Abstract
Stem cell research with biological waste material is an area that holds promise to revolutionize treatment modalities and clinical practice. The interest in surgical remnants is increasing with time as research on human embryonic stem cells remains controversial due to legal and ethical issues. Perhaps, these restrictions are the motivation for the use of alternative mesenchymal stem cell (MSC) sources in the regenerative field. Stem cells (SCs) of Umbilical Cord (UC) and Dental Pulp (DP) have almost similar biological characteristics to other MSCs and can differentiate into a number of cell lineages with enormous potential future prospects. A concise critical observation of UC-MSCs and DP-MSCs is presented here reviewing articles from the last two decades along with other stem cell sources from different biological waste materials.
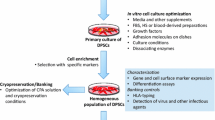
Graphical abstract




Similar content being viewed by others
Availability of data and material
Transparent data.
Code availability
Not applicable.
References:
Cherian E, Nandhini G, Kurian A. Stem cells.2011;Ed.1:1–95.
Bhattachariya N. Phillip stubblefield. Front cord Blood Sci. 2009;1(1):21–2.
Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human Mesenchymal stem cells (MSC): A comparison of Adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9(12):1–14.
Das M, Das A, Barui A, Paul RR. Comparative evaluation of proliferative potential and replicative senescence associated changes in mesenchymal stem cells derived from dental pulp and umbilical cord. Cell Tissue Bank. 2022;23(1):157–70. https://doi.org/10.1007/s10561-021-09926-8. (Epub 2021 Apr 26 PMID: 33900487).
Ren H, Sang Y, Zhang F, Liu Z, Qi N, Chen Y. Comparative analysis of human mesenchymal stem cells from umbilical cord, dental pulp, and menstrual blood as sources for cell therapy. Stem Cells Int. 2016. https://doi.org/10.1155/2016/3516574. (Epub 2016 Jan 10. PMID: 26880954; PMCID: PMC4736971).
Griffiths MJ, Bonnet D, Janes SM. Stem cells of the alveolar epithelium. Lancet. 2005;366:249–60.
Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Asahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76.
Khan FA, Almohazey D, Alomari M, Almofty SA. Isolation, culture, and functional characterization of human embryonic stem cells: current trends and challenges firdos. Stem Cells Int. 2018. https://doi.org/10.1155/218/1429351.
Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;14(9):12. https://doi.org/10.1186/1478-811X-9-12. (PMID:21569606;PMCID:PMC3117820).
Barlow S, Brooke G, Chatterjee K, Price G, Pelekanos R, Rossetti T, Doody M, Venter D, Pain S, Gilshenan K, Atkinson K. Comparison of human placentaand bone marrow-derived multipotent mesenchymal stem cells. Stem Cells Dev. 2008;17:1095–107.
Okamoto K, Miyoshi S, Toyoda M, Hida N, Ikegami Y, Makino H, Nishiyama N, Tsuji H, Cui CH, Segawa K, Uyama T, Kami D, Miyado K, Asada H, Matsumoto K, Saito H, Yoshimura Y, Ogawa S, Aeba R, Yozu R, Umezawa A. Working’ cardiomyocytes exhibiting plateau action potentials from human placenta-derived extraembryonic mesodermal cells. Exp Cell Res. 2007;313:2550–62.
De Coppi P, Bartsch G Jr, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, Furth ME, Soker S, Atala A. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100–6.
Wouters G, Grossi S, Mesoraca A, et al. Isolation of amniotic fluid-derived mesenchymal stem cells. J Prenat Med. 2007;1(3):39–40.
Spitzhorn LS, Rahman MS, Schwindt L, Ho HT. Isolation and molecular characterization of amniotic fluid-derived mesenchymal stem cells obtained from caesarean sections. Stem Cells International. 2017;3:1–15.
Graham CD, Fauza D. Isolation of mesenchymal stem cells from amniotic fluid and placenta. Curr Protoc Stem Cell Biol. 2015;35(1):1–14.
Tsai MS, Lee JL, Chang YJ, Hwang SM. Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod. 2004;19(6):1450–6.
Renda MC, Fecarotta E, Schillaci G, Leto F, Calvaruso G, Garofalo GM, et al. Mesenchymal fetal stem cells (FMSC) from amniotic fluid (AF): expansion and phenotypic characterization. Blood. 2015;126(23):4758.
Prado SD, López EM, Gómez TH, Cicione C, Vázquez ER, Boquete IF, et al. Human amniotic membrane as an alternative source of stem cells for regenerative medicine. Differentiation. 2011;1:162–71.
Ramuta TŽ, Kreft ME. Human amniotic membrane and amniotic membrane-derived cells: how far are we from their use in regenerative and reconstructive urology? Cell Transplant. 2018;27(1):77–92.
Motedayyen H, Esmaeil N, Tajik N, Khadem F, Ghotloo S, Khani B, et al. Method and key points for isolation of human amniotic epithelial cells with high yield, viability and purity. BMC Res Notes. 2017;10:552.
Jaianand K, Palaniyandi M, Iqbal T, Balaji P. Villous chorion: a potential source for pluripotent-like stromal cells. J Natural Sci Biol Med. 2017;8(2):221–8.
Muench MO, Kapidzic M, Gormley M, Gutierrez AG, Kathryn L. The human chorion contains definitive hematopoietic stem cells from the fifteenth week of gestation. Development. 2017;144:1399–2141.
Huang Q, Yang Y, Luo C, Wen Y, Liu R, Li S, et al. An efficient protocol to generate placental chorionic plate-derived mesenchymal stem cells with superior proliferative and immunomodulatory properties. Stem Cell Res Ther. 2019;10:301.
Shaer A, Azarpira N, Aghdaie MH, Esfandiari E. Isolation and characterization of Human mesenchymal stromal cells derived from placental decidua basalis; Umbilical cord Wharton’s Jelly and Amniotic Membrane. Pak J Med Sci Online. 2014;30(5):1022–6.
Chen G, Yue A, Ruan Z, et al. Comparison of biological characteristics of mesenchymal stem cells derived from maternal-origin placenta and Wharton’s jelly. Stem Cell Res Ther. 2015;6:228.
Hassan G, Kasem I, Soukkarieh C, Aljamali M. A simple method to isolate and expand human umbilical cord derived mesenchymal stem cells: using explant method and umbilical cord blood serum. Int J Stem Cells. 2017;10(2):184–92. https://doi.org/10.15283/ijsc17028.
Mennan C, Wright K, Bhattacharjee A, Balain B, Richardson J, Roberts S. Isolation and Characterisation of mesenchymal stem cells from different regions of the human umbilical cord. Biomed Res Int. 2013;2013: 916136.
Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103(5):1669–75. https://doi.org/10.1182/blood-2003-05-1670. (Epub 2003 Oct 23 PMID: 14576065).
Karamzadeh R, Eslaminejad MB, Aflatoonian R. Isolation, characterization and comparative differentiation of human dental pulp stem cells derived from permanent teeth by using two different methods. J Vis Exp. 2012;69:4372. https://doi.org/10.3791/4372.
Pisciotta A, Riccio M, Carnevale G, Lu A, Biasi SD, Gibellini L, et al. Stem cells isolated from human dental pulp and amniotic fluid improve skeletal muscle histopathology in mdx/SCID mice. Stem Cell Res Ther. 2015;6(1):156.
Naz S, Khan FR, Rahat R, Ladhani S, Khan MS, Mohammed N, Ahmad T. Isolation and culture of dental pulp stem cells from permanent and deciduous teeth. Pak J Med Sci. 2019;35(4):997–1002.
Tsai AI, Hong HH, Lin WR, Fu JF, Chang CC, Wang IK, et al. Isolation of mesenchymal stem cells from human deciduous teeth pulp. Biomed Res Int. 2017. https://doi.org/10.1155/2017/2851906.
Marrelli M, Paduano F, Tatullo M. Human periapical cyst-mesenchymal stem cells differentiate into neuronal cells. J Dent Res. 2015;94(6):843–52. https://doi.org/10.1177/0022034515570316. (Epub 2015 Feb 11 PMID: 25672890).
Tatullo M, Codispoti B, Pacifici A, Palmieri F, Marrelli M, Pacifici L, Paduano F. Potential use of human periapical cyst-mesenchymal stem cells (hPCy-MSCs) as a novel stem cell source for regenerative medicine applications. Front Cell Dev Biol. 2017;5(103):5. https://doi.org/10.3389/fcell.2017.00103.
Liao J, Al Shahrani M, Al-Habib M, Tanaka T, Huang GT. Cells isolated from inflamed periapical tissue express mesenchymal stem cell markers and are highly osteogenic. J Endod. 2011;37:1217–24.
Russo V, Yu C, Belliveau P, Hamilton A, Flynn LE. Comparison of human adipose-derived stem cells isolated from subcutaneous, omental, and intrathoracic adipose tissue depots for regenerative applications. Stem Cells Transl Med. 2014;3(2):206–17.
Zollino I, Sibilla MG, Gianesini S, Tessari M, Malagoni AM, Menegatti E. Technique for intraoperatory harvesting of adipose derived stem cells: towards cell treatment of recalcitrant ulcers. Veins Lymph. 2017;6(2):51–5.
Mieczkowska A, Schumacher A, Filipowicz N, et al. Immunophenotyping and transcriptional profiling of in vitro cultured human adipose tissue derived stem cells. Sci Rep. 2018;8:11339. https://doi.org/10.1038/s41598-018-29477-5.
Khaddour K, Hana CK, Mewawalla P. Hematopoietic Stem Cell Transplantation. Jan 2023; StatPearls Publishing; https://www.ncbi.nlm.nih.gov/books/NBK536951/. Accessed 22 Apr 2023.
Broxmeyer HE, Srour E, Orschell C, Ingram DA, Cooper S, Plett PA, Mead LE, Yoder MC. Cord blood stem and progenitor cells. Methods Enzymol. 2006;419:439–73.
Wu C, Chen L, Huang YZ, Huang Y, Parolini O, Zhong Q, Tian X, Deng L. Comparison of the proliferation and differentiation potential of human urine-, placenta decidua basalis-, and bone marrow-derived stem cells. Stem Cells Int. 2018;13(2018):7131532. https://doi.org/10.1155/2018/7131532. (Erratum.In:StemCellsInt.2019Mar10;2019:1651506.PMID:30651734;PMCID:PMC6311712).
Zhang Y, McNeill E, Tian H, et al. Urine derived cells are a potential source for urological tissue reconstruction. J Urol. 2008;180(5):2226–33.
Sun Z, Gu P, Xu H, Zhao W, Zhou Y, Zhou L, Zhang Z, Wang W, Han R, Chai X, An S. Human umbilical cord mesenchymal stem cells improve locomotor function in Parkinson’s disease mouse model through regulating intestinal microorganisms. Front Cell Dev Biol. 2022. https://doi.org/10.3389/fcell.2021.808905.
Rahyussalim AJ, Saleh I, Kurniawati T, Lutfi APWY. Improvement of renal function after human umbilical cord mesenchymal stem cell treatment on chronic renal failure and thoracic spinal cord entrapment: a case report. J Med Case Rep. 2017;11(1):334. https://doi.org/10.1186/s13256-017-1489-7. (PMID:29187247;PMCID:PMC5707902).
Kilpinen L, Impola U, Sankkila L, et al. Extracellular membrane vesicles from umbilical cord blood-derived MSC protect against ischemic acute kidney injury, a feature that is lost after inflammatory conditioning. J Extracell Vesicles. 2013. https://doi.org/10.3402/jev.v2i0.21927.
Zhang C, Liao W, Li W, Li M, Xu X, Sun H, Xue Y, Liu L, Qiu J, Zhang C, Zhang X, Ye J, Du J, Deng D, Deng W, Li T. Human Umbilical cord mesenchymal stem cells derived extracellular vesicles alleviate salpingitis by promoting M1-to-M2 transformation. Front Physiol. 2013;14:1–16. https://doi.org/10.3389/fphys.2023.1131701.
Li Y, Duan X, Chen Y, et al. Dental stem cell-derived extracellular vesicles as promising therapeutic agents in the treatment of diseases. Int J Oral Sci. 2022;14:2. https://doi.org/10.1038/s41368-021-00152-2.
Tayhan SE, Keleş GT, Topçu İ, Mir E, Gürhan SİD. Isolation and in vitro cultivation of human urine-derived cells: an alternative stem cell source. Turk J Urol. 2017;43(3):345–9. https://doi.org/10.5152/tud.2017.
Lin W, Li G. A novel protocol for isolation and culture of multipotent progenitor cells from human urine. J Orthop Transl. 2019;19(3):12–7.
Chen L, Li L, Xing F, Peng J, Peng K, Wang Y, Xiang Z. Human urine-derived stem cells: potential for cell-based therapy of cartilage defects. Stem Cells Int. 2018;2018:4686259. https://doi.org/10.1155/2018/4686259.
Patel AN, Park E, Kuzman M, Benetti F, Silva FJ, Allickson JG. Multipotent menstrual blood stromal stem cells: isolation, characterization, and differentiation. Cell Transplant. 2008;17(3):303–11. https://doi.org/10.3727/096368908784153922. (Erratum.In:CellTransplant.2008;17(6):721.Erratumin:CellTransplant.2008;17(7):875 PMID: 18522233).
Uzieliene I, Urbonaite G, Tachtamisevaite Z, Mobasheri A, Bernotiene E. The potential of menstrual blood-derived mesenchymal stem cells for cartilage repair and regeneration: novel aspects. Stem Cells Int. 2018;3(2018):5748126. https://doi.org/10.1155/2018/5748126. (PMID:30627174;PMCID:PMC6304826).
Sun Y, Ren Y, Yang F, He Y, Liang S, Guan L, Cheng F, Liu Y, Lin J. High-yield isolation of menstrual blood-derived endometrial stem cells by direct red blood cell lysis treatment. Biol Open. 2019;8(5):bio038885. https://doi.org/10.1242/bio.038885. (PMID: 31036750; PMCID: PMC6550070).
Antonio Nanci Ten Cate’s Oral Histology development, structure and function. 6th edition. 2005;192–239.
Chen Y, Li X, Wu J, Lu W, Xu W, Wu B. Dental pulp stem cells from human teeth with deep caries displayed an enhanced angiogenesis potential in vitro. J Dent Sci. 2021;16(1):318–26. https://doi.org/10.1016/j.jds.2020.03.007. (ISSN 1991-7902).
Mattei V, Martellucci S, Pulcini F, Santilli F, Sorice M, Delle Monache S. Regenerative potential of DPSCs and revascularization: direct, paracrine or autocrine effect? Stem Cell Rev Rep. 2021. https://doi.org/10.1007/s12015-021-10162-6.
Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81(8):531–5. https://doi.org/10.1177/154405910208100806. (PMID: 12147742).
Ching HS, Luddin N, Rahman IA, Ponnuraj KT. Expression of odontogenic and osteogenic markers in DPSCs and SHED: a review. Curr Stem Cell Res Ther. 2017;12(1):71–9. https://doi.org/10.2174/1574888x11666160815095733. (PMID: 27527527).
Zhai Q, Dong Z, Wang W, Li B, Jin Y. Dental stem cell and dental tissue regeneration. Front Med. 2019;13:152–9. https://doi.org/10.1007/s11684-018-0628-x.
Fujii Y, Hatori A, Chikazu D, Ogasawara T. Application of dental pulp stem cells for bone and neural tissue regeneration in oral and maxillofacial region. Stem Cells Int. 2023. https://doi.org/10.1155/2023/2026572. (PMID: 37035445; PMCID: PMC10076122).
Cui X, Chen L, Xue T, Jing Y, Liu J, Ji Y, Cheng L. Human umbilical cord and dental pulp-derived mesenchymal stem cells: Biological characteristics and potential roles in vitro and in vivo. Mol Med Rep. 2015;11:3269–78.
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stemcells (DPSCs) in vitro and in vivo. Proc Natl Acadm Sci USA. 2000;97:13625–30.
Mangano C, De Rosa A, Desiderio V, d’Aquino R, Piattelli A, De Francesco F, Tirino V, Mangano F, Papaccio G. The osteoblastic differentiation of dental pulp stem cells and bone formation on different titanium surface textures. Biomaterials. 2010;31:3543–51.
Struys T, Moreels M, Martens W, Donders R, Wolfs E, Lambrichts I. Ultrastructural and immunocytochemical analysis of multilineage differentiated human dental pulp- and umbilical cord-derived mesenchymal stem cells. Cells Tissues Organs. 2010;193:366–78. https://doi.org/10.1159/000321400.
Saben J, Thakali KM, Lindsey FE, Zhong Y, Badger TM, Andres A, Shankar K. Distinct adipogenic differentiation phenotypes of human umbilical cord mesenchymal cells dependent on adipogenic conditions. Exp Biol Med (Maywood). 2011;239(10):1340–51. https://doi.org/10.1177/1535370214539225. (Epub 2014 Jun 20. PMID: 24951473; PMCID: PMC4238288).
Alison MR, Poulsom R, Forbes S, Wright NA. An introduction to stem cells. J Pathol. 2002;197(4):419–23.
Daniel MG, Pereira C-F, Lemischka IR, Moore KA. Making a hematopoietic stem cell. Trends Cell Biol. 2016;26(3):202–14. https://doi.org/10.1016/j.tcb.2015.10.002.
Lane SW, Williams DA, Watt FM. Modulating the stem cell niche for tissue regeneration. Nat Biotechnol. 2014;32(8):795–803. https://doi.org/10.1038/nbt.2978.
Ferraro F, Celso CL, Scadden D. Adult stem cells and their niches. Adv Exp Med Biol. 2010;695:155–68. https://doi.org/10.1007/978-1-4419-7037-4_11. (PMID:21222205;PMCID:PMC4020242).
Biteau B, Hochmuth CE, Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell. 2008;3(4):442–55.
Kretzschmar K, Watt FM. Lineage tracing. Cell. 2012;148(1–2):33–45. https://doi.org/10.1016/j.cell.2012.01.002.
Gage FH, Temple S. Neural stem cells: generating and regenerating the brain. Neuron. 2013;80(3):588–601. https://doi.org/10.1016/j.neuron.2013.10.037.
Yoo KH, Jang IK, Lee MW, Kim HE, Yang MS, Eom Y, Lee JE, Kim YJ, Yang SK, Jung HL, et al. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol. 2009;259:150–6.
Yang CC, Shih YH, Ko MH, Hsu SY, Cheng H, Fu YS. Transplantation of human umbilical mesenchymal stem cells from Wharton’s jelly after complete transection of the rat spinal cord. PLoS ONE. 2008;3: e3336.
Weiss ML, Medicetty S, Bledsoe AR, et al. Stem cells in the umbilical cord. Stem Cells Rev. 2006;24(3):791–2.
Carlin R, Davis D, Weiss ML, Schultz BD. Expression of early transcription factors Oct-4, Sox-2 and Nanog by porcine umbilical cord (PUC) matrix cells. Reprod Biol Endocrinol. 2006;4:8.
Wolfs E, Struys T, Lambrichts I. Ultrastructural and immunocytochemical analysis of multilineage differentiated human dental pulp- and umbilical cord-derived mesenchymal stem cells. Cells Tissues Organs. 2011;96(3):121–6.
Ding DC, Chang YH, Shyu WC, Lin SZ. Human umbilical cord mesenchymal stem cells: A new era for stem cell therapy. Cell Transplant. 2015;24:339–47.
Ya-Bin Yu, Song Y, Chen Ya, Zhang F, Qi F-Z. Differentiation of umbilical cord mesenchymal stem cells into hepatocytes in comparison with bone marrow mesenchymal stem cells. Mol Med Rep. 2018;18(2):2009–16.
Zomer HD, Vidane AS, Gonçalves NN, Ambrósio CE. Mesenchymal and induced pluripotent stem cells: general insights and clinical perspectives. Stem Cells Cloning. 2015;28(8):125–34. https://doi.org/10.2147/SCCAA.S88036. (PMID:26451119;PMCID:PMC4592031).
Laino G, Graziano A, Aquino R, Pirozzi G, Lanza V, Valiante S, De Rosa A, Naro F, Vivarelli E, Papaccio G. An approachable human adult stem cell source for hard-tissue engineering. J Cell Physiol. 2006;206:693–785.
Huang GTJ, Sonoyama W, Chen J, Park S. In vitro characterization of human dental pulp cells: various isolation methods and culturing environments. Cell Tissue Res. 2006;324:225.
Hsieh JY, Wang HW, Chang SJ, Liao KH, Lee IH, Lin WS, Wu CH, Lin WY, Cheng SM. Mesenchymal stem cells from human umbilical cord express preferentially secreted factors related to neuroprotection, neurogenesis, and angiogenesis. PLoS ONE. 2013;8(8): e72604. https://doi.org/10.1371/journal.pone.0072604. (PMID: 23991127; PMCID: PMC3749979).
Teng S-W, Lo Y-S, Liu W-T, Hsuan Y, Lin W. A genome-wide comparison of mesenchymal stem cells derived from human placenta and umbilical cord. Taiwan J Obstet Gynecol. 2017;56(5):664–71.
Kang Q, Sun MH, Cheng H, Peng Y, Montag AG, Deyrup AT, He TC. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther. 2004;17:1312–20.
Kang C-M, Kim H, Song JS, Choi B-J, Kim S-O, Jung H-S, et al. Genetic comparison of stemness of human umbilical cord and dental pulp. Stem Cells Int. 2016;2016:3453890.
Reboredo NM, Díaz A, Castro A, Villaescusa RG. Collection, processing and cryopreservation of umbilical cord blood for unrelated transplantation. Bone Marrow Transplantat. 2001;26:1263–70.
Papaccio G, Graziano A, d’Aquino R, Graziano MF, Pirozzi G, Menditti D, et al. Long-term cryopreservation of dental pulp stem cells (SBP-DPSCs) and their differentiated osteoblasts: a cell source for tissue repair. J Cell Physiol. 2006;208:319–25.
Rai S, Kaur M, Kaur S, Arora SP. Redefining the potential applications of dental stem cells: an asset for future. Indian J Hum Genet. 2012;18(3):276–84. https://doi.org/10.4103/0971-6866.107976. (PMID:23716933;PMCID:PMC3656514).
Mo M, Wang S, Zhou Y, Li H, Wu Y. Mesenchymal stem cell subpopulations: phenotype, property and therapeutic potential. Cell Mol Life Sci. 2016;73(17):3311–21.
Lukomska B, Stanaszek L, Zuba-Surma E, Legosz P, Sarzynska S, Drela K. Challenges and controversies in human mesenchymal stem cell therapy. Stem Cells Int. 2019;9(2019):9628536. https://doi.org/10.1155/2019/9628536. (PMID:31093291;PMCID:PMC6481040).
Petrini C. Umbilical cord blood collection, storage and use: ethical issues. Blood Transfus. 2010;8(3):139–48.
Monitoring stem cell research. Washington, D.C.: The President’s Council on Bioethics [Google Scholar]. 2004. https://bioethicsarchive.georgetown.edu/pcbe/reports/stemcell/. Accessed 22 Apr 2023.
Lyerly AD, Faden RR. Embryonic stem cells. Willingness to donate frozen embryos for stem cell research. Science. 2007;317:46–7.
Clinical trial overview: neuronal ceroid lipofuscinosis (NCL, often called Batten disease). http://www.stemcellsinc.com/clinicaltrials/clinicaltrials.html. Accessed 4 Mar 2009.
European Group on Ethics in Science and New Technologies: Opinion n. 19. Ethical aspects of umbilical cord blood banking. 16 March 2004. http://ec.europa.eu/european_group_ethics/publications/docs/publop19_en.pdf. Accessed 15 Nov 2009.
Azuma K. Regulatory landscape of regenerative medicine in Japan. Curr Stem Cell Rep. 2015;1:118–28. https://doi.org/10.1007/s40778-015-0012-6.
Mathur R. National ethical guidelines for biomedical and health research involving human participants. In: ICMR Guidelines. 2017. (ISBN: 978–81–910091–94).
Caulfield T, Kamenova K, Ogbogu U, Zarzeczny A, Baltz J, Benjaminy S, Cassar PA, Clark M, Isasi R, Knoppers B, Knowles L, Korbutt G, Lavery JV, Lomax GP, Master Z, McDonald M, Preto N, Toews M. Research ethics and stem cells: IS it time to re-think current approaches to oversight? EMBO Rep. 2015;16(1):2–6. https://doi.org/10.15252/embr.201439819. (Epub 2014 Dec 4. PMID: 25476708; PMCID: PMC4304722).
Herberts CA, Kwa MS, Hermsen HP. Risk factors in the development of stem cell therapy. J Transl Med. 2011;9:29. https://doi.org/10.1186/1479-5876-9-29.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72.
Deinsberger J, Reisinger D, Weber B. Global trends in clinical trials involving pluripotent stem cells: a systematic multi-database analysis. npj Regen Med. 2020;5:15. https://doi.org/10.1038/s41536-020-00100-4.
Hynes K, Menicanin D, Han J, et al. Mesenchymal stem cells from iPS cells facilitate periodontal regeneration. J Dent Res. 2013;92(9):833–9.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MD: data collection, writing, correction/addition/alteration. AS: correction/addition/alteration, guidance/supervision/suggestion.
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Das, M., Sloan, A.J. Stem cell sources from human biological waste material: a role for the umbilical cord and dental pulp stem cells for regenerative medicine. Human Cell 36, 1312–1325 (2023). https://doi.org/10.1007/s13577-023-00922-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-023-00922-6