.ncbi.nlm.nih.gov/books/NBK541070/.
Reddon H, Guéant J-L, Meyre D. The importance of gene–environment interactions in human obesity. Clin Sci. 2016;130:1571–97. https://doi.org/10.1042/CS20160221.
Sandholt CH, Hansen T, Pedersen O. Beyond the fourth wave of genome-wide obesity association studies. Nutr Diabetes. 2012;2:e37. https://doi.org/10.1038/nutd.2012.9.
• Kleinendorst L, Abawi O, van der Voorn B, Jongejan MHTM, Brandsma AE, Visser JA, et al. Identifying underlying medical causes of pediatric obesity: Results of a systematic diagnostic approach in a pediatric obesity center. Buchner DA, editor. PLoS One. 2020;15:e0232990. https://doi.org/10.1371/journal.pone.0232990. Investigation of a large pediatric obesity cohort, with multidisciplinary assessment; included biochemical and hormonal evaluation and genetic testing.
•• Kaur Y, de Souza RJ, Gibson WT, Meyre D. A systematic review of genetic syndromes with obesity. Obes Rev. 2017;18:603–34. https://doi.org/10.1111/obr.12531. A systematic review about syndromic obesity. Highlights the genetic heterogeneity of syndromic obesity and that there are many gaps in knowledge, requiring increased clinical and genetic research.
• Kleinendorst L, Massink M, Cooiman MI, Savas M, van der Baan-Slootweg OH, Roelants RJ, et al. Genetic obesity: next-generation sequencing results of 1230 patients with obesity. J Med Genet. 2018;55:578–86. https://doi.org/10.1136/jmedgenet-2018-105315. Investigation of a large obesity cohort using NGS-based gene panel analysis. There was a higher yield for the pediatric group, which reflects that pediatric obesity has a greater probability of having a monogenic origin.
•• D’Angelo CS, Varela MC, De Castro CIE, Otto PA, Perez ABA, Lourenço CM, et al. Chromosomal microarray analysis in the genetic evaluation of 279 patients with syndromic obesity. Mol Cytogenet BioMed central ltd. 2018;11. https://doi.org/10.1186/s13039-018-0363-7. Microarray investigation (for CNVs) of a large syndromic obesity cohort. Highlights the genetic heterogeneity of syndromic obesity, in addition to the difficulty in diagnosis.
Huvenne H, Dubern B, Clément K, Poitou C. Rare genetic forms of obesity: clinical approach and current treatments in 2016. Obes Facts. 2016;9:158–73. https://doi.org/10.1159/000445061.
Clément K, Mosbah H, Poitou C. Rare genetic forms of obesity: from gene to therapy. Physiol Behav. 2020;227:113134. https://doi.org/10.1016/j.physbeh.2020.113134.
Geets E, Meuwissen MEC, Van Hul W. Clinical, molecular genetics and therapeutic aspects of syndromic obesity. Clin Genet. 2019;95:23–40. https://doi.org/10.1111/cge.13367.
Kehinde TA, Bhatia A, Olarewaju B, Shoaib MZ, Mousa J, Osundiji MA. Syndromic obesity with neurodevelopmental delay: opportunities for targeted interventions. Eur J Med Genet. 2022;65:104443. https://doi.org/10.1016/j.ejmg.2022.104443.
Delrue M-A, Michaud J. Fat chance: genetic syndromes with obesity. Clin Genet. 2004;66:83–93. https://doi.org/10.1111/j.0009-9163.2004.00300.x.
Micleaa D, Al-Khzouza C, Osan S, Bucerzan S, Cret V, Popp RA, et al. Genomic study via chromosomal microarray analysis in a group of Romanian patients with obesity and developmental disability/intellectual disability. J Pediatr Endocrinol Metab. 2019;32:667–74. https://doi.org/10.1515/jpem-2018-0439.
Campbell LVC. Genetics of obesity. Aust Fam Physician. 2017;46:456–9. https://www.racgp.org.au/afp/2017/july/genetics-of-obesity/.
Milani D, Cerutti M, Pezzani L, Maffei P, Milan G, Esposito S. Syndromic obesity: clinical implications of a correct diagnosis. Ital J Pediatr. 2014;40:33. https://doi.org/10.1186/1824-7288-40-33.
Wiegand S, Krude H. Monogene und syndromale Krankheitsbilder bei morbider Adipositas. Internist (Berl). 2015;56:111–20. https://doi.org/10.1007/s00108-014-3532-8.
Lionti T, Reid SM, White SM, Rowell MM. A population-based profile of 160 Australians with Prader-Willi syndrome: trends in diagnosis, birth prevalence and birth characteristics. Am J Med Genet Part A. 2015;167:371–8. https://doi.org/10.1002/ajmg.a.36845.
Thomson A, Glasson E, Bittles A. A long-term population-based clinical and morbidity review of Prader-Willi syndrome in Western Australia. J Intellect Disabil Res. 2006;50:69–78. https://doi.org/10.1111/j.1365-2788.2005.00770.x
Vogels A, Van Den Ende J, Keymolen K, Mmortier G, Devriendt K, Legius E, et al. Minimum prevalence, birth incidence and cause of death for Prader-Willi syndrome in Flanders. Eur J Hum Genet. 2004;2012:238–40. https://doi.org/10.1038/sj.ejhg.5201135.
Whittington J, Holland A, Webb T, Butler J, Clarke D, Boer H. Population prevalence and estimated birth incidence and mortality rate for people with Prader-Willi syndrome in one UK health region. J Med Genet. 2001;38:792–8. https://doi.org/10.1136/jmg.38.11.792.
Ehara H, Kousaku O, Kenzo T. Frequency of the Prader –Willi syndrome in the san-in district. Japan Brain Dev. 1995;17:324–6. https://doi.org/10.1016/0387-7604(95)00060-o.
Burd L, Besely B, Martsolf J, Korbeshian J. Prevalence study of Prader-Willi syndrome in North Dakota. Am J Med Genet. 1990;37:97–9. https://doi.org/10.1002/ajmg.1320370122.
Akefeldt A, Gillberg C, Larsson C. Prader-Willi syndrome in a Swedish rural county: epidemiological aspects. Dev Med Child Neurol. 1991;33:715–21. https://doi.org/10.1111/j.1469-8749.1991.tb14950.x.
Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-Willi syndrome. Genet Med. 2012;14:10–26. https://doi.org/10.1038/gim.0b013e31822bead0.
Fridman C, Koiffmann CP. Genomic imprinting: genetic mechanisms and phenotypic consequences in Prader-Willi and Angelman syndromes. Genet Mol Biol. 2000;23:715–24. https://doi.org/10.1590/S1415-47572000000400004.
Butler MG, Miller JL, Forster JL. Prader-Willi Syndrome - clinical genetics, diagnosis and treatment approaches: an update. Curr Pediatr Rev. 2019;15:207–44. https://doi.org/10.2174/1573396315666190716120925.
Driscoll DJ, Miller JL, Cassidy SB. Prader-Willi Syndrome [Internet]. GeneReviews®. 1993. https://www.ncbi.nlm.nih.gov/books/NBK1330/.
Khan MJ, Gerasimidis K, Edwards CA, Shaikh MG. Mechanisms of obesity in Prader–Willi syndrome. Pediatr. Obes. John Wiley and Sons Ltd; 2016. p. 3–13. https://doi.org/10.1111/ijpo.12177.
Einfeld SL, Kavanagh SJ, Smith A, Evans EJ, Tonge BJ, Taffe J. Mortality in Prader-Willi syndrome. Am J Ment Retard. 2006;111:193–8. https://doi.org/10.1352/0895-8017(2006)111[193:MIPS]2.0.CO;2.
Deal CL, Tony M, Höybye C, Allen DB, Tauber M, Christiansen JS, et al. GrowthHormone research society workshop summary: consensus guidelines for recombinant human growth hormone therapy in Prader-Willi syndrome. J Clin Endocrinol Metab. 2013;98:E1072–87. https://doi.org/10.1210/jc.2012-3888.
Alves C, Franco RR. Prader-Willi syndrome: endocrine manifestations and management. Arch. Endocrinol Metab. 2020;64:223–34. https://doi.org/10.20945/2359-3997000000248.
Angulo MA, Butler MG, Cataletto ME. Prader-Willi syndrome: a review of clinical, genetic, and endocrine findings. J Endocrinol Investig. 2015;38:1249–63. https://doi.org/10.1007/s40618-015-0312-9.
Tauber M, Hoybye C. Endocrine disorders in Prader-Willi syndrome: a model to understand and treat hypothalamic dysfunction. Lancet Diabetes Endocrinol. 2021;9:235–46. https://doi.org/10.1016/S2213-8587(21)00002-4.
Ehrhart F, Janssen KJM, Coort SL, Evelo CT, Curfs LMG. Prader-Willi syndrome and Angelman syndrome: visualisation of the molecular pathways for two chromosomal disorders. World J Biol Psychiatry. 2019;20:670–82. https://doi.org/10.1080/15622975.2018.1439594.
Bochukova EG. Transcriptomics of the Prader–Willi syndrome hypothalamus; 2021. p. 369–79. https://doi.org/10.1016/B978-0-12-820683-6.00027-0.
Tauber M, Coupaye M, Diene G, Molinas C, Valette M, Beauloye V. Prader-Willi syndrome: a model for understanding the ghrelin system. J Neuroendocrinol. 2019:31. https://doi.org/10.1111/jne.12728.
Miller JL, Lacroix A, Bird LM, Shoemaker AH, Haqq A, Deal CL, et al. The efficacy, safety, and pharmacology of a ghrelin O-acyltransferase inhibitor for the treatment of Prader-Willi Syndrome. J Clin Endocrinol Metab. 2022;107:e2373–80. https://doi.org/10.1210/clinem/dgac105.
Mahmoud R, Kimonis V, Butler MG. Clinical Trials in Prader–Willi Syndrome: A Review. Int J Mol Sci. 2023;24:2150. https://doi.org/10.3390/ijms24032150.
Elhamamsy AR. Role of DNA methylation in imprinting disorders: an updated review. J Assist Reprod Genet. 2017;34:549–62. https://doi.org/10.1007/s10815-017-0895-5.
Kim S-J, Miller JL, Kuipers PJ, German JR, Beaudet AL, Sahoo T, et al. Unique and atypical deletions in Prader–Willi syndrome reveal distinct phenotypes. Eur J Hum Genet. 2012;20:283–90. https://doi.org/10.1038/ejhg.2011.187.
Butler MG. Magnesium supplement and the 15q11.2 BP1–BP2 microdeletion (Burnside–Butler) Syndrome: a potential treatment? Int J Mol Sci. 2019;20:2914. https://doi.org/10.3390/ijms20122914.
Khan SA, Muhammad N, Khan MA, Kamal A, Rehman ZU, Khan S. Genetics of human Bardet-Biedl syndrome, an updates. Clin Genet. 2016;90:3–15. https://doi.org/10.1111/cge.12737.
Florea L, Caba L, Gorduza EV. Bardet–Biedl Syndrome—Multiple Kaleidoscope Images: Insight into Mechanisms of Genotype–Phenotype Correlations. Genes (Basel). 2021;12:1353. https://doi.org/10.3390/genes12091353.
Beales PL, Warner AM, Hitman GA, Thakker R, Flinter FA. Bardet-Biedl syndrome: a molecular and phenotypic study of 18 families. J Med Genet. 1997;34:92–8. https://doi.org/10.1136/jmg.34.2.92.
Farag TI, Teebi AS. High incidence of Bardet Biedl syndrome among the Bedouin. Clin Genet. 2008;36:463–4. https://doi.org/10.1111/j.1399-0004.1989.tb03378.x.
Klein D, Ammann F. The syndrome of Laurence-moon-Bardet-Biedl and allied diseases in Switzerland. J Neurol Sci. 1969;9:479–513. https://doi.org/10.1016/0022-510X(69)90091-4.
M’hamdi O, Ouertani I, Maazoul F, Chaabouni-Bouhamed H. Prevalence of Bardet–Biedl syndrome in Tunisia. J Community Genet. 2011;2:97–9. https://doi.org/10.1007/s12687-011-0040-6.
Moore S, Green J, Fan Y, et al. Clinical and genetic epidemiology of Bardet–Biedl syndrome in Newfoundland: a 22-year prospective, population-based cohort study. Am J Med Genet A. 2005;132:352–60. https://doi.org/10.1002/ajmg.a.30406.
Suspitsin EN, Imyanitov EN. Bardet-Biedl Syndrome Mol Syndromol. 2016;7:62–71. https://doi.org/10.1159/000445491.
Kim CA, Albano LMJ, Bertola DR. Síndromes genéticas associadas à obesidade. Tratado de obesidade. 2nd ed. Rio de Janeiro – RJ: Guanabara Koogan; 2022. p. 253–61.
Schachat AP, Maumenee IH. Bardet-Biedl Syndrome and related disorders. Arch Ophthalmol. 1982;100:285–8. https://doi.org/10.1001/archopht.1982.01030030287011.
Beales PL, Elcioglu N, Woolf AS, Parker D, Flinter FA. New criteria for improved diagnosis of Bardet-Biedl syndrome: results of a population survey. J Med Genet. 1999;36:437–46. https://doi.org/10.1136/jmg.36.6.437.
Forsythe E, Beales PL. Bardet–Biedl syndrome. Eur J Hum Genet. 2013;21:8–13. https://doi.org/10.1038/ejhg.2012.115.
Melluso A, Secondulfo F, Capolongo G, Capasso G, Zacchia M. Bardet-Biedl Syndrome: current perspectives and clinical outlook. Ther Clin Risk Manag. 2023;19:115–32. https://doi.org/10.2147/TCRM.S338653.
Tsang SH, Aycinena ARP, Sharma T. Ciliopathy: Bardet-Biedl Syndrome; 2018. p. 171–4. https://doi.org/10.1007/978-3-319-95046-4_33.
Berbari NF, Pasek RC, Malarkey EB, Yazdi SMZ, McNair AD, Lewis WR, et al. Leptin resistance is a secondary consequence of the obesity in ciliopathy mutant mice. Proc Natl Acad Sci. 2013;110:7796–801. https://doi.org/10.1073/pnas.1210192110.
Engle SE, Bansal R, Antonellis PJ, Berbari NF. Cilia signaling and obesity. Semin Cell Dev Biol. 2021;110:43–50. https://doi.org/10.1016/j.semcdb.2020.05.006.
Guo D-F, Cui H, Zhang Q, Morgan DA, Thedens DR, Nishimura D, et al. The BBSome Controls Energy Homeostasis by Mediating the Transport of the Leptin Receptor to the Plasma Membrane. Héon E, editor. PLoS Genet. 2016;12:e1005890. https://doi.org/10.1371/journal.pgen.1005890.
Muller J, Stoetzel C, Vincent MC, Leitch CC, Laurier V, Danse JM, et al. Identification of 28 novel mutations in the Bardet–Biedl syndrome genes: the burden of private mutations in an extensively heterogeneous disease. Hum Genet. 2010;127:583–93. https://doi.org/10.1007/s00439-010-0804-9.
Priya S, Nampoothiri S, Sen P, Sripriya S. Bardet–Biedl syndrome: Genetics, molecular pathophysiology, and disease management. Indian J Ophthalmol. 2016;64:620. https://doi.org/10.4103/0301-4738.194328.
Nishimura DY, Fath M, Mullins RF, Searby C, Andrews M, Davis R, et al. Bbs2-null mice have neurosensory deficits, a defect in social dominance, and retinopathy associated with mislocalization of rhodopsin. Proc Natl Acad Sci. 2004;101:16588–93. https://doi.org/10.1073/pnas.0405496101.
Mykytyn K, Mullins RF, Andrews M, Chiang AP, Swiderski RE, Yang B, et al. Bardet-Biedl syndrome type 4 (BBS4)-null mice implicate Bbs4 in flagella formation but not global cilia assembly. Proc Natl Acad Sci. 2004;101:8664–9. https://doi.org/10.1073/pnas.0402354101.
Fath MA, Mullins RF, Searby C, Nishimura DY, Wei J, Rahmouni K, et al. Mkks-null mice have a phenotype resembling Bardet–Biedl syndrome. Hum Mol Genet. 2005;14:1109–18. https://doi.org/10.1093/hmg/ddi123.
Rahmouni K, Fath MA, Seo S, Thedens DR, Berry CJ, Weiss R, et al. Leptin resistance contributes to obesity and hypertension in mouse models of Bardet-Biedl syndrome. J Clin Invest. 2008;118:1458–67. https://doi.org/10.1172/JCI32357.
Thiele S, Mantovani G, Barlier A, Boldrin V, Bordogna P, De Sanctis L, et al. From pseudohypoparathyroidism to inactivating PTH/PTHrP signalling disorder (iPPSD), a novel classification proposed by the EuroPHP network. Eur J Endocrinol. 2016;175:P1–17. https://doi.org/10.1530/EJE-16-0107.
Mantovani G, Bastepe M, Monk D, de Sanctis L, Thiele S, Usardi A, et al. Diagnosis and management of pseudohypoparathyroidism and related disorders: first international consensus statement. Nat Rev Endocrinol. 2018;14:476–500. https://doi.org/10.1038/s41574-018-0042-0.
Underbjerg L, Sikjaer T, Mosekilde L, Rejnmark L. Pseudohypoparathyroidism - epidemiology, mortality and risk of complications. Clin Endocrinol. 2016;84:904–11. https://doi.org/10.1111/cen.12948.
Bilezikian JP. Hypoparathyroidism. J Clin Endocrinol Metab. 2020;105:1722–36. https://doi.org/10.1210/clinem/dgaa113.
Butler MG. Imprinting disorders in humans: a review. Curr Opin Pediatr. 2020;32:719–29. https://doi.org/10.1097/MOP.0000000000000965.
Jüppner H. Molecular definition of Pseudohypoparathyroidism variants. J Clin Endocrinol Metab. 2021;106:1541–52. https://doi.org/10.1210/clinem/dgab060.
Turan S, Bastepe M. GNAS Spectrum of disorders. Curr Osteoporos Rep. 2015;13:146–58. https://doi.org/10.1007/s11914-015-0268-x.
Mantovani G, Elli FM. Multiple hormone resistance and alterations of G-protein-coupled receptors signaling. Best Pract Res Clin Endocrinol Metab. 2018;32:141–54. https://doi.org/10.1016/j.beem.2018.01.002.
Hendy GN, Cole DE, Bastepe M. Hypoparathyroidism and Pseudohypoparathyroidism [internet]. Endotext; 2000. https://www.ncbi.nlm.nih.gov/books/NBK279165/.
Perez KM, Lee EB, Kahanda S, Duis J, Reyes M, Jüppner H, et al. Cognitive and behavioral phenotype of children with pseudohypoparathyroidism type 1A. Am J Med Genet Part A. 2018;176:283–9. https://doi.org/10.1002/ajmg.a.38534.
Mouallem M, Shaharabany M, Weintrob N, Shalitin S, Nagelberg N, Shapira H, et al. Cognitive impairment is prevalent in pseudohypoparathyroidism type Ia, but not in pseudopseudohypoparathyroidism: possible cerebral imprinting of Gs? Clin Endocrinol. 2007;070907132242011 https://doi.org/10.1111/j.1365-2265.2007.03025.x.
Hanna P, Grybek V, Perez de Nanclares G, Tran LC, de Sanctis L, Elli F, et al. Genetic and epigenetic defects at the GNAS locus Lead to distinct patterns of skeletal growth but similar early-onset obesity. J Bone Miner Res. 2018;33:1480–8. https://doi.org/10.1002/jbmr.3450.
Long DN, McGuire S, Levine MA, Weinstein LS, Germain-Lee EL. Body mass index differences in Pseudohypoparathyroidism type 1a versus Pseudopseudohypoparathyroidism May implicate paternal imprinting of Gαs in the development of human obesity. J Clin Endocrinol Metab. 2007;92:1073–9. https://doi.org/10.1210/jc.2006-1497.
Dixit A, Chandler KE, Lever M, Poole RL, Bullman H, Mughal MZ, et al. Pseudohypoparathyroidism type 1b due to paternal uniparental Disomy of chromosome 20q. J Clin Endocrinol Metab. 2013;98:E103–8. https://doi.org/10.1210/jc.2012-2639.
Bastepe M, Altug-Teber Ö, Agarwal C, Oberfield SE, Bonin M, Jüppner H. Paternal uniparental isodisomy of the entire chromosome 20 as a molecular cause of pseudohypoparathyroidism type Ib (PHP-Ib). Bone. 2011;48:659–62. https://doi.org/10.1016/j.bone.2010.10.168.
Fernández-Rebollo E, Lecumberri B, Garin I, Arroyo J, Bernal-Chico A, Goñi F, et al. New mechanisms involved in paternal 20q disomy associated with pseudohypoparathyroidism. Eur J Endocrinol. 2010;163:953–62. https://doi.org/10.1530/EJE-10-0435.
Mendes de Oliveira E, Keogh JM, Talbot F, Henning E, Ahmed R, Perdikari A, et al. Obesity-associated GNAS mutations and the Melanocortin pathway. N Engl J Med. 2021;385:1581–92. https://doi.org/10.1056/NEJMoa2103329.
Hearn T. ALMS1 and Alström syndrome: a recessive form of metabolic, neurosensory and cardiac deficits. J Mol Med. 2019;97:1–17. https://doi.org/10.1007/s00109-018-1714-x.
Marshall JD, Muller J, Collin GB, Milan G, Kingsmore SF, Dinwiddie D, et al. Alström Syndrome: mutation Spectrum of ALMS1. Hum Mutat. 2015;36:660–8. https://doi.org/10.1002/humu.22796.
Tsang SH, Aycinena ARP, Sharma T. Ciliopathy: Alström Syndrome; 2018. p. 179–80. https://doi.org/10.1007/978-3-319-95046-4_35.
Roy Choudhury A, Munonye I, Sanu KP, Islam N, Gadaga C. A review of Alström syndrome: a rare monogenic ciliopathy. Intractable Rare Dis Res. 2021;2021(10):01113. https://doi.org/10.5582/irdr.2021.01113.
Paisey R, Steeds R, Barrett T, Williams D, Geberhiwot T, Gunay-Aygun M. Alström Syndrome. GeneReviews. 2003. https://www.ncbi.nlm.nih.gov/books/NBK1267/.
Tahani N, Maffei P, Dollfus H, Paisey R, Valverde D, Milan G, et al. Consensus clinical management guidelines for Alström syndrome. Orphanet J Rare Dis. 2020;15:253. https://doi.org/10.1186/s13023-020-01468-8.
Rinaldi B, Villa R, Sironi A, Garavelli L, Finelli P, Bedeschi MF. Smith-Magenis Syndrome—clinical review, biological background and related disorders. Genes (Basel). 2022;13:335. https://doi.org/10.3390/genes13020335.
Smith AC, Boyd KE, Brennan C, Charles J, Elsea SH, Finucane BM, et al. Smith-Magenis Syndrome. GeneReviews®; 1993. https://www.ncbi.nlm.nih.gov/books/NBK1310/.
Rive Le Gouard N, Jacquinet A, Ruaud L, Deleersnyder H, Ageorges F, Gallard J, et al. Smith-Magenis syndrome: clinical and behavioral characteristics in a large retrospective cohort. Clin Genet. 2021;99:519–28. https://doi.org/10.1111/cge.13906.
Alaimo JT, Barton LV, Mullegama SV, Wills RD, Foster RH, Elsea SH. Individuals with Smith-Magenis syndrome display profound neurodevelopmental behavioral deficiencies and exhibit food-related behaviors equivalent to Prader-Willi syndrome. Res dev Disabil. In: S.H. Elsea, Department of Molecular and Human Genetics, Baylor College of Medicine, one Baylor plaza, NAB2015, vol. 47. Houston, TX, United States; 2015. p. 27–38. https://doi.org/10.1016/j.ridd.2015.08.011.
Smith ACM, Gropman AL, Bailey-Wilson JE, Goker-Alpan O, Elsea SH, Blancato J, et al. Hypercholesterolemia in children with Smith-Magenis syndrome: del (17)(p11.2p11.2). Genet Med. 2002;4:118–25. https://doi.org/10.1097/00125817-200205000-00004.
Burns B, Schmidt K, Williams SR, Kim S, Girirajan S, Elsea SH. Rai1 haploinsufficiency causes reduced Bdnf expression resulting in hyperphagia, obesity and altered fat distribution in mice and humans with no evidence of metabolic syndrome. Hum Mol Genet. 2010;19:4026–42. https://doi.org/10.1093/hmg/ddq317.
Han JC, Liu Q-R, Jones M, Levinn RL, Menzie CM, Jefferson-George KS, et al. Brain-derived neurotrophic factor and obesity in the WAGR Syndrome. N Engl J Med. 2008;359:918–27. https://doi.org/10.1056/NEJMoa0801119.
Duplomb L, Duvet S, Picot D, Jego G, El Chehadeh-Djebbar S, Marle N, et al. Cohen syndrome is associated with major glycosylation defects. Hum Mol Genet. 2014;23:2391–9. https://doi.org/10.1093/hmg/ddt630.
Rodrigues JM, Fernandes HD, Caruthers C, Braddock SR, Knutsen AP. Cohen Syndrome: review of the literature. Cureus. 2018; https://doi.org/10.7759/cureus.3330.
Roxo-Junior P, Mina I. Cohen Syndrome. Encycl Med Immunol. 2018:1–2. https://doi.org/10.1007/978-1-4614-9209-2_157-1.
Chandler KE. Diagnostic criteria, clinical characteristics, and natural history of Cohen syndrome. J Med Genet. 2003;40:233–41. https://doi.org/10.1136/jmg.40.4.233.
Delcourt M, Riant F, Mancini J, Milh M, Navarro V, Roze E, et al. Severe phenotypic spectrum of biallelic mutations in PRRT2 gene. J Neurol Neurosurg Psychiatry England. 2015;86:782–5. https://doi.org/10.1136/jnnp-2014-309025.
Wang H, Falk M, Wensel C, Traboulsi E. Cohen Syndrome. GeneReview; 2006.
Momtazmanesh S, Rayzan E, Shahkarami S, Rohlfs M, Klein C, Rezaei N. A novel VPS13B mutation in Cohen syndrome: a case report and review of literature. BMC Med Genet. 2020;21:140. https://doi.org/10.1186/s12881-020-01075-1.
Rodrigues JM, Fernandes HD, Caruthers C, Braddock SR, Knutsen AP. Cohen Syndrome: review of the literature. Cureus. Cureus, Inc.; 2018. https://doi.org/10.7759/cureus.3330.
Kääriäinen H, Muilu J, Perola M, Kristiansson K. Genetics in an isolated population like Finland: a different basis for genomic medicine? J Community Genet. 2017;8:319–26. https://doi.org/10.1007/s12687-017-0318-4.
Ioannides Y, Lokulo-Sodipe K, Mackay DJG, Davies JH, Temple IK. Temple syndrome: improving the recognition of an underdiagnosed chromosome 14 imprinting disorder: an analysis of 51 published cases. J Med Genet. 2014;51:495–501. https://doi.org/10.1136/jmedgenet-2014-102396.
Prasasya R, Grotheer KV, Siracusa LD, Bartolomei MS. Temple syndrome and Kagami-Ogata syndrome: clinical presentations, genotypes, models and mechanisms. Hum Mol Genet. 2020;29:R107–16. https://doi.org/10.1093/hmg/ddaa133.
Kagami M, Sekita Y, Nishimura G, Irie M, Kato F, Okada M, et al. Deletions and epimutations affecting the human 14q32.2 imprinted region in individuals with paternal and maternal upd(14)-like phenotypes. Nat Genet. 2008;40:237–42. https://doi.org/10.1038/ng.2007.56.
Bertini V, Fogli A, Bruno R, Azzarà A, Michelucci A, Mattina T, et al. Maternal uniparental Disomy 14 (Temple Syndrome) as a result of a Robertsonian translocation. Mol Syndromol. 2017;8:131–8. https://doi.org/10.1159/000456062.
Wang X, Pang H, Shah BA, Gu H, Zhang L, Wang H. A male case of Kagami-Ogata Syndrome caused by paternal Unipaternal Disomy 14 as a result of a Robertsonian translocation. Front Pediatr. 2020:8. https://doi.org/10.3389/fped.2020.00088.
Suzumori N, Kagami M, Kumagai K, Goto S, Matsubara K, Sano S, et al. Clinical and molecular findings in a patient with 46,XX/47,XX,+14 mosaicism caused by postzygotic duplication of a paternally derived chromosome 14. Am J Med Genet Part A. 2015;167:2474–7. https://doi.org/10.1002/ajmg.a.37194.
Haug MG, Brendehaug A, Houge G, Kagami M, Ogata T. Mosaic upd(14)pat in a patient with mild features of Kagami-Ogata syndrome. Clin Case Reports. 2018;6:91–5. https://doi.org/10.1002/ccr3.1300.
Yakoreva M, Kahre T, Pajusalu S, Ilisson P, Žilina O, Tillmann V, et al. A new case of a rare combination of Temple Syndrome and mosaic trisomy 14 and a literature review. Mol Syndromol. 2018;9:182–9. https://doi.org/10.1159/000489446.
Dauber A, Cunha-Silva M, Macedo DB, Brito VN, Abreu AP, Roberts SA, et al. Paternally inherited DLK1 deletion associated with familial central precocious puberty. J Clin Endocrinol Metab. 2017;102:1557–67. https://doi.org/10.1210/jc.2016-3677.
Macedo DB, Kaiser UB. DLK1, notch signaling and the timing of puberty. Semin Reprod Med. 2019;37:174–81. https://doi.org/10.1055/s-0039-3400963.
Gomes LG, Cunha-Silva M, Crespo RP, Ramos CO, Montenegro LR, Canton A, et al. DLK1 is a novel link between reproduction and metabolism. J Clin Endocrinol Metab. 2019;104:2112–20. https://doi.org/10.1210/jc.2018-02010.
Jordan VK, Zaveri HP, Scott DA. 1p36 deletion syndrome: an update. Appl Clin Genet. 2015;8:189–200. https://doi.org/10.2147/TACG.S65698.
Rocha CF, Vasques RB, Santos SR, Paiva CLA. Mini-review monosomy 1p36 syndrome: reviewing the correlation between deletion sizes and phenotypes. Genet Mol Res. 2016:15. https://doi.org/10.4238/gmr.15017942.
D’Angelo CS, Kohl I, Varela MC, de Castro CIE, Kim CA, Bertola DR, et al. Extending the phenotype of monosomy 1p36 syndrome and mapping of a critical region for obesity and hyperphagia. Am J Med Genet Part A. 2010;152A:102–10. https://doi.org/10.1002/ajmg.a.33160.
Gimeno-Ferrer F, Albuquerque D, Guzmán Luján C, Marcaida Benito G, Torreira Banzas C, Repáraz-Andrade A, et al. The effect of copy number variations in chromosome 16p on body weight in patients with intellectual disability. J Hum Genet. 2019;64:221–31. https://doi.org/10.1038/s10038-018-0545-5.
Zufferey F, Sherr EH, Beckmann ND, Hanson E, Maillard AM, Hippolyte L, et al. A 600 kb deletion syndrome at 16p11.2 leads to energy imbalance and neuropsychiatric disorders. J Med Genet. 2012;49:660–8. https://doi.org/10.1136/jmedgenet-2012-101203.
Chung WK, Roberts TP, Sherr EH, Snyder LG, Spiro JE. 16p11.2 deletion syndrome. Curr Opin Genet Dev. 2021;68:49–56. https://doi.org/10.1016/j.gde.2021.01.011.
Goldenberg P. An update on common chromosome microdeletion and microduplication syndromes. Pediatr Ann United States. 2018;47:e198–203. https://doi.org/10.3928/19382359-20180419-01.
Liangyou R. SH2B1 regulation of energy balance, body weight, and glucose metabolism. World J Diabetes. 2014;5:511–26. https://doi.org/10.4239/wjd.v5.i4.511.
Doche ME, Bochukova EG, Su H-W, Pearce LR, Keogh JM, Henning E, et al. Human SH2B1 mutations are associated with maladaptive behaviors and obesity. J Clin Invest. 2012;122:4732–6. https://doi.org/10.1172/JCI62696.
Jiang L, Su H, Wu X, Shen H, Kim M-H, Li Y, et al. Leptin receptor-expressing neuron Sh2b1 supports sympathetic nervous system and protects against obesity and metabolic disease. Nat Commun. 2020;11:1517. https://doi.org/10.1038/s41467-020-15328-3.
Verhoeven WMA, Kleefstra T, Egger JIM. Kleefstra syndrome: neuropsychiatric sequelae. Eur Psychiatry. 2011;26:819. https://doi.org/10.1016/S0924-9338(11)72524-X.
Kleefstra T, de Leeuw N. Kleefstra Syndrome. GeneReviews. 2010. https://www.ncbi.nlm.nih.gov/books/NBK47079/.
Kleefstra T, et al. Further clinical and molecular delineation of the 9q subtelomeric deletion syndrome supports a major contribution of EHMT1 haploinsufficiency to the core phenotype. J Med Genet. 2009;46:598–606. https://doi.org/10.1136/jmg.2008.062950.
Syndrome K. J Coll Physicians Surg Pak. 2022;32(S76–8) https://doi.org/10.29271/jcpsp.2022.Supp1.S76.
Koemans TS, Kleefstra T, Chubak MC, Stone MH, Reijnders MRF, de Munnik S, et al. Functional convergence of histone methyltransferases EHMT1 and KMT2C involved in intellectual disability and autism spectrum disorder. PLoS Genet. 2017;13:e1006864. https://doi.org/10.1371/journal.pgen.1006864.
Fiszbein A, Giono LE, Quaglino A, Berardino BG, Sigaut L, von Bilderling C, et al. Alternative splicing of G9a regulates neuronal differentiation. Cell Rep. 2016;14:2797–808. https://doi.org/10.1016/j.celrep.2016.02.063.
Deimling SJ, Olsen JB, Tropepe V. The expanding role of the Ehmt2/G9a complex in neurodevelopment. Neurogenesis. 2017;4:e1316888. https://doi.org/10.1080/23262133.2017.1316888.
Balemans MCM, Nadif Kasri N, Kopanitsa MV, Afinowi NO, Ramakers G, Peters TA, et al. Hippocampal dysfunction in the Euchromatin histone methyltransferase 1 heterozygous knockout mouse model for Kleefstra syndrome. Hum Mol Genet. 2013;22:852–66. https://doi.org/10.1093/hmg/dds490.
D’Angelo CS, Kohl I, Varela MC, de Castro CIE, Kim CA, Bertola DR, et al. Obesity with associated developmental delay and/or learning disability in patients exhibiting additional features: report of novel pathogenic copy number variants. Am J Med Genet Part A. 2013;161:479–86. https://doi.org/10.1002/ajmg.a.35761.
Jacobus Gilhuis H, Ma van Ravenswaaij C, Hamel BJC, Gabreëls FJM. Interstitial 6q deletion with a Prader--Willi-like phenotype: a new case and review of the literature. Eur J Paediatr Neurol. 2000;4:39–43. https://doi.org/10.1053/ejpn.1999.0259.
Villa A, Urioste M, Bofarull JM, Martínez-Frías M-L. De novo interstitial deletion q16.2q21 on chromosome 6. Am J Med Genet. 1995;55:379–83. https://doi.org/10.1002/ajmg.1320550326.
Rosenfeld JA, Amrom D, Andermann E, Andermann F, Veilleux M, Curry C, et al. Genotype–phenotype correlation in interstitial 6q deletions: a report of 12 new cases. Neurogenetics. 2012;13:31–47. https://doi.org/10.1007/s10048-011-0306-5.
El Khattabi L, Guimiot F, Pipiras E, Andrieux J, Baumann C, Bouquillon S, et al. Incomplete penetrance and phenotypic variability of 6q16 deletions including SIM1. Eur J Hum Genet. 2015;23:1010–8. https://doi.org/10.1038/ejhg.2014.230.
Holder JL, Butte NF, Zinn AR. Profound obesity associated with a balanced translocation that disrupts the SIM1 gene. Hum Mol Genet. 2000;9:101–8. https://doi.org/10.1093/hmg/9.1.101.
Ramachandrappa S, Raimondo A, Cali AMG, Keogh JM, Henning E, Saeed S, et al. Rare variants in single-minded 1 (SIM1) are associated with severe obesity. J Clin Invest. 2013;123:3042–50. https://doi.org/10.1172/JCI68016.
Zegers D, Beckers S, Hendrickx R, Van Camp JK, de Craemer V, Verrijken A, et al. Mutation screen of the SIM1 gene in pediatric patients with early-onset obesity. Int J Obes. 2014;38:1000–4. https://doi.org/10.1038/ijo.2013.188.
Bonnefond A, Raimondo A, Stutzmann F, Ghoussaini M, Ramachandrappa S, Bersten DC, et al. Loss-of-function mutations in SIM1 contribute to obesity and Prader-Willi–like features. J Clin Invest. 2013;123:3037–41. https://doi.org/10.1172/JCI68035.
Michaud JL, Rosenquist T, May NR, Fan C-M. Development of neuroendocrine lineages requires the bHLH–PAS transcription factor SIM1. Genes Dev. 1998;12:3264–75. https://doi.org/10.1101/gad.12.20.3264.
Matharu N, Rattanasopha S, Tamura S, Maliskova L, Wang Y, Bernard A, et al. CRISPR-mediated activation of a promoter or enhancer rescues obesity caused by haploinsufficiency. Science (80-). 2019;363 https://doi.org/10.1126/science.aau0629.
Kasher PR, Schertz KE, Thomas M, Jackson A, Annunziata S, Ballesta-Martinez MJ, et al. Small 6q16.1 deletions encompassing POU3F2 cause susceptibility to obesity and variable developmental delay with intellectual disability. Am J Hum Genet. 2016;98:363–72. https://doi.org/10.1016/j.ajhg.2015.12.014.
Schönauer R, Jin W, Findeisen C, Valenzuela I, Devlin LA, Murrell J, et al. Monoallelic intragenic POU3F2 variants lead to neurodevelopmental delay and hyperphagic obesity, confirming the gene’s candidacy in 6q16.1 deletions. Am J Hum Genet. 2023; https://doi.org/10.1016/j.ajhg.2023.04.010.
Jahani-Asl A, Cheng C, Zhang C, Bonni A. Pathogenesis of Börjeson-Forssman-Lehmann syndrome: Insights from PHF6 function. Neurobiol Dis. 2016;96:227–35. https://doi.org/10.1016/j.nbd.2016.09.011.
Bellad A, Bandari AK, Pandey A, Girimaji SC, Muthusamy B. A novel missense variant in PHF6 gene causing Börjeson-Forssman-Lehman Syndrome. J Mol Neurosci. 2020;70:1403–9. https://doi.org/10.1007/s12031-020-01560-5.
Kasper BS, Dörfler A, Di Donato N, Kasper EM, Wieczorek D, Hoyer J, et al. Central nervous system anomalies in two females with Borjeson-Forssman-Lehmann syndrome. Epilepsy Behav. 2017;69:104–9. https://doi.org/10.1016/j.yebeh.2017.01.022.
Loontiens S, Dolens A-C, Strubbe S, Van de Walle I, Moore FE, Depestel L, et al. PHF6 expression levels impact human hematopoietic stem cell differentiation. Front cell. Dev Biol. 2020:8. https://doi.org/10.3389/fcell.2020.599472.
Di Donato N, Isidor B, Lopez Cazaux S, Le Caignec C, Klink B, Kraus C, et al. Distinct phenotype of PHF6 deletions in females. Eur J Med Genet. 2014;57:85–9. https://doi.org/10.1016/j.ejmg.2013.12.003.
Wieczorek D, Bögershausen N, Beleggia F, Steiner-Haldenstätt S, Pohl E, Li Y, et al. A comprehensive molecular study on Coffin–Siris and Nicolaides–Baraitser syndromes identifies a broad molecular and clinical spectrum converging on altered chromatin remodeling. Hum Mol Genet. 2013;22:5121–35. https://doi.org/10.1093/hmg/ddt366.
Zweier C, Kraus C, Brueton L, Cole T, Degenhardt F, Engels H, et al. A new face of Borjeson–Forssman–Lehmann syndrome? De novo mutations in PHF6 in seven females with a distinct phenotype. J Med Genet. 2013;50:838–47. https://doi.org/10.1136/jmedgenet-2013-101918.
Cheng C, Deng P-Y, Ikeuchi Y, Yuede C, Li D, Rensing N, et al. Characterization of a mouse model of Börjeson-Forssman-Lehmann Syndrome. Cell Rep. 2018;25:1404–1414.e6. https://doi.org/10.1016/j.celrep.2018.10.043.
Zhang C, Mejia L, et al. The X-linked intellectual disability protein PHF6 associates with the PAF1 complex and regulates neuronal migration in the mammalian brain. Neuron. 2013;78:986–93. https://doi.org/10.1016/j.neuron.2013.04.021.
Gül D, Oğur G, Tunca Y, Özcan O. Third case of WAGR syndrome with severe obesity and constitutional deletion of chromosome (11)(p12p14). Am J Med Genet. 2002;107:70–1. https://doi.org/10.1002/ajmg.10013.
Fischbach BV, Trout KL, Lewis J, Luis CA, Sika M. WAGR Syndrome: a clinical review of 54 cases. Pediatrics. 2005;116:984–8. https://doi.org/10.1542/peds.2004-0467.
Duffy KA, Trout KL, Gunckle JM, Krantz SM, Morris J, Kalish JM. Results from the WAGR Syndrome patient registry: characterization of WAGR Spectrum and recommendations for care management. Front Pediatr. 2021:9. https://doi.org/10.3389/fped.2021.733018.
Unger TJ, Calderon GA, Bradley LC, Sena-Esteves M, Rios M. Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in Hyperphagic behavior and obesity. J Neurosci. 2007;27:14265–74. https://doi.org/10.1523/JNEUROSCI.3308-07.2007.
Haye D, Collet C, Sembely-Taveau C, Haddad G, Denis C, Soulé N, et al. Prenatal findings in carpenter syndrome and a novel mutation in RAB23. Am J Med Genet Part A. 2014;164:2926–30. https://doi.org/10.1002/ajmg.a.36726.
Jenkins D, Baynam G, De Catte L, Elcioglu N, Gabbett MT, Hudgins L, et al. Carpenter syndrome: extended RAB23 mutation spectrum and analysis of nonsense-mediated mRNA decay. Hum Mutat. 2011:32. https://doi.org/10.1002/humu.21457.
Khairat R, Elhossini R, Sobreira N, Wohler E, Otaify G, Mohamed AM, et al. Expansion of the phenotypic and mutational spectrum of carpenter syndrome. Eur J Med Genet. 2022;65:104377. https://doi.org/10.1016/j.ejmg.2021.104377.
Richieri-Costa A, Pirolo L, Cohen MM. Carpenter syndrome with normal intelligence: Brazilian girl born to consanguineous parents. Am J Med Genet. 1993;47:281–3. https://doi.org/10.1002/ajmg.1320470228.
Ingham PW, Nakano Y, Seger C. Mechanisms and functions of hedgehog signalling across the metazoa. Nat Rev Genet. 2011;12:393–406. https://doi.org/10.1038/nrg2984.
Jenkins D, Seelow D, Jehee FS, Perlyn CA, Alonso LG, Bueno DF, et al. RAB23 mutations in carpenter Syndrome imply an unexpected role for hedgehog signaling in cranial-suture development and obesity. Am J Hum Genet. 2007;80:1162–70. https://doi.org/10.1086/518047.
Hor CH, Goh EL. Small GTPases in hedgehog signalling: emerging insights into the disease mechanisms of Rab23-mediated and Arl13b-mediated ciliopathies. Curr Opin Genet Dev. 2019;56:61–8. https://doi.org/10.1016/j.gde.2019.07.009.
Twigg S, et al. Mutations in multidomain protein MEGF8 identify a carpenter syndrome subtype associated with defective lateralization. Am J Hum. 2012;91:897–905. https://doi.org/10.1016/j.ajhg.2012.08.027.
Engelhard C, Sarsfield S, Merte J, Wang Q, Li P, Beppu H, et al. MEGF8 is a modifier of BMP signaling in trigeminal sensory neurons. Elife. 2013:2. https://doi.org/10.7554/eLife.01160.
Yu S, Zhou C, Cao S, He J, Cai B, Wu K, et al. BMP4 resets mouse epiblast stem cells to naive pluripotency through ZBTB7A/B-mediated chromatin remodelling. Nat Cell Biol. 2020;22:651–62. https://doi.org/10.1038/s41556-020-0516-x.
Xu J, Chen M, Yan Y, Zhao Q, Shao M, Huang Z. The effects of altered BMP4 signaling in first branchial-arch-derived murine embryonic orofacial tissues. Int J Oral Sci. 2021;13:40. https://doi.org/10.1038/s41368-021-00142-4.
Jacoby M, Cox JJ, Gayral S, Hampshire DJ, Ayub M, Blockmans M, et al. INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nat Genet. 2009;41:1027–31. https://doi.org/10.1038/ng.427.
Hampshire DJ, Ayub M, Springell K, Roberts E, Jafri H, Rashid Y, et al. MORM syndrome (mental retardation, truncal obesity, retinal dystrophy and micropenis), a new autosomal recessive disorder, links to 9q34. Eur J Hum Genet. 2006;14:543–8. https://doi.org/10.1038/sj.ejhg.5201577.
Drole Torkar A, Avbelj Stefanija M, Bertok S, Trebušak Podkrajšek K, Debeljak M, Stirn Kranjc B, et al. Novel insights into monogenic obesity Syndrome due to INPP5E gene variant: a case report of a female patient. Front Endocrinol (Lausanne). 2021;12. https://doi.org/10.3389/fendo.2021.581134.
Zhang R, Tang J, Li T, Zhou J, Pan W. INPP5E and coordination of signaling networks in cilia. Front Mol Biosci. 2022:9. https://doi.org/10.3389/fmolb.2022.885592.
Coursimault J, Guerrot A-M, Morrow MM, Schramm C, Zamora FM, Shanmugham A, et al. MYT1L-associated neurodevelopmental disorder: description of 40 new cases and literature review of clinical and molecular aspects. Hum Genet. 2022;141:65–80. https://doi.org/10.1007/s00439-021-02383-z.
Carvalho LML, D’Angelo CS, Mustacchi Z, da Silva IT, Krepischi ACV, Koiffmann CP, et al. A novel MYT1L mutation in a boy with syndromic obesity: case report and literature review. Obes Res Clin Pract. 2021. https://doi.org/10.1016/j.orcp.2021.01.001.
Blanchet P, Bebin M, Bruet S, Cooper GM, Thompson ML, Duban-Bedu B, et al. MYT1L mutations cause intellectual disability and variable obesity by dysregulating gene expression and development of the neuroendocrine hypothalamus. PLoS Genet. 2017;13:e1006957. https://doi.org/10.1371/journal.pgen.1006957.
De Rocker N, Vergult S, Koolen D, Jacobs E, Hoischen A, Zeesman S, et al. Refinement of the critical 2p25.3 deletion region: the role of MYT1L in intellectual disability and obesity. Genet med. Nat Publ Group. 2015;17:460–6. https://doi.org/10.1038/gim.2014.124.
Windheuser IC, Becker J, Cremer K, Hundertmark H, Yates LM, Mangold E, et al. Nine newly identified individuals refine the phenotype associated with MYT1L mutations. Am J Med Genet Part A. 2020;182:1021–31. https://doi.org/10.1002/ajmg.a.61515.
Wöhr M, Fong WM, Janas JA, Mall M, Thome C, Vangipuram M, et al. Myt1l haploinsufficiency leads to obesity and multifaceted behavioral alterations in mice. Mol Autism. 2022;13:19. https://doi.org/10.1186/s13229-022-00497-3.
Miskinyte G, Devaraju K, Grønning Hansen M, Monni E, Tornero D, Woods NB, et al. Direct conversion of human fibroblasts to functional excitatory cortical neurons integrating into human neural networks. Stem Cell Res Ther. BioMed Central Ltd.; 2017. p. 8. https://doi.org/10.1186/s13287-017-0658-3.
Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–41. https://doi.org/10.1038/nature08797.
Liang XG, Tan C, Wang CK, Tao RR, Huang YJ, Ma KF, et al. Myt1l induced direct reprogramming of pericytes into cholinergic neurons. CNS Neurosci Ther Blackwell Publishing Ltd. 2018;24:801–9. https://doi.org/10.1111/cns.12821.
Chen J, Yen A, Florian CP, Dougherty JD. MYT1L in the making: emerging insights on functions of a neurodevelopmental disorder gene. Transl Psychiatry. 2022;12:292. https://doi.org/10.1038/s41398-022-02058-x.
White SM, Thompson EM, Kidd A, Savarirayan R, Turner A, Amor D, et al. Growth, behavior, and clinical findings in 27 patients with kabuki (Niikawa-Kuroki) syndrome. Am J Med Genet. 2004;127A:118–27. https://doi.org/10.1002/ajmg.a.20674.
Milani D, Manzoni FMP, Pezzani L, Ajmone P, Gervasini C, Menni F, et al. Rubinstein-Taybi syndrome: clinical features, genetic basis, diagnosis, and management. Ital J Pediatr. 2015;41:4. https://doi.org/10.1186/s13052-015-0110-1.
Suda K, Fukuoka H, Iguchi G, Kanie K, Fujita Y, Odake Y, et al. A case of Luscan-Lumish Syndrome: possible involvement of enhanced GH signaling. J Clin Endocrinol Metab. 2021;106:718–23. https://doi.org/10.1210/clinem/dgaa893.
Zhang Y, Zhang H, Wu W, Wang D, Lv Y, Zhao D, et al. Clinical and genetic features of luscan-lumish syndrome associated with a novel de novo variant of SETD2 gene: case report and literature review. Front Genet. 2023:14. https://doi.org/10.3389/fgene.2023.1081391.
Caputo M, Daffara T, Bellone S, Mancioppi V, Marzullo P, Aimaretti G, et al. Case report: Liraglutide for weight Management in Beckwith-Wiedemann Syndromic Obesity. Front Endocrinol (Lausanne). 2021:12. https://doi.org/10.3389/fendo.2021.687918.
Manor J, Lalani SR. Overgrowth syndromes—evaluation, diagnosis, and management. Front Pediatr. 2020;8 https://doi.org/10.3389/fped.2020.574857.
Kaur H, Panigrahi I. Chung–Jansen Syndrome with obesity. Obes Res Clin Pract. 2021;15:303–5. https://doi.org/10.1016/j.orcp.2021.03.015.
Jansen S, Hoischen A, Coe BP, Carvill GL, Van Esch H, Bosch DGM, et al. A genotype-first approach identifies an intellectual disability-overweight syndrome caused by PHIP haploinsufficiency. Eur J Hum Genet. 2018;26:54–63. https://doi.org/10.1038/s41431-017-0039-5.
White SM, Bhoj E, Nellåker C, Lachmeijer AMA, Marshall AE, Boycott KM, et al. A DNA repair disorder caused by de novo monoallelic DDB1 variants is associated with a neurodevelopmental syndrome. Am J Hum Genet. 2021;108:749–56. https://doi.org/10.1016/j.ajhg.2021.03.007.
Cali E, Suri M, Scala M, Ferla MP, Alavi S, Faqeih EA, et al. Biallelic PRMT7 pathogenic variants are associated with a recognizable syndromic neurodevelopmental disorder with short stature, obesity, and craniofacial and digital abnormalities. Genet Med. 2023;25:135–42. https://doi.org/10.1016/j.gim.2022.09.016.
Yang L, Zhang W, Peng J, Yin F. Heterozygous KIDINS220 mutation leads to spastic paraplegia and obesity in an Asian girl. Eur J Neurol. 2018;25:e53–4. https://doi.org/10.1111/ene.13600.
Josifova DJ, Monroe GR, Tessadori F, de Graaff E, van der Zwaag B, Mehta SG, et al. Heterozygous KIDINS220/ARMS nonsense variants cause spastic paraplegia, intellectual disability, nystagmus, and obesity. Hum Mol Genet. 2016;25:2158–67. https://doi.org/10.1093/hmg/ddw082.
Aerden M, Denommé-Pichon A-S, Bonneau D, Bruel A-L, Delanne J, Gérard B, et al. The neurodevelopmental and facial phenotype in individuals with a TRIP12 variant. Eur J Hum Genet. 2023; https://doi.org/10.1038/s41431-023-01307-x.
Bosch E, Hebebrand M, Popp B, Penger T, Behring B, Cox H, et al. BDV Syndrome: an emerging Syndrome with profound obesity and neurodevelopmental delay resembling Prader-Willi Syndrome. J Clin Endocrinol Metab. 2021; https://doi.org/10.1210/clinem/dgab592.
Durmaz A, Aykut A, Atik T, Özen S, Ayyıldız Emecen D, Ata A, et al. A new cause of obesity Syndrome associated with a mutation in the carboxypeptidase gene detected in three siblings with obesity, intellectual disability and hypogonadotropic hypogonadism. J Clin Res Pediatr Endocrinol. 2021;13:52–60. https://doi.org/10.4274/jcrpe.galenos.2020.2020.0101.
Vuillaume ML, Naudion S, Banneau G, Diene G, Cartault A, Cailley D, et al. New candidate loci identified by array-CGH in a cohort of 100 children presenting with syndromic obesity. Am J Med Genet Part A Wiley-Liss Inc. 2014;164:1965–75. https://doi.org/10.1002/ajmg.a.36587.
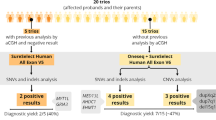
•• Carvalho LML, D’Angelo CS, Villela D, da Costa SS, de Lima Jorge AA, da Silva IT, et al. Genetic investigation of syndromic forms of obesity. Int J Obes. 2022. https://doi.org/10.1038/s41366-022-01149-5. First study applying a comprehensive genomic investigation of an syndromic obesity cohort, showing a high diagnostic yield (~47%). Relevant to understanding that methods of genome-wide investigation should be prioritized over target sequencing of panels. Furthermore, this publication demonstrates that it is necessary to consider the possibility that the etiology of the syndromic condition may be independent of obesity.
Emerson E, Robertson J, Baines S, Hatton C. Obesity in British children with and without intellectual disability: cohort study. BMC Public Health. 2016;16:644. https://doi.org/10.1186/s12889-016-3309-1.
Hsieh K, Rimmer JH, Heller T. Obesity and associated factors in adults with intellectual disability. J Intellect Disabil Res. 2014;58:851–63. https://doi.org/10.1111/jir.12100.
Melville CA, Cooper S-A, Morrison J, Allan L, Smiley E, Williamson A. The prevalence and determinants of obesity in adults with intellectual disabilities. J Appl Res Intellect Disabil. 2008;21:425–37. https://doi.org/10.1111/j.1468-3148.2007.00412.x.
Melville CA, Oppewal A, Schäfer Elinder L, Freiberger E, Guerra-Balic M, Hilgenkamp TIM, et al. Definitions, measurement and prevalence of sedentary behaviour in adults with intellectual disabilities — a systematic review. Prev Med (Baltim). 2017;97:62–71. https://doi.org/10.1016/j.ypmed.2016.12.052.
de Winter CF, Bastiaanse LP, Hilgenkamp TIM, Evenhuis HM, Echteld MA. Overweight and obesity in older people with intellectual disability. Res Dev Disabil. 2012;33:398–405. https://doi.org/10.1016/j.ridd.2011.09.022.
Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63:824. https://doi.org/10.1001/archpsyc.63.7.824.
Chao AM, Wadden TA, Berkowitz RI. Obesity in adolescents with psychiatric disorders. Curr Psychiatry Rep. 2019;21:3. https://doi.org/10.1007/s11920-019-0990-7.
Avila C, Holloway AC, Hahn MK, Morrison KM, Restivo M, Anglin R, et al. An overview of links between obesity and mental health. Curr Obes Rep. 2015;4:303–10. https://doi.org/10.1007/s13679-015-0164-9.
Martins LB, Monteze NM, Calarge C, Ferreira AVM, Teixeira AL. Pathways linking obesity to neuropsychiatric disorders. Nutrition. 2019;66:16–21. https://doi.org/10.1016/j.nut.2019.03.017.
Edwards G, Jones C, Pearson E, Royston R, Oliver C, Tarver J, et al. Prevalence of anxiety symptomatology and diagnosis in syndromic intellectual disability: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2022;138:104719. https://doi.org/10.1016/j.neubiorev.2022.104719.
Fusco SDFB, Amancio SCP, Pancieri AP, Alves MVMFF, Spiri WC, Braga EM. Ansiedade, qualidade do sono e compulsão alimentar em adultos com sobrepeso ou obesidade. Rev da Esc Enferm da USP. 2020:54. https://doi.org/10.1590/s1980-220x2019013903656.
Yang L, Zhou Q, Ma B, Mao S, Dai Y, Zhu M, et al. Perinatal features of Prader-Willi syndrome: a Chinese cohort of 134 patients. Orphanet J Rare Dis. 2020;15:24. https://doi.org/10.1186/s13023-020-1306-z.
Gross N, Rabinowitz R, Gross-Tsur V, Hirsch HJ, Eldar-Geva T. Prader-Willi syndrome can be diagnosed prenatally. Am J Med Genet Part A. 2015;167:80–5. https://doi.org/10.1002/ajmg.a.36812.
Mackay DJG, Temple IK. Human imprinting disorders: principles, practice, problems and progress. Eur J Med Genet. 2017;60:618–26. https://doi.org/10.1016/j.ejmg.2017.08.014.
Lewis EM, Kroll KL. Development and disease in a dish: the epigenetics of neurodevelopmental disorders. Epigenomics. 2018;10:219–31. https://doi.org/10.2217/epi-2017-0113.
Mariman ECM, Vink RG, Roumans NJT, Bouwman FG, Stumpel CTRM, Aller EEJG, et al. The cilium: a cellular antenna with an influence on obesity risk. Br J Nutr. 2016;116:576–92. https://doi.org/10.1017/S0007114516002282.
Blesson A, Cohen JS. Genetic counseling in neurodevelopmental disorders. Cold Spring Harb Perspect Med. 2020;10:a036533. https://doi.org/10.1101/cshperspect.a036533.
Availability of Data and Materials
Not applicable
Funding
This study was supported by São Paulo Research Foundation - FAPESP (2022/03980–5, 2018/08486–3 and 2013/08028–1), National Council for Scientific and Technological Development - CNPq (3051012022–6 and 303294/2020–5) and Coordination for the Improvement of Higher Education Personnel - CAPES (1805008).
Author information
Authors and Affiliations
Contributions
LMLC prepared the first version of the manuscript, figures, and tables. All other authors reviewed and made relevant subsequent contributions.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there are no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Carvalho, L.M.L., Jorge, A.A., Bertola, D.R. et al. A Comprehensive Review of Syndromic Forms of Obesity: Genetic Etiology, Clinical Features and Molecular Diagnosis. Curr Obes Rep (2024). https://doi.org/10.1007/s13679-023-00543-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s13679-023-00543-y