Abstract
Purpose of Review
Bronchoscopy-related infection is recognized as a leading healthcare hazard, and this review delves into its incidence, causes, and prevention.
Recent Findings
Due to both the recognition of the scale of bronchoscopy-related infection and the COVID-19 pandemic, single-use or disposable bronchoscopy (SUFB) technology has progressed beyond the ICU setting to the bronchoscopy suite. A review of data related to currently available scopes, bench-top, and clinical data is also presented.
Summary
Not only does SUFB technology provide a portable and immediately accessible bronchoscope for procedures in the ICU and endoscopy suite, it also is a sterile option for standard bronchoscopy procedures thus avoiding infection related to scope contamination. Cost-effectiveness comparisons vary between single-use and reusable scopes depending on calculated incidence of scope-related infection. Although no one should suffer an infection related to bronchoscopy, SUFB technology lacks proper comparison to reusable scope quality. In the meantime, the most important intervention is proper cleaning and assessment of reusable scopes for damage.
Similar content being viewed by others
Introduction
Prior to the COVID-19 pandemic, there was gathering evidence regarding the risk of infection secondary to bronchoscopy procedures [1••]. It was also recognized that primary causes of these infections were scope damage and inadequate cleaning [2•]. International bodies subsequently recognized and reported their concerns regarding this health hazard [3]. In parallel, single-use or disposable bronchoscopy (SUFB) technology was improving with progression in use from the intensive care unit [4] to the bronchoscopy suite [5,6,7]. Due to the portability, accessibility, sterility, and the lack of requirement for the staff to prepare or clean the equipment, the COVID-19 pandemic was associated with an exponential growth in the use of SUFB [8, 9].
Both infection risk related to the reusable flexible bronchoscopes (RFB) and an up-to-date assessment of SUFB technology are included in this review.
Infectious Risk Related to Reusable Bronchoscopes
Bronchoscopes are classified as semi-critical goods by the Spaulding Classification System because they encounter healthy skin and mucous membranes [10]. Semi-critical instruments must go through at least a high-level disinfection (HLD), which entails the total elimination of all microorganisms in or on an instrument, except for a tiny number of spores, following the published norms and manufacturer’s instructions. It is important to state that this differs from sterilization either through autoclaving or a chemical process. A RFB would have a limited lifespan if it was chemically sterilized after each use. Besides, the components of modern bronchoscopes are not compatible with temperatures required for autoclaving. According to the Food and Drug Administration (FDA), high-level disinfection comprises using a sterilant for a shorter contact time to kill an appropriate Mycobacterium species by 6-log10. Enough pathogens should be removed through manual cleaning and high-level disinfection to stop the spread of infection [11, 12].
Reusable endoscopes [including RFBs] are associated with more outbreaks than any other medical equipment [12]. A consensus statement from 2005 suggested that the risk of bronchoscope-related infections was also underrecognized in addition to being underreported and generally lacking active surveillance intended to find post-procedure infections [13].
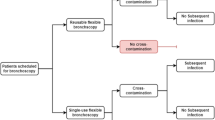
This questions the adequacy of current reprocessing and sterilization standards to maintain the safety of the patients. Reprocessing bronchoscopes is a gargantuan task and involves several steps, including pre-cleaning, leak testing, manual cleaning, visual inspection, disinfection, rinsing, drying, and storage (Fig. 1A) [11]. When bronchoscopes fail the leak test, they must be returned to the manufacturer for repairs and restoration. All these steps make the process of reprocessing a time-consuming and costly affair [2•]. Lack of adherence to the recommended reprocessing procedure has been associated with nearly all the reported cases of cross-infection [1••].

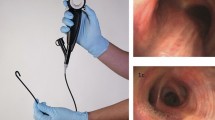
© Vortex, Vathin® H-Steriscope™ Large and Boston Scientific® EXALT™ Model B. C Ambu®single-use bronchoscopes. On the left is the Ambu® aScope™ 5 Broncho Large. On the right is the Ambu® aScope 4 Large. Note the progression in scope handle design with ability to rotate 120° left and right. D Axess Vision™ Single Use Flexible 2.8 mm Channel Vortex with the Screeni® viewing system placed in a mannequin used for bronchoscopic training
Reusable scope processing and single-use bronchoscopy. A Pathway depicting reprocessing step for a reusable bronchoscope. Steps at risk for contamination and cleaning failure are indicated by the red exclamation mark and warning triangle. B Five commercially available single-use bronchoscopes, from left to right: Pentax® Medical ONE Pulmo™, Ambu® aScope 4 Large, The Surgical Company Broncoflex
Bronchoscopes that are not promptly cleaned after usage can develop a biofilm. A biofilm describes an accumulated collection of microorganisms and extracellular matter that is firmly attached to a surface and difficult to remove. Bronchoscopes’ inner, constrictive tubes and any fissures or abnormalities on their surfaces are infamous places for forming biofilms. They frequently harbor harmful pathogens, such as carbapenem-resistant Enterobacteriaceae and related multi-drug resistance organisms (sometimes known as “superbugs”) and have been linked to numerous outbreaks that have been documented [1, 11].
Retrospective data has also demonstrated infection with multidrug-resistant organisms (MDROs) attributed to exposure to a common bronchoscope. In one study, 23 out of 33 patients were exposed to MDR Pseudomonas aeruginosa and Klebsiella pneumoniae via a common bronchoscope. These organisms were recovered from the lumen of the bronchoscope, and evaluation with borescopy revealed a luminal defect [14]. Infections caused by MDROs, particularly carbapenem-resistant Enterobacteriaceae (CRE), are especially worrisome as they have shown mortality rates as high as 50% [15]. A study from 2013 linked a bronchoscope to an outbreak of CRE [16].
Despite HLD, the implicated bronchoscope, persistently demonstrated contamination. The bronchoscope was returned to the manufacturer for repairs, demonstrating worn-out components and surface flaws in both bronchoscopes’ functioning channels. After repair, no further infections were identified [1••].
While SUFBs have been around since the mid-2000s, the COVID-19 pandemic has emphasized infection control during the endoscopic examination because it is an aerosol-generating procedure. This has created an increased focus on this relatively novel instrument [17].
Reprocessing
In 2015, the US Food and Drug Administration (FDA) published a safety communication describing reports of residual contamination and risk of infection following bronchoscopy with RFBs. The FDA investigation analyzed medical device reports (MDRs) submitted to FDA from manufacturers and healthcare facilities. Between January 2010 and June 2015, the FDA received 109 MDRs concerning infections or device contamination associated with flexible bronchoscopes. Compared to the number of bronchoscopy operations performed in the USA each year, this is considered a minimal number of MDRs. However, in 2014, the FDA received 50 MDRs that cited illnesses or device contamination related to reprocessed flexible bronchoscopes, which spurred additional studies on this issue. Despite following the manufacturer’s recommendations for reprocessing, a tiny percentage of these complaints point to ongoing device contamination. To determine whether device contamination persisted despite meticulous adherence to the manufacturer’s reprocessing instructions and whether other factors may have contributed to these events, the FDA continues to assess these reports through follow-ups with healthcare facilities and manufacturers [18].
In addition to the FDA investigations, the Centre for Disease Control (CDC) Division of Healthcare Quality Promotion (DHQP) conducted 15 separate investigations of bronchoscope-associated outbreaks between 2014 and 2019 involving around 150 patients. Organisms implicated in these outbreaks included nontuberculous mycobacteria and various bacterial and fungal species [19].
A recent high impact study included evaluations of storage conditions, manual cleaning evaluations, high-level disinfection evaluations, and direct observation of reprocessing techniques for flexible bronchoscopes [2•]. Lighted magnification and borescopes were used to examine ports and channels visually. They used microbial cultures and assays for protein, hemoglobin, and adenosine triphosphate (ATP) to evaluate for evidence of contamination. Researchers analyzed 24 bronchoscopes used in clinical settings across three sites.
Of those 24 bronchoscopes, 100% still had residual contamination after manual cleaning. Fourteen fully reprocessed bronchoscopes (58%) had mold, Stenotrophomonas maltophilia, and Escherichia coli/Shigella species growing. The bronchoscopes had noticeable flaws, such as residual fluid, dark, red, or greasy residue, scratches, broken insertion tubes and distal ends, and filamentous debris in channels. At two out of three sites, reprocessing procedures fell short of standards.
These reports show that reprocessing failures are frequent and cause significant patient harm. This is even more relevant in the immunocompromised population, such as lung transplant recipients who require frequent bronchoscopies [1••].
Recontamination
Endoscopy has been highlighted multiple times in the Emergency Care Research Institute (ECRI) Top 10 Health Technology Hazards since 2016 [3]. Points of concern include endoscope reprocessing failures and inadequate cleaning to recently noted recontamination after disinfection. The handling of an endoscope following disinfection can become compromised at multiple points. Endoscopes must be wholly dried following high-level disinfection to prevent viable leftover germs from quickly multiplying and colonizing the equipment. After reprocessing, the ECRI and pertinent professional organizations advise purging endoscope channels with clean air to encourage drying [3].
Endoscopes can lose their disinfected condition if handled with dirty gloves, a practice that the ECRI has noted. Gloves used to handle an endoscope at that stage must not be used to remove the scope from the reprocessing machine since those endoscopes are still contaminated with live germs even after being cleaned but before high-level disinfection [20]. Recontamination can also happen when endoscopes are moved around and kept in storage. Those cleaned and dried should be transported in a clean container only. They should also avoid meeting any potentially dirty surfaces [3].
Single-Use Flexible Bronchoscopy
Single-use flexible bronchoscopy (SUFB) use scopes which connect typically to a portable monitor allowing sterile procedures not limited by poorly mobile burdensome equipment (Fig. 1B, C). Scopes are for single use and completely sterile thus bypassing any risk of infection related to improper high-level disinfection or scope damage. Although not scientifically proven, it is hypothesized that SUFBs should reduce the risk of bronchoscopy-related infection [21•]. SUFBs have many other advantages including facilitating the procedure in and outside the ICU or bronchoscopy suite, eliminating the need for bulky and expensive processor, and monitoring requirement, storage space, and ongoing maintenance especially during pandemics and off hours emergency procedures [10].
Other advantages include reducing damage to the reusable bronchoscopes during training or its use through a rigid bronchoscope.
The FDA has made a recommendation to consider SUFBs where there is increased risk of spreading infection (for example, multidrug resistant microorganisms, immunocompromised patients, or patients with prion disease) or when there is no support for immediate reprocessing of the bronchoscope [22]. During the COVID-19 pandemic, several international bodies released consensus statements suggesting the use of SUFBs [23,24,25,26,27]. Upon recent internet search, up to 15 companies have either released or developed SUFB iterations.
Initial Development and ICU Data
The first scope was developed by Ambu® in 2009 for intubation, and subsequent developments have been associated with reports regarding intubation, percutaneous tracheostomy, bronchoalveolar lavage, and management of hemoptysis [4, 10]. However, data includes predominantly case series and retrospective studies without prospective trials with rigorous outcome analysis [28,29,30,31,32]. On assessment of the structure of the initial Ambu® aScope 1–4 designs, the handle of the scope was significantly different in design to standard flexible bronchoscopes (Fig. 1D). However, further scope development (including the Ambu® aScope 5 (Fig. 1D) by many medical technology companies have led to better design, suction, flexibility, and procedure capabilities [33].
Benchtop Comparisons
Scope development has included several benchtop comparisons [34, 35]. Our institution published a study examining the specifications, user ratings, and benchtop performance of SUFBs with CE approval between mid-2021 and 2022. Bronchoscopes included in the study were Ambu® aScope 4™ Large, Boston Scientific® EXALT™ Model B Large, The Surgical Company Broncoflex© Vortex, Vathin® H-Steriscope™ Large, and Pentax® Medical ONE Pulmo™. Scope channel diameters were 2.8–3 mm [33]. Testing included simulation tests on a low fidelity simulator (including intensive care and pulmonary physicians) and benchtop analysis including size and weight measurements, handle design, detailed analysis of scope flexion, extension, and turning angle with and without in situ devices. A pseudo-mucus liquid was also used to test suction capabilities with and without device in situ. Scopes differed in gender and hand size preference and maintenance of turning angle with device in situ. The Broncoflex© Vortex was preferred in simulation testing (without suction or image analysis); however, the Boston Scientific® EXALT™ Model B radically outperformed all other scopes including a reusable 3.2-mm channel scope in suction capabilities. Other benchtop and pilot studies have commented on BAL adequacy in comparison to historical controls [36]. Differences in benchtop and simulation testing should translate into clinical applications.
SUFB in Bronchoscopy Suite
Several studies have been published regarding SUFB use in the bronchoscopy suite [5,6,7].
The first was a prospective Spanish multi-center study of 300 patients using the AMBU® aScope 4 [7]. Thirty-six bronchoscopists participated, and most procedures were lavages or washings. Post procedure questionnaires were completed and sent into the primary center. Only 17 patients had a biopsy, and there were no needle aspirations, brushings, or any advanced techniques. Most procedures were satisfactory with reversion to a reusable scope in 5.7%. Reasons for changing the scope included scope damage, suction, and image quality. Our center subsequently published two prospective single-center studies regarding our experience [5, 6]. The first trial collected data prospectively on 139 procedures using the Surgical Company Broncoflex© range of SUFBs [5]. The majority were carried out for infection (45%) and malignancy (32%). Procedures included advanced bronchoscopy techniques such as transbronchial needle aspiration (TBNA), electrocautery, endobronchial and transbronchial biopsy, and cryobiopsy. Most were performed in the endoscopy suite, and 8% were COVID positive or suspected. Most procedures reported the highest score in satisfaction (83%) with technical limitations reported in 15% (predominately related to scope suction or inadequate image quality) reverting to a reusable scope in 3%. Our center also recently published our experience with the Boston Scientific® EXALT™ series of scopes [5]. Data was collected on 24 sequential cases including endobronchial, transbronchial biopsy, and cryobiopsy. Two debulking procedures were performed through a rigid bronchoscope including biopsy, mechanical debulking, argon plasma coagulation, and electrocautery using the 2.8 EXALT™ SUFB. In similar fashion to pre-clinical studies [33, 35], superior suction with impressive capabilities of managing hemoptysis was noted.
Environmental Impact
The environmental impact is an ongoing concern as the medical field moves further toward single-use devices. It is pertinent to evaluate the environmental implications of SUFBs. A comparative study published in 2018 evaluated the carbon dioxide (CO2)-equivalent emissions and resource consumption from a single-use bronchoscope (Ambu® aScope™ 4) versus RFBs. This study noted that the use of personal protective equipment (PPE) along with washing and drying processes for the RFBs contributed significantly to the environmental burden. If one set of PPE was used per RFB reprocessing, this had an equivalent or even higher environmental impact than SUFBs. However, the authors noted that reprocessing practices for RFBs vary highly and so could not conclude as to which device had a more significant environmental impact overall [37]. Further environmental impact assessments are needed, but it is reassuring that this initial study did not find that SUFBs were inferior from an environmental viewpoint.
Role in Training
SUFB has great potential in their role for training and in particular simulation-based learning. Given the high cost of RFBs including the reprocessing and repairs, it is not economical to use RFBs as training devices. Our institution has published on the use of single-use bronchoscopes coupled with a low-cost bio-simulator ALFIE™ as a training tool. This model allowed trainees to improve their scope handling techniques and for simulated procedures such as endobronchial biopsy and brushing [38].
Cost Comparisons of Single Use and Reusable Bronchoscopes Table 1
If a healthcare system is considering switching from RFBs to SUFBs, one of the most important factors is the cost. Several cost-effective analyses have been published including meta-analysis and systematic reviews (Table 1) [21, 39,40,41,42].
Multiple factors other than cost of the equipment need to be considered. While comparing SUFBs and RFBs, less staff are required for SUFBs [28, 42]. Furthermore, SUFBs reduce the risk of infection to staff, delays between the procedures, and reliance on staff and logistical support associated with repair, reprocessing, microbial surveillance, and maintenance of accreditation/certification of reprocessing rooms [42].
One analysis estimated the cost of SUFB at €232 per procedure, similar to that reported elsewhere in the literature [42]. They concluded that SUFBs were associated with a significantly higher cost, and the reason their analysis differed to other published literature was likely the result of the economy of performing large number of procedures (1500 bronchoscopies annually). Their estimated cost of €78 per procedure with RFBs at their institution may be less than other centers. The authors concluded in a hospital performing less than 328 bronchoscopies a year, the use of SUFBs could become economically viable. However, the authors highlighted that assessing SUFBs from a cost viewpoint alone does not factor in many other organizational factors such as risk of infection, simplified institutional processes, staff stress, ease of access during an emergency, teaching opportunities, risk to infection to the staff, and the impact on high-risk patient groups [42]. Two more recent studies mirrored these findings [39, 41]. Another analysis concluded that in a larger academic institution with high procedural numbers, SUFBs may be more expensive. Cost comparison depends on a number of annual procedures, cost and incidence of scope-related infection, and cost of SUFBs [39]. The other identified no difference in cost [41].
While considering cross-contamination and potential follow-up infections, a previously published cost-effectiveness study of SUFBs in a typical intensive care unit (ICU) in the USA showed that implementation is cost-effective and linked to improved patient safety [43].
A unique systematic review of sixteen studies sought to calculate the risk of cross-contamination or infection following using RFBs in any clinical setting [21•]. Further, it determined the cost of treating the clinical consequences of such infections. In this cost-effectiveness analysis, the effect measure was the averted risk of infection while using SUFBs instead of RFBs. To represent a more accurate estimate, the risk of infection and cross-contamination was calculated using a weighted average with a fixed-effect model. Since no incidents of cross-contamination with SUFBs have been recorded to date, the risk is anticipated to be 0% [21•]. Their systematic evaluation showed the risk of patient infection following bronchoscopy with a cost per use of an RFB of £249 and a cost per use of a SUFB of £220. However, if a 2.8% risk of bronchoscopy-related infection is included with added costs of treating an infection, this cost-effectiveness analysis increased the cost per patient usage of £511 for RFB compared to £220 cost per use of SUFBs.
Conclusions
No patient should suffer from preventable nosocomial infections due to bronchoscopy. Using bronchoscopes with physical defects and harboring viruses, bacteria, or fungi places patients at risk and could adversely affect public health. Institutions must verify that bronchoscope reprocessing procedures follow regulations and manufacturer instructions to protect patients and reprocessing workers. These challenges related to infection control and reprocessing of RFBs in current clinical practice coupled with the COVID-19 pandemic have led to an exciting surge in research and innovation in the field of SUFBs. Cost-effectiveness analysis varies. Without estimation of cost of scope-related infection, cost analysis supports RFBs in centers with high procedure numbers (> 300). However, if cost of infection is added in, the argument sways towards SUFBs. A change is required from single-center studies to proper randomized trials between SUFBs and RFBs to identify whether this potential reduction in infection and cost does not come at the expense of the quality of standard and advanced bronchoscopy procedures.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Mehta AC, Muscarella LF. Bronchoscope-related “superbug” infections. Chest. 2020;157(2):454–69. https://doi.org/10.1016/J.CHEST.2019.08.003. This landmark review article simply presents the bronchoscope related risk of infection to our patients.
• Ofstead CL, Quick MR, Wetzler HP, et al. Effectiveness of reprocessing for flexible bronchoscopes and endobronchial ultrasound bronchoscopes. Chest. 2018;154(5):1024–34. https://doi.org/10.1016/j.chest.2018.04.045. Results from this highly cited research identify real world pitfalls in bronchoscope cleaning.
Top 10 Health Technology Hazards for 2019. Accessed 25 Mar 2022. https://www.ecri.org/top-ten-tech-hazards.
Ho E, Wagh A, Hogarth K, Murgu S. Single-use and reusable flexible bronchoscopes in pulmonary and critical care medicine. Diagnostics (Basel). 2022;12(1). https://doi.org/10.3390/diagnostics12010174.
O’Reilly EM, Sweeney AM, Deasy KF, Kennedy MP. A pilot clinical evaluation of a new single use bronchoscope. J Bronchology Interv Pulmonol. 2022;1. https://doi.org/10.1097/LBR.0000000000000904.
Sweeney AM, Kavanagh G, Deasy KF, et al. Single-use or disposable flexible bronchoscopy in advanced bronchoscopy procedures: experience in a quaternary referral centre. Respiration. 2022;101(10):931–8. https://doi.org/10.1159/000526214.
Flandes J, Giraldo-Cadavid LF, Alfayate J, et al. Bronchoscopist’s perception of the quality of the single-use bronchoscope (Ambu aScope4TM) in selected bronchoscopies: a multicenter study in 21 Spanish pulmonology services. Respir Res. 2020;21(1). https://doi.org/10.1186/s12931-020-01576-w.
Barron S, Kennedy MP. Single-use bronchoscopes: applications in COVID-19 pandemic. J Bronchology Interv Pulmonol. 2021;28(1):E3–4. https://doi.org/10.1097/LBR.0000000000000685.
Barron S, Kennedy MP. Can single-use bronchoscopes help prevent nosocomial COVID-19 infections? Expert Rev Med Devices. 2021;18(5):439–43. https://doi.org/10.1080/17434440.2021.1920924.
Barron SP, Kennedy MP. Single-use (disposable) flexible bronchoscopes: the future of bronchoscopy? Adv Ther. 2020;37(11):4538–48. https://doi.org/10.1007/S12325-020-01495-8.
Chhabria MS, Maldonado F, Mehta AC. Infection control in the bronchoscopy suite: effective reprocessing and disinfection of reusable bronchoscopes. Curr Opin Pulm Med. 2023;29(1):21–8. https://doi.org/10.1097/MCP.0000000000000925.
Rutala W, Weber D. Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008; Miscellaneous Inactivating Agents.
Mehta AC, Prakash UBS, Garland R, et al. American College of Chest Physicians and American Association for Bronchology [corrected] consensus statement: prevention of flexible bronchoscopy-associated infection. Chest. 2005;128(3):1742–55. https://doi.org/10.1378/CHEST.128.3.1742.
Galdys AL, Marsh JW, Delgado E, et al. Bronchoscope-associated clusters of multidrug-resistant Pseudomonas aeruginosa and carbapenem-resistant Klebsiella pneumoniae. Infect Control Hosp Epidemiol. 2019;40(1):40–6. https://doi.org/10.1017/ICE.2018.263.
Muscarella LF. Risk of transmission of carbapenem-resistant Enterobacteriaceae and related “superbugs” during gastrointestinal endoscopy. World J Gastrointest Endosc. 2014;6(10):457. https://doi.org/10.4253/WJGE.V6.I10.457.
Klefisch F. A flexible bronchoscope as a source of an outbreak with OXA-48 carbapenemase producing Klebsiella pneumoniae. Hygiene + Medizin. 2015;1(40):8–34.
Ofstead CL, Hopkins KM, Binnicker MJ, Poland GA. Potential impact of contaminated bronchoscopes on novel coronavirus disease (COVID-19) patients. Infect Control Hosp Epidemiol. 2020;41(7):862–4. https://doi.org/10.1017/ice.2020.102.
Infections Associated with Reprocessed Flexible Bronchoscopes: FDA Safety Communication. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm462949.htm.
Bardossy A, Novosad S, Perkins K, Moulton-Meissner HA, Arduino M, Benowitz I. Bronchoscope-related outbreaks and pseudo-outbreaks: CDC Consultations—United States, 2014–2019. Infect Control Hosp Epidemiol. 2020;41(S1):s144–s144. https://doi.org/10.1017/ice.2020.660.
A Brief Look at ECRI Institute’s 2019 Top 10 Health Technology Hazards. J Radiol Nurs. 2019;38(1). https://doi.org/10.1016/j.jradnu.2019.01.001.
• Mouritsen JM, Ehlers L, Kovaleva J, Ahmad I, El-Boghdadly K. A systematic review and cost effectiveness analysis of reusable vs. single-use flexible bronchoscopes. Anaesthesia. 2020;75(4):529–40. https://doi.org/10.1111/anae.14891. This article offers a robust cost-effective analysis of single use and reusable bronchoscopy.
Administration UF and D. Flexible Bronchoscopes and Updated Recommendations for Reprocessing: FDA Safety Communication.
Society IT. Irish Thoracic Society Statement on Bronchoscopy and SARS COVID-19. 2020.
Guedes F, Boléo-Tomé JP, Rodrigues LV, et al. Recommendations for interventional pulmonology during COVID-19 outbreak: a consensus statement from the Portuguese Pulmonology Society. Pulmonology. 2020;26(6):386–97. https://doi.org/10.1016/j.pulmoe.2020.07.007.
Pritchett MA, Oberg CL, Belanger A, et al. Society for Advanced Bronchoscopy Consensus Statement and Guidelines for bronchoscopy and airway management amid the COVID-19 pandemic. J Thorac Dis. 2020;12(5):1781–1798. https://doi.org/10.21037/jtd.2020.04.32.
Wahidi MM, Lamb C, Murgu S, et al. American Association for Bronchology and Interventional Pulmonology (AABIP) Statement on the Use of Bronchoscopy and Respiratory Specimen Collection in Patients With Suspected or Confirmed COVID-19 Infection. J Bronchology Interv Pulmonol. 2020;27(4):e52–4. https://doi.org/10.1097/LBR.0000000000000681.
Chang SH, Jiang J, Kon ZN, et al. Safety and efficacy of bronchoscopy in critically ill patients with coronavirus disease 2019. Chest. 2021;159(2):870–2. https://doi.org/10.1016/j.chest.2020.09.263.
Marshall DC, Dagaonkar RS, Yeow C, et al. Experience with the use of single-use disposable bronchoscope in the ICU in a tertiary referral center of Singapore. J Bronchology Interv Pulmonol. 2017;24(2):136–43. https://doi.org/10.1097/LBR.0000000000000335.
Kriege M, Dalberg J, McGrath BA, et al. Evaluation of intubation and intensive care use of the new Ambu® aScopeTM 4 broncho and Ambu® aViewTM compared to a customary flexible endoscope a multicentre prospective, non-interventional study. Trends in Anaesthesia and Critical Care. 2020;31:35–41. https://doi.org/10.1016/j.tacc.2020.02.001.
Gao CA, Bailey JI, Walter JM, et al. Bronchoscopy on intubated patients with COVID-19 is associated with low infectious risk to operators. Ann Am Thorac Soc. 2021;18(7):1243–6. https://doi.org/10.1513/AnnalsATS.202009-1225RL.
Niroula A, Van Nostrand KM, Khullar OV, et al. Percutaneous tracheostomy with apnea during coronavirus disease 2019 era: a protocol and brief report of cases. Crit Care Explor. 2020;2(5):e0134. https://doi.org/10.1097/CCE.0000000000000134.
Reynolds S, Zurba J, Duggan L. A single-centre case series assessing the Ambu(®) aScopeTM 2 for percutaneous tracheostomies: a viable alternative to fibreoptic bronchoscopes. Can J Respir Ther. 2015;51(2):43–5.
Deasy K, Sweeney. Ane-Marie, Danish H, O’ Reilly E, Ibrahim H, Kennedy MP. Single Use or disposable flexible bronchoscopes: bench top and pre-clinical comparison of currently available devices. J Intensive Care Med. 2023;38(6):519–28
Liu L, Wahidi M, Mahmood K, Giovacchini C, Shofer S, Cheng G. Operator perception of a single-use flexible bronchoscope: comparison with current standard bronchoscopes. Respir Care. 2020;65(11):1655–62. https://doi.org/10.4187/respcare.07574.
Lamb CR, Yavarovich E, Kang V, et al. Performance of a new single-use bronchoscope versus a marketed single-use comparator: a bench study. BMC Pulm Med. 2022;22(1):189. https://doi.org/10.1186/S12890-022-01982-4.
Singh S, Shah PL. Safe and efficient practice of bronchoscopic sampling from mechanically ventilated patients: a structured evaluation of the Ambu Bronchosampler-Ascope 4 Integrated System. Respiration. 2021;100(1):27–33. https://doi.org/10.1159/000511982.
Lilholt Sørensen B. comparative study on environmental impacts of reusable and single-use bronchoscopes. Am J Environ Prot. 2018;7(4):55. https://doi.org/10.11648/j.ajep.20180704.11.
Sweeney, A., Deasy, K. and Kennedy M. Combining a low-cost bio-simulator and single use or disposable bronchoscope: a platform for remote training. Open J Respir Dis. 2022;12(2). https://doi.org/10.4236/ojrd.2022.122006.
Kristensen AE, Kurman JS, Hogarth DK, Sethi S, Sørensen SS. systematic review and cost-consequence analysis of ambu ascope 5 broncho compared with reusable flexible bronchoscopes: insights from two US university hospitals and an academic institution. Pharmacoecon Open. Published online May 15, 2023. https://doi.org/10.1007/s41669-023-00417-y.
El-Boghdadly K, Mouritsen JM, Ehlers L, Kovaleva J, Ahmad I. Cost analysis of reusable vs. single-use flexible bronchoscopes: a reply. Anaesthesia. 2020;75(5):696–697. https://doi.org/10.1111/anae.15005.
Andersen CØ, Travis H, Dehlholm-Lambertsen E, Russell R, Jørgensen EP. The cost of flexible bronchoscopes: a systematic review and meta-analysis. Pharmacoecon Open. 2022;6(6):787–97. https://doi.org/10.1007/s41669-022-00356-0.
Châteauvieux C, Farah L, Guérot E, et al. Single-use flexible bronchoscopes compared with reusable bronchoscopes: positive organizational impact but a costly solution. J Eval Clin Pract. 2018;24(3):528–35. https://doi.org/10.1111/jep.12904.
Terjesen CL, Kovaleva J, Ehlers L. Early assessment of the likely cost effectiveness of single-use flexible video bronchoscopes. Pharmacoecon Open. 2017;1(2):133–41. https://doi.org/10.1007/S41669-017-0012-9.
Funding
Open Access funding provided by the IReL Consortium.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Authors NL, CY, MC, and AM declare that they have no conflict of interest. Author MK has received speaker fees and/or honorariums from Pentax® Medical, Ambu® Medical, The Surgical Company©, and Boston Scientific®. His institution also received equipment from The Surgical Company© for teaching physicians in training.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Logan, N., Yurosko, C., Mehta, A. et al. Bronchoscopy-Related Infection and the Development of Single-Use Bronchoscopy Technology. Curr Pulmonol Rep 12, 190–197 (2023). https://doi.org/10.1007/s13665-023-00328-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13665-023-00328-7