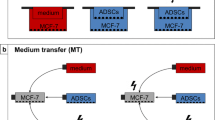
Abstract
Cell–cell interactions between cancer cells and neighboring adipose tissue-derived stromal cells (ATSCs) are known to regulate the aggressiveness of cancer cells. In addition, the radiation-induced bystander effect is an important modulator of cancer cell kinetics. Radiation therapy is often given for urinary cancer, but the biological effects of the irradiated cancer stroma, including adipose tissue, on urothelial carcinoma (UC) remain unclear. We investigated the bystander effect of irradiated ATSCs on UC using a collagen gel culture method to replicate irradiated ATSC–cancer cell interactions after a single 12-Gy dose of irradiation. Proliferative activity, invasive capacity, protein expression and nuclear translocation of p53 binding protein-1 (53BP1) were analyzed. Irradiated ATSCs significantly inhibited the growth and promoted the apoptosis of UC cells in comparison to non-irradiated controls. The invasiveness of UC cells was increased by irradiated ATSCs, but not irradiated fibroblasts. Nuclear translocation of 53BP1 protein due to the bystander effect was confirmed in the irradiated group. Irradiated ATSCs regulated the expressions of the insulin receptor, insulin-like growth factor-1 and extracellular signal-regulated kinase-1/2 in UC. In conclusion, the bystander effect of irradiated ATSCs is a critical regulator of UC, and the actions differed depending on the type of mesenchymal cell involved. Our alternative culture model is a promising tool for further investigations into radiation therapy for many types of cancer.









Similar content being viewed by others
References
Cumberbatch MGK, Jubber I, Black PC, Esperto F, Figueroa JD, Kamat AM, et al. Epidemiology of bladder cancer: a systematic review and contemporary update of risk factors in 2018. Eur Urol. 2018;74(6):784–95. https://doi.org/10.1016/j.eururo.2018.09.001.
De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008;123(10):2229–38. https://doi.org/10.1002/ijc.23925.
Nagase K, Akutagawa T, Rikitake-Yamamoto M, Morito S, Futamata M, Tobu S, et al. Cellular and physical microenvironments regulate the aggressiveness and sunitinib chemosensitivity of clear cell renal cell carcinoma. J Pathol. 2021;254(1):46–56.
Akutagawa T, Aoki S, Yamamoto-Rikitake M, Iwakiri R, Fujimoto K, Toda S. Cancer–adipose tissue interaction and fluid flow synergistically modulate cell kinetics, HER2 expression, and trastuzumab efficacy in gastric cancer. Gastric Cancer. 2018;21(6):946–55.
Miyake M, Hori S, Morizawa Y, Tatsumi Y, Nakai Y, Anai S, et al. CXCL1-mediated interaction of cancer cells with tumor-associated macrophages and cancer-associated fibroblasts promotes tumor progression in human bladder cancer. Neoplasia. 2016;18(10):636–46. https://doi.org/10.1016/j.neo.2016.08.002.
Deschavanne PJ, Fertil B. A review of human cell radiosensitivity in vitro. Inte J Radiat Oncol* Biol* Phys. 1996;34(1):251–66.
Burdak-Rothkamm S, Rothkamm K. Radiation-induced bystander and systemic effects serve as a unifying model system for genotoxic stress responses. Mutat Res. 2018;778:13–22. https://doi.org/10.1016/j.mrrev.2018.08.001.
Prise KM, Folkard MD, Michael B. A review of the bystander effect and its implications for low-dose exposure. Radiat Prot Dosim. 2003;104(4):347–55.
Wang H, Yu KN, Hou J, Liu Q, Han W. Radiation-induced bystander effect: early process and rapid assessment. Cancer Lett. 2015;356(1):137–44. https://doi.org/10.1016/j.canlet.2013.09.031.
Zimmermann M, de Lange T. 53BP1: pro choice in DNA repair. Trends Cell Biol. 2014;24(2):108–17.
FitzGerald JE, Grenon M, Lowndes NF. 53BP1: function and mechanisms of focal recruitment. Biochem Soc Trans. 2009;37(Pt 4):897–904. https://doi.org/10.1042/BST0370897.
Matsuda K, Kawasaki T, Akazawa Y, Hasegawa Y, Kondo H, Suzuki K, et al. Expression pattern of p53-binding protein 1 as a new molecular indicator of genomic instability in bladder urothelial carcinoma. Sci Rep. 2018;8(1):15477. https://doi.org/10.1038/s41598-018-33761-9.
Deng T, Lyon CJ, Bergin S, Caligiuri MA, Hsueh WA. Obesity, inflammation, and cancer. Annu Rev Pathol. 2016;11:421–49.
Nieman KM, Romero IL, Van Houten B. Lengyel E (2013) Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochimica et Biophysica Acta (BBA)-Mol Cell Biol Lipids. 1831;10:1533–41.
Kawasaki-Nanri M, Aoki S, Uchihashi K, Yamamoto M, Udo K, Nishijima-Matsunobu A, et al. Differential effects of adipose tissue stromal cells on the apoptosis, growth and invasion of bladder urothelial carcinoma between the superficial and invasive types. Int J Urol. 2016;23(6):510–9. https://doi.org/10.1111/iju.13086.
Akutagawa T, Aoki S, Yamamoto-Rikitake M, Iwakiri R, Fujimoto K, Toda S. Cancer-adipose tissue interaction and fluid flow synergistically modulate cell kinetics, HER2 expression, and trastuzumab efficacy in gastric cancer. Gastric Cancer. 2018;21(6):946–55. https://doi.org/10.1007/s10120-018-0829-7.
Nomoto-Kojima N, Aoki S, Uchihashi K, Matsunobu A, Koike E, Ootani A, et al. Interaction between adipose tissue stromal cells and gastric cancer cells in vitro. Cell Tissue Res. 2011;344(2):287–98. https://doi.org/10.1007/s00441-011-1144-3.
Aoki S, Udo K, Morimoto H, Ikeda S, Takezawa T, Uchihashi K, et al. Adipose tissue behavior is distinctly regulated by neighboring cells and fluid flow stress: a possible role of adipose tissue in peritoneal fibrosis. J Artif Organs. 2013;16(3):322–31. https://doi.org/10.1007/s10047-013-0702-8.
Toda S, Yamada S, Aoki S, Inokuchi A, Sugihara H. Air-liquid interface promotes invasive growth of laryngeal squamous cell carcinoma with or without hypoxia. Biochem Biophys Res Commun. 2005;326(4):866–72. https://doi.org/10.1016/j.bbrc.2004.11.122.
Matsuyama A, Toda S, Yamada S, Inokuchi A, Sugihara H. Effects of irradiation on biological behavior of carcinoma cells under carcinoma-stromal cell interaction and air-liquid interface: a possible model for testing radiosensitivity of carcinoma of the upper aerodigestive tract using a collagen gel culture system. Pathol Res Practice. 2002;198(7):469–78. https://doi.org/10.1078/0344-0338-00284.
Kamochi N, Nakashima M, Aoki S, Uchihashi K, Sugihara H, Toda S, et al. Irradiated fibroblast-induced bystander effects on invasive growth of squamous cell carcinoma under cancer–stromal cell interaction. Cancer Sci. 2008;99(12):2417–27. https://doi.org/10.1111/j.1349-7006.2008.00978.x.
Hamada N, Matsumoto H, Hara T, Kobayashi Y. Intercellular and intracellular signaling pathways mediating ionizing radiation-induced bystander effects. J Radiat Res. 2007;48(2):87–95. https://doi.org/10.1269/jrr.06084.
Kobayashi M, Kadota J, Hashimoto Y, Fujisato T, Nakamura N, Kimura T, et al. Elastic modulus of ECM hydrogels derived from decellularized tissue affects capillary network formation in endothelial cells. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21176304.
Yu Y, Skocaj M, Kreft ME, Resnik N, Veranic P, Franceschi P, et al. Comparative lipidomic study of urothelial cancer models: association with urothelial cancer cell invasiveness. Mol Biosyst. 2016;12(11):3266–79. https://doi.org/10.1039/c6mb00477f.
Hansel DE, Platt E, Orloff M, Harwalker J, Sethu S, Hicks JL, et al. Mammalian target of rapamycin (mTOR) regulates cellular proliferation and tumor growth in urothelial carcinoma. Am J Pathol. 2010;176(6):3062–72. https://doi.org/10.2353/ajpath.2010.090872.
Yamada T, Ueda T, Shibata Y, Ikegami Y, Saito M, Ishida Y, et al. TRPV2 activation induces apoptotic cell death in human T24 bladder cancer cells: a potential therapeutic target for bladder cancer. Urology. 2010;76(2):509 e1-7. https://doi.org/10.1016/j.urology.2010.03.029.
Fang D, Kitamura H. Cancer stem cells and epithelial-mesenchymal transition in urothelial carcinoma: possible pathways and potential therapeutic approaches. Int J Urol. 2018;25(1):7–17. https://doi.org/10.1111/iju.13404.
Ching CB, Hansel DE. Expanding therapeutic targets in bladder cancer: the PI3K/Akt/mTOR pathway. Lab Invest. 2010;90(10):1406–14. https://doi.org/10.1038/labinvest.2010.133.
Malaguarnera R, Belfiore A. The insulin receptor: a new target for cancer therapy. Front Endocrinol (Lausanne). 2011;2:93. https://doi.org/10.3389/fendo.2011.00093.
Vella V, Milluzzo A, Scalisi NM, Vigneri P, Sciacca L. Insulin receptor isoforms in cancer. Int J Mol Sci. 2018;19(11):3615.
Zhang L, Zhou F, ten Dijke P. Signaling interplay between transforming growth factor-β receptor and PI3K/AKT pathways in cancer. Trends Biochem Sci. 2013;38(12):612–20.
Syed V. TGF-β signaling in cancer. J Cell Biochem. 2016;117(6):1279–87.
Farhood B, Khodamoradi E, Hoseini-Ghahfarokhi M, Motevaseli E, Mirtavoos-Mahyari H, Eleojo Musa A, et al. TGF-β in radiotherapy: mechanisms of tumor resistance and normal tissues injury. Pharmacol Res. 2020. https://doi.org/10.1016/j.phrs.2020.104745.
Seymour CB, Mothersill C. Radiation-induced bystander effects—implications for cancer. Nat Rev Cancer. 2004;4(2):158–64.
Cheung DT, Perelman N, Tong D, Nimni ME. The effect of γ-irradiation on collagen molecules, isolated α-chains, and crosslinked native fibers. J Biomed Mater Res. 1990;24(5):581–9.
Montella M, Di Maso M, Crispo A, Grimaldi M, Bosetti C, Turati F, et al. Metabolic syndrome and the risk of urothelial carcinoma of the bladder: a case-control study. BMC Cancer. 2015;15(1):1–7.
Kumar V, Abbas AK, Fausto N, Aster JC. Robbins and Cotran pathologic basis of disease, professional edition e-book. Elsevier health sciences; 2014.
Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Can Res. 2011;71(7):2455–65.
Wu Q, Li B, Li Z, Li J, Sun S, Sun S. Cancer-associated adipocytes: key players in breast cancer progression. J Hematol Oncol. 2019;12(1):1–15.
Reiss K, D’Ambrosio C, Tu X, Tu C, Baserga R. Inhibition of tumor growth by a dominant negative mutant of the insulin-like growth factor I receptor with a bystander effect. Clin Cancer Res. 1998;4(11):2647–55.
Najafi M, Fardid R, Hadadi G, Fardid M. The mechanisms of radiation-induced bystander effect. J Biomed Phys Eng. 2014;4(4):163.
Li L, Wang L, Prise KM, Yu K, Chen G, Chen L, et al. Akt/mTOR mediated induction of bystander effect signaling in a nucleus independent manner in irradiated human lung adenocarcinoma epithelial cells. Oncotarget. 2017;8(11):18010.
Acknowledgements
We thank M. Itoh, T. Sakumoto, S. Morito, M. Nishida, F. Mutoh and S. Nakahara for their excellent technical assistance.
Funding
This work was funded by the Japanese Ministry of Education, Culture, Sports, Science and Technology for Scientific Research (Grant no. 18K09138).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest. This research was supported in part by a Grant-in-Aid from the Japanese Ministry of Education, Culture, Sports, Science and Technology for Scientific Research (No. 18K09138).
Ethical approval
All procedures involving human or animal materials were performed in accordance with the Ethical Guidelines of Saga University.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13577_2022_668_MOESM1_ESM.eps
Supplementary file1 Figure S1. Extracellular matrix structure of collagen gels cultured for 7 days or 14 days. (a) Transmission electron microscopy image of a non-irradiated collagen gel cultured for 7 days. (b) Electron micrograph of an irradiated collagen gel cultured for 7 days. Non-irradiated collagen fibers exhibited helical structures, whereas the irradiated collagen gel consisted mainly of finely fragmented fibers. (c) The elastic moduli of non-irradiated and irradiated (12 Gy) collagen gels with or without NIH3T3 cells on day 7 and day 14. When compared with gels not containing NIH3T3 cells, gels embedded with NIH3T3 cells had a significantly lower elastic modulus on day 7 and a significantly higher elastic modulus on day 14 (both non-irradiated and irradiated conditions). (EPS 728 KB)
13577_2022_668_MOESM2_ESM.eps
Supplementary file2 Figure S2. Irradiation and mesenchymal cells regulate the phosphoinositide 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway and the expressions of the insulin receptor (IR) and insulin-like growth factor-1 receptor (IGF-1R) in RT4 cells. Total IR expression was significantly greater in RT4 cells co-cultured with non-irradiated ATSCs than in RT4 cells monocultured on a non-irradiated gel. Irradiated ATSCs decreased the phosphorylated/total IR ratio in RT4 cells compared to the monoculture group. Total IGF-1R expression was significantly greater in RT4 cells co-cultured with non-irradiated ATSCs than in RT4 cells monocultured on a non-irradiated gel or RT4 cells co-cultured with irradiated ATSCs. Irradiation of ATSCs significantly decreased the phosphorylated/total IR ratio in RT4 cells. Co-culture with ASTCs increased total JNK expression in RT4 cells under both non-irradiated and irradiated conditions, and irradiated ATSCs decreased total JNK expression in RT4 cells compared to non-irradiated ATSCs. The phosphorylated/total JNK ratio was increased by irradiation in the presence and absence of ATSCs and by co-culture with ATSCs under both non-irradiated and irradiated conditions. Total Akt expression in RT4 cells was increased by co-culture with ATSCs under both non-irradiated and irradiated conditions. In the absence of ATSCs, irradiation decreased the phosphorylated/total Akt ratio in RT4 cells. Both non-irradiated and irradiated ATSCs significantly increased the phosphorylated/total Akt ratio in RT4 cells compared to the respective monoculture group. Non-irradiated and irradiated ATSCs significantly upregulated total mTOR expression in RT4 cells compared to the respective monoculture group. Irradiation significantly decreased total mTOR expression in RT4 cells both in the presence and absence of ATSCs. There were no significant change in the phosphorylated/total mTOR ratio in RT4 cells under any experimental conditions. Relative expression is depicted as the ratio of the target protein expression to α/β-tubulin expression. Data represent the mean ± SD of 5 determinations. *P < 0.05, **P < 0.01, ***P < 0.001. (EPS 895 KB)
13577_2022_668_MOESM3_ESM.eps
Supplementary file3 Figure S3. Irradiation and mesenchymal cells regulate the phosphoinositide 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway and the expressions of the insulin receptor (IR) and insulin-like growth factor-1 receptor (IGF-1R) in T24 cells. Total IR and IGF-1R expressions in T24 cells were significantly increased after co-culture with ATSCs under both non-irradiated and irradiated conditions. The phosphorylated/total IR ratio and phosphorylated/total IGF-1R ratio were significantly greater in T24 cells co-cultured with non-irradiated ATSCs than in T24 cells monocultured on a non-irradiated gel or T24 cells co-cultured with irradiated ATSCs. Total JNK expression in T24 cells was significantly increased after co-culture with ATSCs under both non-irradiated and irradiated conditions. The phosphorylated/total JNK ratio was significantly greater in T24 cells monocultured on a non-irradiated collagen gel than in T24 cells monocultured on an irradiated gel or T24 cells co-cultured with non-irradiated ATSCs. Total Akt expression in T24 cells was significantly reduced by ATSCs under both non-irradiated and irradiated conditions, and by irradiation both in the presence and absence of ATSC co-culture. Non-irradiated and irradiated ATSCs significantly increased the phosphorylated/total Akt ratio in T24 cells compared to the respective monoculture group. Non-irradiated and irradiated ATSCs significantly upregulated total mTOR expression in T24 cells in comparison to the respective monoculture group. The phosphorylated/total mTOR ratio in T24 cells was not affected by irradiation or ATSCs. Relative expression is depicted as the ratio of target protein expression to α/β-tubulin expression. Data represent the mean ± SD of 5 determinations. *P < 0.05, **P < 0.01, ***P < 0.001. (EPS 892 KB)
Rights and permissions
About this article
Cite this article
Kawasaki, M., Nagase, K., Aoki, S. et al. Bystander effects induced by the interaction between urothelial cancer cells and irradiated adipose tissue-derived stromal cells in urothelial carcinoma. Human Cell 35, 613–627 (2022). https://doi.org/10.1007/s13577-022-00668-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-022-00668-7