Abstract
Rosacea is a common, chronic inflammatory disease characterized by both fluctuating and fixed heterogeneous signs such as facial erythema, papules/pustules, telangiectasia, acute vasodilation (flushing), and phymatous changes, and symptoms such as cutaneous stinging and burning. The shift to a phenotype-based approach to rosacea management has improved the consistency of recommendations across recent published guidelines. Consistent and thorough guidance for the classification, diagnosis, and management of the disease is difficult, as the mechanisms underlying the development of rosacea are still not completely understood nor universally accepted. Here, we provide a critical review of current published guidance, and gaps in the knowledge and management of rosacea. We present the recently approved microencapsulated benzoyl peroxide as an effective topical treatment option for papulopustular rosacea. Benzoyl peroxide (BPO) has been used in acne management for many years; however, many clinicians perceive treatment of rosacea with any BPO formulation to be counterintuitive because of concerns of potential skin irritation, while the lack of an accepted mechanism of action on rosacea pathophysiology means that others may be hesitant to use BPO as a treatment. Minocycline foam 1.5% is also an option for the treatment of inflammatory lesions in rosacea, with a decreased risk of systemic adverse events compared with oral minocycline.
Similar content being viewed by others
Future recommendations and guideline updates for rosacea should aim to establish consensus and promote consistent management across the dermatologic community, including the importance of patient-centric management and education to improve adherence and treatment success. |
The recent approval of new treatment options for rosacea, including microencapsulated benzoyl peroxide (E-BPO) as an effective and tolerable treatment for papulopustular rosacea and minocycline foam for inflammatory lesions of rosacea, warrant a revision to current published guidelines. |
Introduction
Rosacea is a common, chronic disease that, despite having an estimated prevalence of 3.2% in the USA, is still poorly understood [1, 2]. It is characterized by heterogeneous signs and symptoms that cycle between remission and exacerbation, including fixed and transient facial erythema, flushing, papules, pustules, phymatous changes, telangiectasias, and stinging and burning [1, 3,4,5]. Management should be individualized to the patient, primarily on the basis of current clinical presentation (phenotype); and, when appropriate, multiple therapies should be integrated to optimally target the patient-specific clinical manifestations of rosacea [3,4,5]. In this opinion piece, we provide a critical review of published guidelines, particularly those published by the American Acne and Rosacea Society (AARS) and the Global Rosacea Consensus (ROSCO) panel. We also discuss the more recently approved microencapsulated benzoyl peroxide (E-BPO) and minocycline foam as effective and well-tolerated treatment options for rosacea. The existence of safe and effective treatments for rosacea that were not available at the time of the last AARS and ROSCO guideline updates warrants revision. This article is based on previous studies and does not include new research by the authors involving human participants or animals.
Knowledge Gaps in Rosacea
Psychosocial associations Since the last AARS and ROSCO updates in 2019 [4, 6], the 2020 Beyond the Visible report has highlighted the psychological and invisible burden of rosacea [7]. It revealed that 89% of patients with rosacea considered their disease to be uncontrolled to some extent, and 58% experienced a significant daily life impact. Patients with rosacea had missed 4% of work time in the past year, equating to nearly 10.5 working days annually [7].
Beyond the psychological burden There are several gaps in our rosacea understanding that necessitate attention. Understanding the development of rosacea is pivotal for effective treatment for patients; however, data gaps persist in its pathophysiology and classification of severity for ocular, phymatous, and granulomatous rosacea, which can limit treatment options. Additionally, the quality of evidence for the treatment of phymatous and granulomatous rosacea is low, as there are limited trial data [6].
Association between phyma and carcinomas Previous research has suggested that there is an unexplored link between phyma and skin cancer. Rhinophyma has been observed to hide the emergence of basal and squamous cell carcinomas developing in the nasal area of the elderly population; however, it is possible that this is coincidental [8, 9]. Regardless, vigilance by dermatologists during treatment of phymatous rosacea is crucial for early cancer detection [8].
Cutaneous microbiome Another incompletely understood area of rosacea is the effect of the skin microbiome on the pathophysiology and presentation of the disease. Woo et al. found a link between lower Cutibacterium acnes and increased rosacea severity [10]. Proliferation of Demodex folliculorum and its associated bacteria (e.g., Bacillus oleronius) may contribute to early inflammation in rosacea [11, 12]. The secretome of Gram-negative Bacillus oleronius may stimulate peripheral blood cell proliferation and neutrophil accumulation in rosacea skin, leading to inflammation and, in the case of the latter, tissue degradation [13, 14]. However, the role of B. oleronius in rosacea is not as widely accepted as D. folliculorum, warranting additional research. Additionally, increased facial skin temperature in patients with rosacea, caused by increased blood flow, is thought to promote inflammatory β-hemolytic protein production by Staphylococcus epidermidis [15].
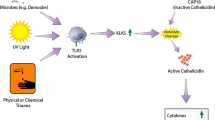
Cathelicidin cascade The role of cathelicidin antimicrobial peptides in rosacea warrants further exploration—Yamasaki et al. discovered that patients with rosacea often express higher levels of abnormally processed cathelicidins in their facial skin, correlating to increased inflammation [16]. Toll-like receptor activation may contribute to this process, triggering various immune and vascular responses that promote angiogenesis, inflammation, and skin microbiome changes [16, 17].
Transient receptor potential (TRP) protein channels Immunostaining has shown increased TRPV gene expression in dermal inflammatory cells in patients with rosacea [18]. Activation of some TRPV channels owing to heat may explain rosacea symptoms triggered by environmental warmth or dietary factors [18,19,20]. This activation may trigger an inflammatory cascade, releasing proinflammatory cytokines such as interleukin-1, prostaglandin E2, and matrix metalloproteinases 1 [18]. Further investigation may elucidate this link.
Overview of Current Published Guidance
Since its introduction in 2008, there have been several updates to the AARS guidelines to address the use of newly approved therapies and other management options for the treatment of rosacea [6, 21,22,23,24,25]. However, since the last guideline update in 2019, new treatments have been approved for the treatment of rosacea, including E-BPO cream and minocycline foam, which highlights the need for updated guidelines.
Several consensus publications support a shift from a subtype-led to a phenotype-based approach for rosacea management [1, 25,26,27,28]. Published data support the use of topical alpha-agonists and/or device therapy for the management of rosacea presenting with persistent central facial erythema (PFE), while there is a plethora of data suggesting that papulopustular lesions with perilesional erythema should be treated with topical and/or oral treatments that target inflammation [1, 4, 6, 29].
Combination therapy approaches Clinical evidence supports the use of combination therapies to treat signs and symptoms of rosacea effectively, especially for severe or unresponsive cases [4,5,6, 23, 24, 26, 30]. An individualized phenotypic approach to rosacea treatment is essential to improve the overall effectiveness of therapy; additional research and real-world data collection can help us understand how to optimize treatment outcomes by combining available treatments in the rosacea armamentarium. Various combination treatments have been explored: oral and topical therapies for faster control of papulopustular rosacea; alpha-agonist and topical therapies for specific unresponsive manifestations of papulopustular rosacea; and alpha-agonist treatment with a selective physical modality to address PFE and telangiectasias [30,31,32,33].
Skin barrier dysfunction Patients with rosacea have an impaired skin barrier and sensitive skin [34]. Discussions surrounding skin barrier function often focus on the epidermal permeability barrier; however, alterations in the microbiome and immune-response barriers may also contribute [10,11,12,13,14,15, 35]. A comprehensive skin care routine is essential for successful rosacea treatment [5, 36]. Using appropriate cleansers, moisturizers, and photoprotection can reduce skin irritation and barrier impairment, improving therapeutic outcomes and patient adherence [5, 6, 21, 22, 25, 26].
More uncommon rosacea types Current publications describe management options for ocular rosacea, including lid hygiene, various topical ophthalmic agents, oral doxycycline therapy, and topical ivermectin specifically for the treatment of blepharitis [4, 6, 37,38,39,40,41,42]. Existing recommended management options for phymatous and granulomatous rosacea are limited, with guidance suggesting the use of oral tetracyclines and low-dose isotretinoin or device therapy and surgical therapy, respectively [6, 23].
Physical devices The use of energy devices to improve skin quality and manage PFE and telangiectasias of rosacea is explored in the ROSCO and AARS guidelines [4, 24]. The AARS guidelines cover physical modalities and devices, including tangential excision, electroscalpel, and dermabrasion [24]. However, treatment with energy devices can lead to paradoxical cutaneous concerns such as swelling, erythema, bruising, dyspigmentation, and scarring, highlighting the importance of proper device use by an experienced operator [24, 43, 44]. Additionally, intradermal botulinum A can treat rosacea with PFE and flushing that are poorly responsive to other therapies or prone to tolerability issues [6, 45].
Gaps in Clinical Evidence, Guidelines, and Overall Rosacea Treatment
There are several gaps in the current management landscape of rosacea, as summarized in Table 1. First, patients who desire fast-acting, long-term control of their rosacea have limited treatment options. Establishing realistic patient expectations at treatment initiation is crucial, especially for those hoping for a rosacea “cure.” Patients seeking rapid improvement, including those who have previously used topical therapies, are often prescribed oral systemic agents alongside topical treatments. This is continued until symptom control is achieved, with an eventual transition to topical monotherapy for maintenance [46]. Generally, topical medications are preferred in most cases for the treatment of rosacea, considering the chronic nature of the disease; oral systemic therapy has risks such as systemic adverse events and antibiotic resistance with prolonged full-dose antibiotic use [46]. Therefore, there is a need to explore quick yet effective and long-lasting topical therapies for papulopustular rosacea.
There are limited data available from well-designed, two-arm clinical studies evaluating the efficacy and safety of long-term treatment of rosacea beyond the typical clinical trial length of pivotal studies (12–16 weeks) [47, 48]. In addition, the limited data available primarily address rosacea presenting with papulopustular lesions. This has led to a limited understanding of real-world rosacea presentations, and inconsistencies in recommendations related to standardized care, especially for the long-term management of rosacea [25, 48]. To improve this, we recommend conducting well-designed comparative retrospective studies to establish optimal first-line and maintenance treatments for rosacea [49].
Published guidelines lack specific guidance on alternative therapies, treatment adjustments, and treatment for specific patient types. Current guidance was developed using clinical trial data from treatment-naïve patients or those who had been off treatment for multiple weeks/months [25]. Additional data are needed regarding successful treatment of nonresponders to inform future guideline updates. Emphasizing the diagnosis and management of rosacea in patients with skin of color is also recommended, as facial erythema and telangiectasias can be more difficult to visualize in darker skin, leading to underdiagnosis, delayed diagnosis and treatment, and worsening chronic manifestations of rosacea, such as ocular or phymatous changes [50].
Although the consistency of recommendations for rosacea management has improved with the phenotype approach, it is important to standardize definitions and specific criteria to avoid confusion among clinicians. Guidelines should also include patient-centric recommendations as vital management components, including patient education, psychosocial support, and individualized treatment plans that consider patients’ preferences, needs, and expectations [48]. Developing a shared decision-making model that incorporates social determinants of health, such as home environment, medical care access, and education level, may accommodate a comprehensive patient-centric approach to rosacea management. Respective of the prior scientific and clinical updates, considerations for the availability, accessibility, and affordability of treatment options may have a significant impact on patients’ ability to access care, receive optimal therapy, and adhere to treatment. Foremost, however, is the need for more frequent updates to consensus recommendations and guidelines that include new, clinically relevant information on rosacea pathophysiology, diagnosis, skin care, potential comorbidities, and therapeutic advances, including emerging treatments and management options.
Novel topical treatments in the rosacea armamentarium Microencapsulated benzoyl peroxide (E-BPO): a novel topical therapy option for papulopustular rosacea
In April 2022, the Food and Drug Administration (FDA) approved E-BPO cream, 5% for the treatment of papulopustular lesions of rosacea [51]. Since then, visible and well-tolerated improvements have been observed with long-term treatment, as demonstrated in our real-world case study in Fig. 1; further information is provided in Supplementary Material 1.
Clinical photographs of a patient showing improvement in visible signs of rosacea before and after combination treatment with once-daily E-BPO cream, 5%. The patient presented with papules, PFE, and perilesional erythema with intermittent flushing episodes, and sensory symptoms of burning. Once-daily combination treatment of oral doxycycline 40 mg, carvedilol, oxymetazoline cream, 1% and E-BPO, 5% markedly improved the patient’s overall facial erythema by 12-month follow-up, with flushing and burning symptoms also well controlled. a Pretreatment. b Posttreatment (12-month follow-up). Further details of this patient case study can be found in the Supplementary Material
Unencapsulated traditional formulations of benzoyl peroxide (BPO) have been recognized as an effective topical management option for rosacea since 1961 [52]. Upon contact with skin, it is believed that BPO penetrates the stratum corneum and enters the pilosebaceous duct, degrading into benzoic acid and oxygen [53]. Additionally, reduction in D. folliculorum was observed in a clinical study of BPO and erythromycin versus metronidazole [54]. Efficacy for the treatment of moderate-to-severe rosacea was highlighted in another study assessing once-daily application of BPO, 5%, and clindamycin, 1% topical gel, with adverse application-site reactions occurring in 14.8% of active-arm patients [55]. Although BPO efficacy has been proven in clinical trials, use in clinical practice has historically been limited by tolerability [53]. Direct skin application can cause high transient exposure leading to local cutaneous reactions, including erythema, stinging, burning, and itching.
Silica microencapsulation of BPO (E-BPO) is a novel technology demonstrated in clinical trials to be effective and tolerable in the skin of patients with rosacea [56,57,58]. This microencapsulation process sequesters BPO in an amorphous silica shell of predetermined size and thickness, creating a permeable barrier between the medication and skin for gradual release to control the rate of skin exposure and decrease the risk of local adverse reactions [56, 59,60,61].
In two phase 3 trials, E-BPO cream, 5%, was statistically superior to vehicle in treating subjects with papulopustular rosacea [57, 58]. E-BPO exhibited a rapid onset of clinical effects in both co-primary endpoints, viz. Investigator’s Global Assessment (IGA) success and mean inflammatory lesion count change. IGA scoring included the number of papules/pustules and erythema severity, while success was defined as a patient scoring 0 (“clear”) or 1 (“almost clear”) on a five-point scale (0–4). IGA success was achieved by over 25% of subjects treated with E-BPO cream, 5%, by week 4 in both phase 3 trials, versus 6.5% and 14.1% in the vehicle groups (P < 0.001 and P = 0.009). Subjects who received E-BPO cream, 5%, demonstrated a 67.9% greater reduction in the mean number of inflammatory lesions from baseline to week 12 versus vehicle treatment in both trials (−17.4 versus −9.5 and −20.3 versus −13.3, respectively; P < 0.001). Generally, E-BPO cream, 5%, was safe and well tolerated in phase 3 trials [57]. Additionally, a phase 3 extension study demonstrated that E-BPO is effective in the reduction of papules, pustules, and erythema, and well tolerated for up to 52 weeks of treatment with limited cutaneous irritation [58].
We have provided a case study that depicts visible improvements in a patient presenting with papules, pustules, and PFE after 1 year of continued combination therapy including once-daily use of E-BPO cream, 5%, which clinically suggests a reduction in perilesional erythema and PFE. However, more data are needed to evaluate the potential therapeutic contributions of E-BPO cream, 5%, for the reduction of overall facial erythema with continued use in patients with inflammatory lesions, as monotherapy or in combination with other therapies. There is scientific basis for this consideration: cathelicidin-induced inflammation in lesions can contribute to the progressive increase in PFE via mechanisms induced by variant peptides [16, 62]. Similar to what has been observed with ivermectin treatment for mild–moderate inflamed rhinophyma [63], further investigations could be conducted into the use of E-BPO cream, 5%, for the treatment of clinically inflamed phyma.
Symptoms of rosacea such as burning, stinging, and itching were captured as tolerability parameters in phase 3 clinical trials, showing improvement with E-BPO cream, 5%, treatment. The case study corroborates findings from the phase 3 trials: improvements in burning symptoms accompanying flushing episodes were observed within 3 months of once-daily topical E-BPO treatment initiation.
E-BPO cream, 5%, is a relatively new rosacea treatment with limited available data. Unpublished data suggest enduring changes to the skin barrier and microbiome after 8 weeks of E-BPO treatment, with decreases in the relative abundance of Staphylococcus and increases in Cutibacterium; however, the significance of these changes in pathophysiology requires further investigation. Moreover, the efficacy of E-BPO on inflammatory lesions and associated erythema support antiinflammatory activity and warrant further characterization, particularly its role in addressing specific sources of erythema and for long-term management, including in patients with early and/or visible inflamed phyma. Further research is encouraged for the effect of E-BPO cream, 5%, on granulomatous rosacea, and its utilization for treating papulopustular rosacea in nonresponders.
Minocycline Foam, 1.5%: Another Topical Option for the Treatment of Inflammatory Lesions in Rosacea
Minocycline foam, 1.5%, is a topical tetracycline-class drug approved in 2020 for the treatment of inflammatory lesions in adult patients with moderate-to-severe rosacea [64, 65]. Tetracyclines can provide therapeutic relief for rosacea through their antiinflammatory properties, including regulating cathelicidin production. One study has shown that minocycline can significantly reduce cathelicidins in human bone marrow-derived mesenchymal stromal/stem cells (P < 0.001) [66].
Previously, oral tetracyclines were indicated for the treatment of papulopustular rosacea but have been associated with systemic adverse events such as pill esophagitis, dose-related phototoxicity, and cutaneous hyperpigmentation. Oral minocycline treatment has been associated with cutaneous hyperpigmentation and acute vestibular adverse events such as vertigo and dizziness, and uncommon immunologic adverse events such as drug-induced lupus-like syndrome and autoimmune hepatitis [23].
Topical administration of minocycline was found to circumvent these systemic adverse events. Two pivotal phase 3 studies found that there were no reported cases of hyperpigmentation after once-daily application of minocycline foam, 1.5%, for 12 weeks. Generally, minocycline foam, 1.5%, was safe and well tolerated in phase 3 trials. Most treatment-emergent adverse events were mild to moderate, with diarrhea, pruritus, and viral upper respiratory tract infection being the most frequently reported events [64, 65]. Subjects had improved local tolerability signs at week 12 when treated with minocycline foam, 1.5%. Localized symptoms such as erythema, telangiectasia, and flushing were mild-to-moderate at weeks 12 and 40, as observed in an open-label extension study [65].
Additionally, the proven efficacy of minocycline for the treatment of inflammatory lesions in rosacea was maintained with topical application, with clinical efficacy established as early as week 4 [65]. Subjects treated with minocycline foam, 1.5%, demonstrated a 18.4% greater reduction in the mean number of inflammatory lesions versus vehicle treatment from baseline to week 12 in both trials (−17.57 versus −15.65; P = 0.0031 and −18.54 versus −14.88; P < 0.0001, respectively). At week 12, IGA success was achieved by roughly half of subjects treated with minocycline foam, 1.5%, in both trials, compared with 43% and 39% treated with vehicle (P = 0.0273 and P = 0.0077) [65].
Conclusions
It is important to note that current gaps in published consensus recommendations and guidelines are primarily driven by limitations in data from clinical trials, including evaluation of long-term treatment, phenotype-specific combination therapy approaches, and treatments for nonresponders. Moreover, patient and clinician education are important to improve the overall management of rosacea and inform on the full spectrum of general management suggestions and available treatments. Future updates could focus on the importance of patient-centric management and education, including the need for an optimized skin care routine (for barrier repair and sun protection), and its impact on adherence and success. They should also aim to promote consistent therapeutic approaches by establishing up-to-date consensus for the classification, diagnosis, and treatment of rosacea.
Our understanding of rosacea, its pathophysiology, and the current treatment landscape have come a long way since the original publication of rosacea subtypes in 2002 [67]. In addition, with their recent approvals, we believe that any updates to management guidelines should include E-BPO cream, 5%, as an available option for the topical treatment of papulopustular rosacea with limited cutaneous irritation, and minocycline foam, 1.5%, for the treatment of inflammatory lesions in papulopustular rosacea, with a decreased risk of systemic adverse events compared with oral minocycline [65]. This article also suggests how additional investigations of E-BPO cream, 5%, may help to address some of the identified gaps in our understanding of rosacea pathophysiology and its management.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Thiboutot D, Anderson R, Cook-Bolden F, Draelos Z, Gallo RL, Granstein RD, et al. Standard management options for rosacea: the 2019 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2020;82(6):1501–10. https://doi.org/10.1016/j.jaad.2020.01.077.
Gether L, Overgaard LK, Egeberg A, Thyssen JP. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179(2):282–9. https://doi.org/10.1111/bjd.16481.
Rainer BM, Kang S, Chien AL. Rosacea: epidemiology, pathogenesis, and treatment. Dermatoendocrinol. 2017;9(1): e1361574. https://doi.org/10.1080/19381980.2017.1361574.
Schaller M, Almeida LMC, Bewley A, Cribier B, Del Rosso J, Dlova NC, et al. Recommendations for rosacea diagnosis, classification and management: update from the global ROSacea COnsensus 2019 panel. Br J Dermatol. 2020;182(5):1269–76. https://doi.org/10.1111/bjd.18420.
Del Rosso JQ, Thiboutot D, Gallo R, Webster G, Tanghetti E, Eichenfield L, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 1: a status report on the disease state, general measures, and adjunctive skin care. Cutis. 2013;92(5):234–40.
Del Rosso JQ, Tanghetti E, Webster G, Stein Gold L, Thiboutot D, Gallo RL. Update on the management of rosacea from the American Acne & Rosacea Society (AARS). J Clin Aesthet Dermatol. 2019;12(6):17–24.
Steinhoff, M, Harper J, Gieler U, Tan J. Beyond the Visible: Rosacea and psoriasis of the face 2020. https://hosted.bmj.com/rosacea/assets/beyond-the-visible-rosacea-and-psoriasis-of-the-face.pdf. Accessed 18 May 2023.
Lazzeri D, Colizzi L, Licata G, Pagnini D, Proietti A, Alì G, et al. Malignancies within rhinophyma: report of three new cases and review of the literature. Aesthetic Plast Surg. 2012;36(2):396–405. https://doi.org/10.1007/s00266-011-9802-0.
Chlebicka I, Stefaniak AA, Bieniek A, Matusiak Ł, Woźniak Z, Szepietowski JC. Basal cell carcinoma within rhinophyma: coincidence or relationship? Postepy Dermatol Alergol. 2021;38(5):855–7. https://doi.org/10.5114/ada.2020.99367.
Woo YR, Lee SH, Cho SH, Lee JD, Kim HS. Characterization and analysis of the skin microbiota in rosacea: Impact of systemic antibiotics. J Clin Med. 2020;9(1):185. https://doi.org/10.3390/jcm9010185.
Forton FMN. The pathogenic role of demodex mites in rosacea: a potential therapeutic target already in erythematotelangiectatic rosacea? Dermatol Ther (Heidelb). 2020;10(6):1229–53. https://doi.org/10.1007/s13555-020-00458-9.
Jarmuda S, O’Reilly N, Żaba R, Jakubowicz O, Szkaradkiewicz A, Kavanagh K. Potential role of demodex mites and bacteria in the induction of rosacea. J Med Microbiol. 2012;61(Pt 11):1504–10. https://doi.org/10.1099/jmm.0.048090-0.
Lacey N, Delaney S, Kavanagh K, Powell FC. Mite-related bacterial antigens stimulate inflammatory cells in rosacea. Br J Dermatol. 2007;157(3):474–81. https://doi.org/10.1111/j.1365-2133.2007.08028.x.
O’Reilly N, Bergin D, Reeves EP, McElvaney NG, Kavanagh K. Demodex-associated bacterial proteins induce neutrophil activation. Br J Dermatol. 2012;166(4):753–60. https://doi.org/10.1111/j.1365-2133.2011.10746.x.
Dahl MV, Ross AJ, Schlievert PM. Temperature regulates bacterial protein production: possible role in rosacea. J Am Acad Dermatol. 2004;50(2):266–72. https://doi.org/10.1016/j.jaad.2003.05.005.
Yamasaki K, Nardo AD, Bardan A, Murakami M, Ohtake T, Coda A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13(8):975–80. https://doi.org/10.1038/nm1616.
Holmes AD, Steinhoff M. Integrative concepts of rosacea pathophysiology, clinical presentation and new therapeutics. Exp Dermatol. 2017;26(8):659–67. https://doi.org/10.1111/exd.13143.
Chan KTM. Transient receptors potential (TRP) channels and neuroimmunology: mutation pathways of Rosacea. J Cell Sci Mut. 2018;2(1):39–42. https://doi.org/10.35841/cell-science.2.1.39-42.
Zhou X, Su Y, Wu S, Wang H, Jiang R, Jiang X. The temperature-sensitive receptors TRPV4 and TRPM8 have important roles in the pruritus of rosacea. J Dermatol Sci. 2022;108(2):68–76. https://doi.org/10.1016/j.jdermsci.2022.11.004.
Weiss E, Katta R. Diet and rosacea: the role of dietary change in the management of rosacea. Dermatol Pract Concept. 2017;7(4):31–7. https://doi.org/10.5826/dpc.0704a08.
Del Rosso JQ, Baldwin H, Webster G, American Acne & Rosacea Society. American Acne & Rosacea Society rosacea medical management guidelines. J Drugs Dermatol. 2008;7(6):531–3.
Del Rosso JQ, Thiboutot D, Gallo R, Webster G, Tanghetti E, Eichenfield LF, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 2: a status report on topical agents. Cutis. 2013;92(6):277–84.
Del Rosso JQ, Thiboutot D, Gallo R, Webster G, Tanghetti E, Eichenfield LF, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 3: a status report on systemic therapies. Cutis. 2014;93(1):18–28.
Tanghetti E, Del Rosso JQ, Thiboutot D, Gallo R, Webster G, Eichenfield LF, et al. Consensus recommendations from the American acne & rosacea society on the management of rosacea, part 4: a status report on physical modalities and devices. Cutis. 2014;93(2):71–6.
Del Rosso JQ, Thiboutot D, Gallo R, Webster G, Tanghetti E, Eichenfield LF, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 5: a guide on the management of rosacea. Cutis. 2014;93(3):134–8.
Schaller M, Almeida LMC, Bewley A, Cribier B, Dlova NC, Kautz G, et al. Rosacea treatment update: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176(2):465–71. https://doi.org/10.1111/bjd.15173.
Del Rosso JQ, Gallo RL, Tanghetti E, Webster G, Thiboutot D. An evaluation of potential correlations between pathophysiologic mechanisms, clinical manifestations, and management of rosacea. Cutis. 2013;91(3 Suppl):1–8.
Tan J, Berg M, Gallo RL, Del Rosso JQ. Applying the phenotype approach for rosacea to practice and research. Br J Dermatol. 2018;179(3):741–6. https://doi.org/10.1111/bjd.16815.
van Zuuren EJ, Fedorowicz Z, Tan J, van der Linden MMD, Arents BWM, Carter B, et al. Interventions for rosacea based on the phenotype approach: an updated systematic review including GRADE assessments. Br J Dermatol. 2019;181(1):65–79. https://doi.org/10.1111/bjd.17590.
Bhatia ND, Del Rosso JQ. Optimal management of papulopustular rosacea: rationale for combination therapy. J Drugs Dermatol. 2012;11(7):838–44.
Stein Gold L, Papp K, Lynde C, Lain E, Gooderham M, Johnson S, et al. Treatment of rosacea with concomitant use of topical ivermectin 1% cream and brimonidine 0.33% gel: a randomized, vehicle-controlled study. J Drugs Dermatol. 2017;16(9):909–16.
Del Rosso JQ, Levin J. The clinical relevance of maintaining the functional integrity of the stratum corneum in both healthy and disease-affected skin. J Clin Aesthet Dermatol. 2011;4(9):22–42.
Tanghetti EA, Goldberg DJ, Dover JS, Geronemus RG, Bai Z, Alvandi N, et al. Oxymetazoline and energy-based therapy in patients with rosacea: evaluation of the safety and tolerability in an open-label, interventional study. Lasers Surg Med. 2021;53(1):55–65. https://doi.org/10.1002/lsm.23253.
Alexis A, Woolery-Lloyd H, Andriessen A, Desai S, Han G, Rodriguez D. Improving rosacea outcomes in skin of color patients: a review on the nuances in the treatment and the use of cleansers and moisturizers. J Drugs Dermatol. 2022;21(6):574–80. https://doi.org/10.36849/JDD.6838.
Dirschka T, Tronnier H, Fölster-Holst R. Epithelial barrier function and atopic diathesis in rosacea and perioral dermatitis. Br J Dermatol. 2004;150(6):1136–41. https://doi.org/10.1111/j.1365-2133.2004.05985.x.
Zip C. The role of skin care in optimizing treatment of acne and rosacea. Skin Therapy Lett. 2017;22(3):5–7.
Webster G, Schaller M. Ocular rosacea: a dermatologic perspective. J Am Acad Dermatol. 2013;69(6 Suppl 1):S42–3. https://doi.org/10.1016/j.jaad.2013.04.040.
Mahmud H, Keenan JD, Gonzales J, Schallhorn J, Chan M, Arnold B, et al. Ocular Rosacea microBiome Study (ORBS)-sub-microbial versus antibiotic dosing of doxycycline versus placebo in treatment of symptomatic ocular rosacea: study protocol for a parallel-arm randomized clinical trial. Trials. 2022;23(1):1033. https://doi.org/10.1186/s13063-022-06948-9.
Quarterman MJ, Johnson DW, Abele DC, Lesher JL Jr, Hull DS, Davis LS. Ocular rosacea. Signs, symptoms, and tear studies before and after treatment with doxycycline. Arch Dermatol. 1997;133(1):49–54. https://doi.org/10.1001/archderm.133.1.49.
Sobolewska B, Doycheva D, Deuter C, Pfeffer I, Schaller M, Zierhut M. Treatment of ocular rosacea with once-daily low-dose doxycycline. Cornea. 2014;33(3):257–60. https://doi.org/10.1097/ICO.0000000000000051.
Arman A, Demirseren DD, Takmaz T. Treatment of ocular rosacea: comparative study of topical cyclosporine and oral doxycycline. Int J Ophthalmol. 2015;8(3):544–9. https://doi.org/10.3980/j.issn.2222-3959.2015.03.19.
Sobolewska B, Doycheva D, Deuter C, Schaller M, Zierhut M. Efficacy of topical ivermectin for the treatment of cutaneous and ocular rosacea. Ocul Immunol Inflamm. 2021;29(6):1137–41. https://doi.org/10.1080/09273948.2020.1727531.
Yepuri V, Patil AD, Fritz K, Salavastru C, Kroumpouzos G, Nisticò SP, et al. Light-based devices for the treatment of facial erythema and telangiectasia. Dermatol Ther (Heidelb). 2021;11(6):1879–87. https://doi.org/10.1007/s13555-021-00607-8.
Taub AF, DeVita EC. Successful treatment of erythematotelangiectatic rosacea with pulsed light and radiofrequency. J Clin Aesthet Dermatol. 2008;1(1):37–40.
Luque A, Rojas AP, Ortiz-Florez A, Perez-Bernal J. Botulinum toxin: an effective treatment for flushing and persistent erythema in rosacea. J Clin Aesthet Dermatol. 2021;14(3):42–5.
Rivero AL, Whitfeld M. An update on the treatment of rosacea. Aust Prescr. 2018;41(1):20–4. https://doi.org/10.18773/austprescr.2018.004.
Draelos ZD, Gold MH, Weiss RA, Baumann L, Grekin SK, Robinson DM, et al. Efficacy and safety of oxymetazoline cream 1.0% for treatment of persistent facial erythema associated with rosacea: Findings from the 52-week open label REVEAL trial. J Am Acad Dermatol. 2018;78(6):1156–63. https://doi.org/10.1016/j.jaad.2018.01.027.
Schaller M, Tan J, Webster G. How to optimize rosacea treatment for better patient outcomes: An opinion piece. J Clin Aesthet Dermatol. 2022;15(7):E60–2.
Kim JS, Seo BH, Cha DR, Suh HS, Choi YS. Maintenance of remission after oral metronidazole add-on therapy in rosacea treatment: a retrospective, comparative study. Ann Dermatol. 2022;34(6):451–60. https://doi.org/10.5021/ad.22.093.
Maliyar K, Abdulla SJ. Dermatology: how to manage rosacea in skin of colour. Drugs Context. 2022. https://doi.org/10.7573/dic.2021-11-1.
Epsolay Prescribing Information. Galderma. 2022. https://www.galderma.com/us/sites/default/files/2022-03/Epsolay_PI.pdf. Accessed 18 May 2023.
Pace WE. A benzoyl peroxide-sulfur cream for acne vulgaris. Can Med Assoc J. 1965;93(6):252–4.
Baldwin H, Elewski B, Hougeir F, Yamauchi P, Levy-Hacham O, Hamil K, et al. Sixty years of benzoyl peroxide use in dermatology. J Drugs Dermatol. 2023;22(1):54–9. https://doi.org/10.36849/JDD.7150.
Oztürkcan S, Ermertcan AT, Sahin MT, Afşar FS. Efficiency of benzoyl peroxide-erythromycin gel in comparison with metronidazole gel in the treatment of acne rosacea. J Dermatol. 2004;31(8):610–7. https://doi.org/10.1111/j.1346-8138.2004.tb00566.x.
Breneman D, Savin R, VadePol C, Vamvakias G, Levy S, Leyden J. Double-blind, randomized, vehicle-controlled clinical trial of once-daily benzoyl peroxide/clindamycin topical gel in the treatment of patients with moderate to severe rosacea. Int J Dermatol. 2004;43(5):381–7. https://doi.org/10.1111/j.1365-4632.2004.02283.x.
Erlich M, Arie T, Koifman M, Talmon Y. Structure elucidation of silica-based core-shell microencapsulated drugs for topical applications by cryogenic scanning electron microscopy. J Colloid Interface Sci. 2020;579:778–85. https://doi.org/10.1016/j.jcis.2020.06.114.
Bhatia N et al. Efficacy and safety of microencapsulated benzoyl peroxide (E-BPO) cream, 5% in papulopustular rosacea: results from two phase 3, vehicle-controlled trials. https://ir.sol-gel.com/static-files/efbfefd1-93fb-405b-9dca-011df6c438e9. Accessed 18 May 2023.
Bhatia N, et al. Long-term efficacy and safety of benzoyl peroxide cream, 5% prepared with microencapsulation in papulopustular rosacea: results from an extension of two phase 3, vehicle-controlled trials. In: Poster Presented at Maui Derm Live Dermatology CME Conference 2021, Maui, Hawaii.
Green LJ, Lain E. Enhancing topical pharmacotherapy for acne and rosacea: vehicle choices and outcomes. J Clin Aesthet Dermatol. 2022;15(5):36–40.
Ciriminna R, Sciortino M, Alonzo G, de Schrijver A, Pagliaro M. From molecules to systems: sol-gel microencapsulation in silica-based materials. Chem Rev. 2011;111(2):765–89. https://doi.org/10.1021/cr100161x.
Lam PL, Gambari R. Advanced progress of microencapsulation technologies: in vivo and in vitro models for studying oral and transdermal drug deliveries. J Control Release. 2014;178:25–45. https://doi.org/10.1016/j.jconrel.2013.12.028.
Yamasaki K, Gallo RL. Rosacea as a disease of cathelicidins and skin innate immunity. J Investig Dermatol Symp Proc. 2011;15(1):12–5. https://doi.org/10.1038/jidsymp.2011.4.
Micali G, et al. 42109—clinical and erythema-directed imaging evaluation of inflamed rhinophyma with topical ivermectin: a case series. In: Poster Presented at American Academy of Dermatology 2023, New Orleans, U.S.
ZILXI Prescribing Information. VYNE Therapeutics Inc; 2020. https://zilxi.com/sites/default/files/documents/prescribing-information.pdf. Accessed 7 June 2023.
Stein Gold L, Del Rosso JQ, Kircik L, Bhatia ND, Hooper D, Nahm WK, et al. Minocycline 1.5% foam for the topical treatment of moderate to severe papulopustular rosacea: results of 2 phase 3, randomized, clinical trials. J Am Acad Dermatol. 2020;82(5):1166–73. https://doi.org/10.1016/j.jaad.2020.01.043.
Del Rosso JQ, Webster G, Weiss JS, Bhatia ND, Stein Gold L, Kircik L. Nonantibiotic properties of tetracyclines in rosacea and their clinical implications. J Clin Aesthet Dermatol. 2021;14(8):14–21.
Wilkin J, Dahl M, Detmar M, Drake L, Feinstein A, Odom R, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46(4):584–7. https://doi.org/10.1067/mjd.2002.120625.
Acknowledgements
Medical Writing and Editorial Assistance Under the close direction of the authors, medical writing support was provided by Charlotte Lewis, BSc, of Ogilvy Health UK and funded by Galderma. The journal’s Rapid Service fee was also funded by Galderma.
Funding
Authors were invited by Galderma who funded the planning and delivery of this project. Medical writing services, provided by Ogilvy Health UK, and the Rapid Service Fee were funded by Galderma.
Author information
Authors and Affiliations
Contributions
JDR, HB, NB, RC, JPY, JH, FGH, JMJ, ON, DAR, TS and JW participated in the drafting, critical revision, and approval of the final version of the manuscript. Galderma provided a formal review of the publication (reviewed for accuracy), but the authors had final authority.
Corresponding author
Ethics declarations
Conflict of Interest
All panel members received honoraria from Galderma for participating in this project. James Del Rosso has served as a research investigator, consultant and/or speaker for Almirall, Bausch Health (Ortho Dermatology), BiopharmX, Dermata, Dr. Reddy, EPI Health, Evommune, Ferndale, Galderma, JEM Health, LaRoche Posay, LEO Pharma, L’Oréal, Mayne Pharma, Primus, Sente, Sol–Gel, Sonoma, Sun Pharma, and Vyne Therapeutics (Foamix). Hilary Baldwin has acted as an investigator, consultant, and/or speaker for Almirall, Bausch Health, Cassiopeia, EPI Health, Galderma, La Roche-Posay, L’Oréal, Mayne Pharma, Sol–Gel, Sun Pharma, and Vyne. Neal Bhatia has served as advisor, consultant, and/or investigator for Abbvie, Almirall, Arcutis, Arena, Biofrontera, BMS, BI, Brickell, Dermavant, EPI Health, Ferndale, Galderma, Genentech, InCyte, ISDIN, J&J, LaRoche-Posay, Leo, Lilly, Mayne, Novartis, Ortho, Pfizer, P&G, Regeneron, Sanofi, Stemline, SunPharma, Verrica, and Vyne. Rajeev Chavda and Jean Phillipe York are employees of Galderma. Julie Harper has received honoraria from Almirall, Bausch Health (Ortho Dermatologics), BiopharmX, Cassiopeia, Cutanea, Dermira, EPI Health, Galderma, LaRoche Posay, SolGel, Sun Pharma, and Vyne Therapeutics (Foamix). Firas George Hougeir has served as an investigator, consultant, advisor and/or speaker for Galderma, Abbvie, Aclaris, Almirall, Amgen, Arcutis, Dermavant, Intraderm, ISDIN, Janssen, LEO Pharma, Lilly, Menarini, Novartis, Orthodermatologics, Pfizer, Promius, Regeneron, Sanofi-Genzyme, UCB, and Verrica. J. Mark Jackson has received research, honoraria, and/or consulting support from Abbvie, Arcuitis, BMS, Dermavant, Evommune, Galderma, Janssen, Lilly, Novartis, Pfizer, Sanofi/Genzyme, and UCB. Omar Noor has worked as a consultant for Abbvie Inc., Almirall LLC, Amgen Inc., Galderma, Lilly USA, Ortho Dermatologics, Novartis Pharmaceuticals Corporation, Pfizer Inc., Procter & Gamble Ventures, Regeneron Pharmaceuticals Inc., and Sanofi Genzyme. David Rodriguez has received honoraria, has consultancy agreements with, and/or has served as a speaker for Allergan, Inc, Galderma, Genentech, LEO Pharma, and Merz. Todd Schlesinger has acted in a consulting, speaking or advisory capacity for AbbVie, Allergan, Almirall, Amgen, ASLAN Pharma, Arcutis, Biofrontera AG, Bristol Myers Squibb, Castle Biosciences, Cara Therapeutics, Celgene, CMS Aesthetics DCME, Concert Pharmaceuticals (now SUN), Lilly, Galderma, Genentech, Greenway Therapeutix (shareholder), Janssen, Kintor, MED Learning Group, Merz, MJH Associates, Nextphase Therapeutics, Novartis, Ortho Dermatologics, Pfizer, Pierre Fabre, Plasmed, Prolacta Bioscience, RBC, Regeneron, Sanofi Genzyme, SkinCeuticals/L’Oréal, Sisaf, SUN Pharma, UCB and Verrica. Jonathan Weiss has acted as an investigator/advisor and/or speaker for Almirall, Dr Reddy’s, EPI Health, Foamix, Galderma, Novartis, and Ortho Derm.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. The patient presented in the case study has consented to their anonymized medical details, treatment plan, and patient photos to be published.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Del Rosso, J., Baldwin, H., Bhatia, N. et al. A Review of the Diagnostic and Therapeutic Gaps in Rosacea Management: Consensus Opinion. Dermatol Ther (Heidelb) 14, 271–284 (2024). https://doi.org/10.1007/s13555-023-01087-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-023-01087-8