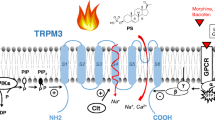
Abstract
Environmental temperature and cellular mechanical force are the inherent factors that participate in various biological processes and regulate cancer progress, which have been hot topics worldwide. They occupy a dominant part in the cancer tissues through different approaches. However, extensive investigation regarding pathological mechanisms in the carcinogenic field. After research, we found cold stress via two means to manipulate tumors: neuroscience and mechanically sensitive ion channels (MICHs) such as TRP families to regulate the physiological and pathological activities. Excessive cold stimulation mediated neuroscience acting on every cancer stage through the hypothalamus-pituitary-adrenocorticoid (HPA) to reach the target organs. Comparatively speaking, mechanical force via Piezo of MICHs controls cancer development. The progression of cancer depends on the internal activation of proto-oncogenes and the external tumorigenic factors; the above two means eventually lead to genetic disorders at the molecular level. This review summarizes the interaction of bidirectional communication between them and the tumor. It covers the main processes from cytoplasm to nucleus related to metastasis cascade and tumor immune escape.



Similar content being viewed by others
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70.
Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396(6712):643–9.
Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579–91.
Nikolaev YA, Cox CD, Ridone P, Rohde PR, Cordero-Morales JF, Vásquez V, et al. Mammalian TRP ion channels are insensitive to membrane stretch. J Cell Sci. 2019. https://doi.org/10.1242/jcs.238360.
Voskarides K. The, “cancer-cold” hypothesis and possible extensions for the Nordic populations. Scandinavian J Public Health. 2019;47(5):477–81.
Sharma A, Verma HK, Joshi S, Panwar MS, Mandal CC. A link between cold environment and cancer. Tumour Biol. 2015;36(8):5953–64.
Chatra K, Kuppili V, Edla DR, Verma AK. Cancer data classification using binary bat optimization and extreme learning machine with a novel fitness function. Med Biol Eng Comput. 2019;57(12):2673–82.
Wang Y, Guan J, Wang H, Wang Y, Leeper D, Iliakis G. Regulation of dna replication after heat shock by replication protein a-nucleolin interactions. J Biol Chem. 2001;276(23):20579–88.
Estrada LD, Ağaç D, Farrar JD. Sympathetic neural signaling via the β2-adrenergic receptor suppresses T-cell receptor-mediated human and mouse CD8(+) T-cell effector function. Eur J Immunol. 2016;46(8):1948–58.
Bucsek MJ, Giridharan T, MacDonald CR, Hylander BL, Repasky EA. An overview of the role of sympathetic regulation of immune responses in infectious disease and autoimmunity. Int J Hypertherm. 2018;34(2):135–43.
Robinson EL, Bagchi RA, Major JL, Bergman BC, Matsuda JL, McKinsey TA. HDAC11 inhibition triggers bimodal thermogenic pathways to circumvent adipocyte catecholamine resistance. J Clin Investig. 2023. https://doi.org/10.1172/JCI168192.
Jin J, Miao C, Wang Z, Zhang W, Zhang X, Xie X, et al. Design and synthesis of aryloxypropanolamine as β(3)-adrenergic receptor antagonist in cancer and lipolysis. Eur J Med Chem. 2018;150:757–70.
Sereni F, Dal Monte M, Filippi L, Bagnoli P. Role of host β1- and β2-adrenergic receptors in a murine model of B16 melanoma: functional involvement of β3-adrenergic receptors. Naunyn Schmiedebergs Arch Pharmacol. 2015;388(12):1317–31.
Bellinger DL, Lorton D. Autonomic regulation of cellular immune function. Auton Neurosci. 2014;182:15–41.
Cohen S, Levi-Montalcini R, Hamburger V. A nerve growth-stimulating factor isolated from Sarcom as 37 and 180. Proc Natl Acad Sci USA. 1954;40(10):1014–8.
Heitz F, Hengsbach A, Harter P, Traut A, Ataseven B, Schneider S, et al. Intake of selective beta blockers has no impact on survival in patients with epithelial ovarian cancer. Gynecol Oncol. 2017;144(1):181–6.
Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population- based study. J Clin Oncol. 2011;29(19):2635–44.
Melhem-Bertrandt A, Chavez-Macgregor M, Lei X, Brown EN, Lee RT, Meric-Bernstam F, et al. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol. 2011;29(19):2645–52.
Wang HM, Liao ZX, Komaki R, Welsh JW, O’Reilly MS, Chang JY, et al. Improved survival outcomes with the incidental use of beta-blockers among patients with non-small-cell lung cancer treated with definitive radiation therapy. Annal Oncol. 2013;24(5):1312–9.
Lee KW, Tsai YS, Chiang FY, Huang JL, Ho KY, Yang YH, et al. Lower ataxia telangiectasia mutated (ATM) mRNA expression is correlated with poor outcome of laryngeal and pharyngeal cancer patients. Annal Oncol. 2011;22(5):1088–93.
Ghanemi M, Pourshohod A, Ghaffari MA, Kheirollah A, Amin M, Zeinali M, et al. Specific Targeting of HER2-Positive Head and Neck Squamous Cell Carcinoma Line HN5 by Idarubicin-ZHER2 Affibody Conjugate. Curr Cancer Drug Targets. 2019;19(1):65–73.
Huang Z, Li G, Zhang Z, Gu R, Wang W, Lai X, et al. β2AR-HIF-1α-CXCL12 signaling of osteoblasts activated by isoproterenol promotes migration and invasion of prostate cancer cells. BMC Cancer. 2019;19(1):1142.
Eng JW, Reed CB, Kokolus KM, Pitoniak R, Utley A, Bucsek MJ, et al. Housing temperature-induced stress drives therapeutic resistance in murine tumour models through β2-adrenergic receptor activation. Nat Commun. 2015;6:6426.
Al-Astal HI, Massad M, AlMatar M, Ekal H. Cellular functions of RNA-binding Motif protein 3 (RBM3): clues in hypothermia, cancer biology and apoptosis. Protein Pept Lett. 2016;23(9):828–35.
Bandyopadhayaya S, Ford B, Mandal CC. Cold-hearted: a case for cold stress in cancer risk. J Therm Biol. 2020;91: 102608.
Hirata K, Nagasaka T. Enhancement of calorigenic response to cold and to norepinephrine in physically trained rats. Jpn J Physiol. 1981;31(5):657–65.
Afsarian O, Shahir MH, Lourens A, Akhlaghi A, Lotfolahian H, Hoseini A, et al. Eggshell temperature manipulations during incubation and in ovo injection of thyroxine are associated with a decreased incidence of cold-induced ascites in broiler chickens. Poult Sci. 2018;97(1):328–36.
Shmakov DN, Nuzhny VP, Kibler NA, Kharin SN. Changes in total cholesterol and heart rate in normotensive and hypertensive rats under combined influence of cold exposure and hypokinesia. Bull Exp Biol Med. 2020;169(6):738–41.
Zareei E, Karami F, Gholami M, Ershadi A, Avestan S, Aryal R, et al. Physiological and biochemical responses of strawberry crown and leaf tissues to freezing stress. BMC Plant Biol. 2021;21(1):532.
Shi H, Tan SJ, Zhong H, Hu W, Levine A, Xiao CJ, et al. Winter temperature and UV are tightly linked to genetic changes in the p53 tumor suppressor pathway in Eastern Asia. Am J Hum Genet. 2009;84(4):534–41.
So CL, Milevskiy MJG, Monteith GR. Transient receptor potential cation channel subfamily V and breast cancer. Labor Investig. 2020;100(2):199–206.
Wang Y, Yin S, Mei L, Yang Y, Xu S, He X, et al. A dual receptors-targeting and size-switchable “cluster bomb” co-loading chemotherapeutic and transient receptor potential ankyrin 1 (TRPA-1) inhibitor for treatment of triple negative breast cancer. J Controll Release. 2020;321:71–83.
Zhan C, Shi Y. TRPC channels and cell proliferation. Adv Exp Med Biol. 2017;976:149–55.
Lin R, Bao X, Wang H, Zhu S, Liu Z, Chen Q, et al. TRPM2 promotes pancreatic cancer by PKC/MAPK pathway. Cell Death Dis. 2021;12(6):585.
Santoni G, Farfariello V. TRP channels and cancer: new targets for diagnosis and chemotherapy. Endocr Metab Immune Disord Drug Targets. 2011;11(1):54–67.
Kärki T, Tojkander S. TRPV protein family-from mechanosensing to cancer invasion. Biomolecules. 2021;11(7):1019.
Chen J, Luan Y, Yu R, Zhang Z, Zhang J, Wang W. Transient receptor potential (TRP) channels, promising potential diagnostic and therapeutic tools for cancer. Biosci Trends. 2014;8(1):1–10.
Su KH, Lin SJ, Wei J, Lee KI, Zhao JF, Shyue SK, et al. The essential role of transient receptor potential vanilloid 1 in simvastatin-induced activation of endothelial nitric oxide synthase and angiogenesis. Acta Physiol (Oxf). 2014;212(3):191–204.
Wong KK, Banham AH, Yaacob NS, Nur Husna SM. The oncogenic roles of TRPM ion channels in cancer. J Cell Physiol. 2019. https://doi.org/10.1002/jcp.28168.
Grolez GP, Chinigò G, Barras A, Hammadi M, Noyer L, Kondratska K, et al. TRPM8 as an anti-tumoral target in prostate cancer growth and metastasis dissemination. Int J Mol Sci. 2022;23(12):6672.
Anderson KJ, Cormier RT, Scott PM. Role of ion channels in gastrointestinal cancer. World J Gastroenterol. 2019;25(38):5732–72.
Lan X, Zhao J, Song C, Yuan Q, Liu X. TRPM8 facilitates proliferation and immune evasion of esophageal cancer cells. 2019. Biosci Rep. https://doi.org/10.1042/BSR20191878.
Yee NS. TRPM8 ion channels as potential cancer biomarker and target in pancreatic cancer. Adv Protein Chem Struct Biol. 2016;104:127–55.
Lunardi A, Barbareschi M, Carbone FG, Morelli L, Brunelli M, Fortuna N, et al. TRPM8 protein expression in hormone naïve local and lymph node metastatic prostate cancer. Pathologica. 2021;113(2):95–101.
Gkika D, Lemonnier L, Shapovalov G, Gordienko D, Poux C, Bernardini M, et al. TRP channel-associated factors are a novel protein family that regulates TRPM8 trafficking and activity. J Cell Biol. 2015;208(1):89–107.
Zhang L, Barritt GJ. Evidence that TRPM8 is an androgen-dependent Ca2+ channel required for the survival of prostate cancer cells. Can Res. 2004;64(22):8365–73.
Bidaux G, Roudbaraki M, Merle C, Crépin A, Delcourt P, Slomianny C, et al. Evidence for specific TRPM8 expression in human prostate secretory epithelial cells: functional androgen receptor requirement. Endocr Relat Cancer. 2005;12(2):367–82.
Thebault S, Lemonnier L, Bidaux G, Flourakis M, Bavencoffe A, Gordienko D, et al. Novel role of cold/menthol-sensitive transient receptor potential melastatine family member 8 (TRPM8) in the activation of store-operated channels in LNCaP human prostate cancer epithelial cells. J Biol Chem. 2005;280(47):39423–35.
Bidaux G, Flourakis M, Thebault S, Zholos A, Beck B, Gkika D, et al. Prostate cell differentiation status determines transient receptor potential melastatin member 8 channel subcellular localization and function. J Clin Investig. 2007;117(6):1647–57.
Tsavaler L, Shapero MH, Morkowski S, Laus R. Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Can Res. 2001;61(9):3760–9.
Fuessel S, Sickert D, Meye A, Klenk U, Schmidt U, Schmitz M, et al. Multiple tumor marker analyses (PSA, hK2, PSCA, trp-p8) in primary prostate cancers using quantitative RT-PCR. Int J Oncol. 2003;23(1):221–8.
Yee NS, Zhou W, Lee M. Transient receptor potential channel TRPM8 is over-expressed and required for cellular proliferation in pancreatic adenocarcinoma. Cancer Lett. 2010;297(1):49–55.
Yee NS, Li Q, Kazi AA, Yang Z, Berg A, Yee RK. Aberrantly over-expressed TRPM8 channels in pancreatic adenocarcinoma: correlation with tumor size/stage and requirement for cancer cells invasion. Cells. 2014;3(2):500–16.
Cucu D, Chiritoiu G, Petrescu S, Babes A, Stanica L, Duda DG, et al. Characterization of functional transient receptor potential melastatin 8 channels in human pancreatic ductal adenocarcinoma cells. Pancreas. 2014;43(5):795–800.
Yee NS, Chan AS, Yee JD, Yee RK. TRPM7 and TRPM8 ion channels in pancreatic adenocarcinoma: potential roles as cancer biomarkers and targets. Scientifica. 2012;2012: 415158.
Chodon D, Guilbert A, Dhennin-Duthille I, Gautier M, Telliez MS, Sevestre H, et al. Estrogen regulation of TRPM8 expression in breast cancer cells. BMC Cancer. 2010;10:212.
Liu J, Chen Y, Shuai S, Ding D, Li R, Luo R. TRPM8 promotes aggressiveness of breast cancer cells by regulating EMT via activating AKT/GSK-3β pathway. Tumour Biol. 2014;35(9):8969–77.
Du GJ, Li JH, Liu WJ, Liu YH, Zhao B, Li HR, et al. The combination of TRPM8 and TRPA1 expression causes an invasive phenotype in lung cancer. Tumour Biol. 2014;35(2):1251–61.
Wang Y, Yang Z, Meng Z, Cao H, Zhu G, Liu T, et al. Knockdown of TRPM8 suppresses cancer malignancy and enhances epirubicin-induced apoptosis in human osteosarcoma cells. Int J Biol Sci. 2013;10(1):90–102.
Okamoto Y, Ohkubo T, Ikebe T, Yamazaki J. Blockade of TRPM8 activity reduces the invasion potential of oral squamous carcinoma cell lines. Int J Oncol. 2012;40(5):1431–40.
Louhivuori LM, Bart G, Larsson KP, Louhivuori V, Näsman J, Nordström T, et al. Differentiation dependent expression of TRPA1 and TRPM8 channels in IMR-32 human neuroblastoma cells. J Cell Physiol. 2009;221(1):67–74.
Wondergem R, Bartley JW. Menthol increases human glioblastoma intracellular Ca2+, BK channel activity and cell migration. J Biomed Sci. 2009;16(1):90.
Mergler S, Strowski MZ, Kaiser S, Plath T, Giesecke Y, Neumann M, et al. Transient receptor potential channel TRPM8 agonists stimulate calcium influx and neurotensin secretion in neuroendocrine tumor cells. Neuroendocrinology. 2007;85(2):81–92.
Koike C, Obara T, Uriu Y, Numata T, Sanuki R, Miyata K, et al. TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc Natl Acad Sci USA. 2010;107(1):332–7.
Chinigò G, Fiorio Pla A, Gkika D. TRP channels and small GTPases interplay in the main hallmarks of metastatic cancer. Front Pharmacol. 2020;11: 581455.
Kolisek M, Beck A, Fleig A, Penner R. Cyclic ADP-ribose and hydrogen peroxide synergize with ADP-ribose in the activation of TRPM2 channels. Mol Cell. 2005;18(1):61–9.
Liao M, Cao E, Julius D, Cheng Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature. 2013;504(7478):107–12.
Kim M, Sisco NJ, Hilton JK, Montano CM, Castro MA, Cherry BR, et al. Evidence that the TRPV1 S1–S4 membrane domain contributes to thermosensing. Nat Commun. 2020;11(1):4169.
Doñate-Macián P, Álvarez-Marimon E, Sepulcre F, Vázquez-Ibar JL, Perálvarez-Marín A. The membrane proximal domain of TRPV1 and TRPV2 channels mediates protein_protein interactions and lipid binding in vitro. Int J Mol Sci. 2019;20(3):682.
Ramer R, Hinz B. Inhibition of cancer cell invasion by cannabinoids via increased expression of tissue inhibitor of matrix metalloproteinases-1. J Natl Cancer Inst. 2008;100(1):59–69.
Xu S, Zhang L, Cheng X, Yu H, Bao J, Lu R. Capsaicin inhibits the metastasis of human papillary thyroid carcinoma BCPAP cells through the modulation of the TRPV1 channel. Food Funct. 2018;9(1):344–54.
Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16(9):582–98.
Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6(5):392–401.
Atsumi T, Singh R, Sabharwal L, Bando H, Meng J, Arima Y, et al. Inflammation amplifier, a new paradigm in cancer biology. Can Res. 2014;74(1):8–14.
Wang S, Liu Z, Wang L, Zhang X. NF-kappaB signaling pathway, inflammation and colorectal cancer. Cell Mol Immunol. 2009;6(5):327–34.
Munaron L. Intracellular calcium, endothelial cells and angiogenesis. Recent Pat Anti-Cancer Drug Discovery. 2006;1(1):105–19.
Earley S, Brayden JE. Transient receptor potential channels in the vasculature. Physiol Rev. 2015;95(2):645–90.
Faehling M, Kroll J, Föhr KJ, Fellbrich G, Mayr U, Trischler G, et al. Essential role of calcium in vascular endothelial growth factor A-induced signaling: mechanism of the antiangiogenic effect of carboxyamidotriazole. FASEB J. 2002;16(13):1805–7.
Moccia F, Negri S, Shekha M, Faris P, Guerra G. Endothelial Ca(2+) signaling, angiogenesis and vasculogenesis: just what it takes to make a blood vessel. Int J Mol Sci. 2019;20(16):3962.
Negri S, Faris P, Rosti V, Antognazza MR, Lodola F, Moccia F. Endothelial TRPV1 as an emerging molecular target to promote therapeutic angiogenesis. Cells. 2020;9(6):1341.
Voets T, Prenen J, Vriens J, Watanabe H, Janssens A, Wissenbach U, et al. Molecular determinants of permeation through the cation channel TRPV4. J Biol Chem. 2002;277(37):33704–10.
Watanabe H, Vriens J, Janssens A, Wondergem R, Droogmans G, Nilius B. Modulation of TRPV4 gating by intra- and extracellular Ca2+. Cell Calcium. 2003;33(5–6):489–95.
Zhao P, Lieu T, Barlow N, Metcalf M, Veldhuis NA, Jensen DD, et al. Cathepsin S causes inflammatory pain via biased agonism of PAR2 and TRPV4. J Biol Chem. 2014;289(39):27215–34.
Walter BA, Purmessur D, Moon A, Occhiogrosso J, Laudier DM, Hecht AC, et al. Reduced tissue osmolarity increases TRPV4 expression and pro-inflammatory cytokines in intervertebral disc cells. Eur Cell Mater. 2016;32:123–36.
Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457(7225):102–6.
Tang B, Wu J, Zhu MX, Sun X, Liu J, Xie R, et al. VPAC1 couples with TRPV4 channel to promote calcium-dependent gastric cancer progression via a novel autocrine mechanism. Oncogene. 2019;38(20):3946–61.
Cappelli HC, Kanugula AK, Adapala RK, Amin V, Sharma P, Midha P, et al. Mechanosensitive TRPV4 channels stabilize VE-cadherin junctions to regulate tumor vascular integrity and metastasis. Cancer Lett. 2019;442:15–20.
Shi M, Du F, Liu Y, Li L, Cai J, Zhang GF, et al. Glial cell-expressed mechanosensitive channel TRPV4 mediates infrasound-induced neuronal impairment. Acta Neuropathol. 2013;126(5):725–39.
Ye L, Kleiner S, Wu J, Sah R, Gupta RK, Banks AS, et al. TRPV4 is a regulator of adipose oxidative metabolism, inflammation, and energy homeostasis. Cell. 2012;151(1):96–110.
Thodeti CK, Matthews B, Ravi A, Mammoto A, Ghosh K, Bracha AL, et al. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ Res. 2009;104(9):1123–30.
Nan L, Zheng Y, Liao N, Li S, Wang Y, Chen Z, et al. Mechanical force promotes the proliferation and extracellular matrix synthesis of human gingival fibroblasts cultured on 3D PLGA scaffolds via TGF-β expression. Mol Med Rep. 2019;19(3):2107–14.
Romani P, Valcarcel-Jimenez L, Frezza C, Dupont S. Crosstalk between mechanotransduction and metabolism. Nat Rev Mol Cell Biol. 2021;22(1):22–38.
Iskratsch T, Wolfenson H, Sheetz MP. Appreciating force and shape—the rise of mechanotransduction in cell biology. Nat Rev Mol Cell Biol. 2014;15(12):825–33.
Campellone KG, Welch MD. A nucleator arms race: cellular control of actin assembly. Nat Rev Mol Cell Biol. 2010;11(4):237–51.
Hotulainen P, Lappalainen P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J Cell Biol. 2006;173(3):383–94.
Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, et al. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol. 2001;153(6):1175–86.
Jiang G, Huang AH, Cai Y, Tanase M, Sheetz MP. Rigidity sensing at the leading edge through alphavbeta3 integrins and RPTPalpha. Biophys J. 2006;90(5):1804–9.
Roca-Cusachs P, Iskratsch T, Sheetz MP. Finding the weakest link: exploring integrin-mediated mechanical molecular pathways. J Cell Sci. 2012;125(Pt 13):3025–38.
Rognoni L, Stigler J, Pelz B, Ylänne J, Rief M. Dynamic force sensing of filamin revealed in single-molecule experiments. Proc Natl Acad Sci USA. 2012;109(48):19679–84.
Prager-Khoutorsky M, Lichtenstein A, Krishnan R, Rajendran K, Mayo A, Kam Z, et al. Fibroblast polarization is a matrix-rigidity-dependent process controlled by focal adhesion mechanosensing. Nat Cell Biol. 2011;13(12):1457–65.
Mohammadi H, Sahai E. Mechanisms and impact of altered tumour mechanics. Nat Cell Biol. 2018;20(7):766–74.
Hannigan G, Troussard AA, Dedhar S. Integrin-linked kinase: a cancer therapeutic target unique among its ILK. Nat Rev Cancer. 2005;5(1):51–63.
Grootaert MOJ, Moulis M, Roth L, Martinet W, Vindis C, Bennett MR, et al. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc Res. 2018;114(4):622–34.
Mehta HM, Malandra M, Corey SJ. G-CSF and GM-CSF in neutropenia. J Immunol. 2015;195(4):1341–9.
Hasday JD, Garrison A, Singh IS, Standiford T, Ellis GS, Rao S, et al. Febrile-range hyperthermia augments pulmonary neutrophil recruitment and amplifies pulmonary oxygen toxicity. Am J Pathol. 2003;162(6):2005–17.
Capitano ML, Nemeth MJ, Mace TA, Salisbury-Ruf C, Segal BH, McCarthy PL, et al. Elevating body temperature enhances hematopoiesis and neutrophil recovery after total body irradiation in an IL-1-, IL-17-, and G-CSF-dependent manner. Blood. 2012;120(13):2600–9.
Ostberg JR, Repasky EA. Comparison of the effects of two different whole body hyperthermia protocols on the distribution of murine leukocyte populations. Int J Hyperther. 2000;16(1):29–43.
Tulapurkar ME, Almutairy EA, Shah NG, He JR, Puche AC, Shapiro P, et al. Febrile-range hyperthermia modifies endothelial and neutrophilic functions to promote extravasation. Am J Respir Cell Mol Biol. 2012;46(6):807–14.
Huse M. Mechanical forces in the immune system. Nat Rev Immunol. 2017;17(11):679–90.
Jairaman A, Othy S, Dynes JL, Yeromin AV, Zavala A, Greenberg ML, et al. Piezo1 channels restrain regulatory T cells but are dispensable for effector CD4(+) T cell responses. Sci Adv. 2021. https://doi.org/10.1126/sciadv.abg5859.
Horn LA, Chariou PL, Gameiro SR, Qin H, Iida M, Fousek K, et al. Remodeling the tumor microenvironment via blockade of LAIR-1 and TGF-β signaling enables PD-L1-mediated tumor eradication. J Clin Investig. 2022. https://doi.org/10.1172/JCI155148.
Mennens SFB, Bolomini-Vittori M, Weiden J, Joosten B, Cambi A, van den Dries K. Substrate stiffness influences phenotype and function of human antigen-presenting dendritic cells. Sci Rep. 2017;7(1):17511.
Choi Y, Kwon JE, Cho YK. Dendritic cell migration is tuned by mechanical stiffness of the confining space. Cells. 2021;10(12):3362.
Chakraborty M, Chu K, Shrestha A, Revelo XS, Zhang X, Gold MJ, et al. Mechanical stiffness controls dendritic cell metabolism and function. Cell Rep. 2021;34(2): 108609.
Huang Z, Sun Z, Zhang X, Niu K, Wang Y, Zheng J, et al. Loss of stretch-activated channels, PIEZOs, accelerates non-small cell lung cancer progression and cell migration. 2019. Biosci Rep. https://doi.org/10.1042/BSR20181679.
Pappu P, Madduru D, Chandrasekharan M, Modhukur V, Nallapeta S, Suravajhala P. Next generation sequencing analysis of lung cancer datasets: a functional genomics perspective. Indian J Cancer. 2016;53(1):1–7.
Harsch M, Bendrat K, Hofmeier G, Branscheid D, Niendorf A. A new method for histological microdissection utilizing an ultrasonically oscillating needle: demonstrated by differential mRNA expression in human lung carcinoma tissue. Am J Pathol. 2001;158(6):1985–90.
Chen X, Wanggou S, Bodalia A, Zhu M, Dong W, Fan JJ, et al. A feedforward mechanism mediated by mechanosensitive ion channel PIEZO1 and tissue mechanics promotes glioma aggression. Neuron. 2018;100(4):799-815.e7.
Zhang J, Zhou Y, Huang T, Wu F, Liu L, Kwan JSH, et al. PIEZO1 functions as a potential oncogene by promoting cell proliferation and migration in gastric carcinogenesis. Mol Carcinog. 2018;57(9):1144–55.
Xu H, Chen Z, Li C. The prognostic value of Piezo1 in breast cancer patients with various clinicopathological features. Anticancer Drugs. 2021;32(4):448–55.
Lou W, Liu J, Ding B, Jin L, Xu L, Li X, et al. Five miRNAs-mediated PIEZO2 downregulation, accompanied with activation of Hedgehog signaling pathway, predicts poor prognosis of breast cancer. Aging. 2019;11(9):2628–52.
Han Y, Liu C, Zhang D, Men H, Huo L, Geng Q, et al. Mechanosensitive ion channel Piezo1 promotes prostate cancer development through the activation of the Akt/mTOR pathway and acceleration of cell cycle. Int J Oncol. 2019;55(3):629–44.
Etem E, Ceylan GG, Özaydın S, Ceylan C, Özercan I, Kuloğlu T. The increased expression of Piezo1 and Piezo2 ion channels in human and mouse bladder carcinoma. Adv Clin Experim Med. 2018;27(8):1025–31.
De Felice D, Alaimo A. Mechanosensitive piezo channels in cancer: focus on altered calcium signaling in cancer cells and in tumor progression. Cancers. 2020;12(7):1780.
Yang XN, Lu YP, Liu JJ, Huang JK, Liu YP, Xiao CX, et al. Piezo1 is as a novel trefoil factor family 1 binding protein that promotes gastric cancer cell mobility in vitro. Dig Dis Sci. 2014;59(7):1428–35.
Li C, Rezania S, Kammerer S, Sokolowski A, Devaney T, Gorischek A, et al. Piezo1 forms mechanosensitive ion channels in the human MCF-7 breast cancer cell line. Sci Rep. 2015;5:8364.
Liu CSC, Ganguly D. Mechanical cues for T Cell Activation: role of Piezo1 mechanosensors. Crit Rev Immunol. 2019;39(1):15–38.
Forget A, Gianni-Barrera R, Uccelli A, Sarem M, Kohler E, Fogli B, et al. Mechanically defined microenvironment promotes stabilization of microvasculature, which correlates with the enrichment of a novel Piezo-1(+) population of circulating CD11b(+) /CD115(+) monocytes. Adv Mater. 2019;31(21): e1808050.
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science (New York, NY). 1997;275(5302):964–7.
Funding
This work was supported by grants from the National Nature Science Foundation of China (82072985), Heilongjiang Province Innovation Base Award Project (JD2023SJ03), Wu-Jieping Medical Foundation (320.6750.19089-22,320.6750.19089-48), Beijing Medical Award Foundation (YXJL-2019-1416-0069), Hai Yan Youth Fund of Harbin Medical University Cancer Hospital (JJQN2021-02), the Fundamental Research Funds for the Provincial Universities (2021-KYYWF-0253), Natural Science Foundation of Heilongjiang Province (LH2022H065), Scientific research project of the Heilongjiang Provincial Health Commission (20210808020126).
Author information
Authors and Affiliations
Contributions
Yun-jing Hou, Xin-xin Yang, Lin He, and Hong-xue Meng. Yun-jing Hou, Xin-xin Yang reviewed the literature and wrote the first draft. Lin He contributed to the conception and design of the manuscript. Hong-xue Meng administrated and finalized the manuscript. All authors read and approved the final version of the manuscript. Data authentication is not applicable.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hou, Yj., Yang, Xx., He, L. et al. Pathological mechanisms of cold and mechanical stress in modulating cancer progression. Human Cell 37, 593–606 (2024). https://doi.org/10.1007/s13577-024-01049-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-024-01049-y