Abstract
Methyl aminolevulinate (MAL) is a topical compound approved for use with photodynamic therapy (PDT) for the treatment of actinic keratosis (AK) and field cancerization in certain countries. There exists a high burden of disease for patients with AK: repeated treatments are required, there is a known risk of progression to keratinocyte carcinoma, and cosmetic appearance is affected. Delivery of PDT using MAL is a flexible treatment strategy available in many forms; red light, daylight, or artificial daylight can be used for illumination, all of which result in high AK clearance rates and low recurrence. MAL-PDT protocols continue to evolve to further improve adherence and treatment outcomes. Here, we used PubMed to search MEDLINE to identify guidelines, consensus recommendations, and studies describing the use of MAL for the treatment of AK. The aim of this targeted review is to consider various MAL-PDT treatment strategies on the basis of published literature, with a focus on personalizing treatment for the heterogeneous AK population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Actinic keratosis (AK) has a negative impact on patients’ physical and psychological quality of life due to disease chronicity, fear of progression to keratinocyte carcinoma, and effect on cosmetic appearance. |
Methyl aminolevulinate (MAL), used as a topical precursor in conjunction with photodynamic therapy (PDT), is an approved and recommended treatment option for patients with AK in certain countries. |
As a field-directed therapy, MAL-PDT has demonstrated high efficacy in clearing AK lesions with low recurrence rates, is generally well tolerated, and provides favorable cosmetic outcomes. |
MAL-PDT is a safe and effective treatment option for both immunocompetent patients and those who are immunosuppressed, such as organ transplant recipients, with or without additional pre-treatments. |
MAL is efficacious when used in conjunction with any of the available PDT modalities (conventional, daylight, or artificial daylight). |
AK is a chronic disease requiring repeated treatment sessions; MAL-PDT provides flexible treatment strategies to suit the needs of the patient, potentially optimizing treatment adherence. |
Introduction
Actinic keratosis (AK) is a common skin condition caused by chronic exposure to ultraviolet (UV) radiation and appearing clinically as scaly erythematous lesions [1, 2]. The presence of multiple actinic keratoses (AKs) surrounded by an area of photodamaged skin is referred to as a “field of cancerization” [1, 3].
The prevalence of AK varies widely between countries [4]. It is estimated to affect 6% of women and 15% of men aged over 40 years in England, increasing to 18% and 34%, respectively, for those aged over 70 years [4, 5]. In Australia, AK is estimated to affect 11–40% of white people aged over 40 years [1], and in the Netherlands 28% of women and 49% of men aged over 45 years [5].
There is a known risk of AK progression to keratinocyte carcinoma (KC) [1, 3]. KCs are the most common tumors in the Western world and are categorized as basal cell carcinomas (80%) or cutaneous squamous cell carcinomas (cSCC; 20%) [6]; progression of AK is usually to cSCC [1, 3]. Although most cSCCs are successfully treated by surgery and radiation therapy, up to 7% of cases may metastasize, making treatment more challenging [6].
With no way of knowing which AK lesions will progress, treatment of all AKs is recommended [1,2,3]. Clinical studies estimate that up to 16% of AK lesions may progress to invasive SCC per year, with an increasing transformation risk associated with a greater number of existing AK lesions [4]. The burden of disease for patients is underestimated: risk of AK progression to KC, effect on cosmetic appearance, and chronicity of disease requiring repeated treatments all negatively affect patients’ physical and psychological quality of life and impact their confidence and well-being [5, 7,8,9].
Several lesion-directed or field-directed therapies are available for the treatment of AK including cryosurgery, laser treatment, topical drugs, and photodynamic therapy (PDT) [1, 3, 10]. The expert consensus-based recommendation is that treatment of AKs should be based on a multitude of factors, including clinical presentation, risk factors (e.g., immunosuppression, number of lesions, cumulative UV exposure), comorbidities, life expectancy, and the patient’s preference [3].
Photodynamic therapy (PDT) is a widely approved treatment for AK and field cancerization and uses visible light to react with topical precursors of the heme biosynthetic pathway, namely methyl aminolevulinate (MAL) and 5-aminolevulinic acid (ALA) [2, 10]. These precursors are preferentially taken up by atypical epidermal keratinocytes to enhance the formation and accumulation of protoporphyrin IX (PpIX) within the target cells to produce reactive oxygen species upon photoactivation, causing apoptosis of precancerous cells [2, 3, 10, 11]. Conventional PDT (cPDT) adopts the use of an artificial, red light-emitting diode (LED) light source to activate the 630/635 nm PpIX absorption peaks to improve tissue penetration, whereas daylight PDT (dlPDT) utilizes natural daylight, and artificial daylight PDT (adlPDT) exposes the skin to an artificial broad-band white light source [10, 12].
The aim of this targeted review is to consider different aspects surrounding MAL-PDT on the basis of published literature, with a focus on personalizing its use across the heterogeneous AK population. We discuss its efficacy and its many available forms, including potential amendments to PDT delivery, such as the use of c/dl/adlPDT and adapting incubation/illumination times, aimed at easing the burden of treatment for patients with AK.
Literature Review
Three photosensitizing agents are licensed for use in Europe to treat AK in combination with a red LED light: MAL (160 mg/g); a nanoemulsion of ALA; and an ALA patch [10].
This targeted literature review was conducted to identify guidelines, consensus recommendations, and studies describing the use of MAL for the treatment of AK. PubMed was used to search MEDLINE with terms that would define various aspects of this narrative review (e.g., “methyl aminolevulinate”, “efficacy”, “long-term efficacy”, “field cancerization”, “photodynamic therapy”), between the dates of 1 January 2010 and 25 October 2022. There were no restrictions to study design. Studies from the USA were excluded due to discontinued availability of MAL for AK, and only English language articles were included. The full list of search strings and filters can be found in Tables S1 and S2.
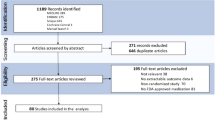
From the 639 search results received, 226 publications met the inclusion criteria for this review and were further assessed to ensure the key results were reported; a total of 38 publications were selected for this targeted literature review (Fig. 1).
Flowchart of publication selection. *Citations that were: duplicated; not related to AK/Bowen’s disease/BCC; had no available abstracts; commentaries, letters to editors, etc.; not inclusive of MAL with PDT. †Key objectives included: guidelines and consensus recommendations from Europe and Australia; MAL-c/dl/adlPDT, including the influence of patients’ blood serum profile; protocols relating to incubation/illumination times and different treatment modalities; long-term efficacy of MAL-PDT; prevention of field cancerization; protocol amendments required, and differences in efficacy observed, for OTR cohorts; patient and physician perceptions of treatment satisfaction and outcomes. adlPDT artificial daylight PDT, AK actinic keratosis, BCC basal cell carcinoma, cPDT conventional PDT, dlPDT daylight PDT, MAL methyl aminolevulinate, OTR organ transplant recipient, PDT photodynamic therapy
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
MAL-PDT is a Recommended Treatment for AK
Current guidelines recommend MAL-cPDT or MAL-dlPDT for the treatment of single or multiple grade I–II AK (Olsen classification) and for field cancerization of the face and scalp in immunocompetent individuals [3, 10]. Although current recommendations suggest use of MAL-cPDT for immunosuppressed individuals, a recent study demonstrates good efficacy, tolerability, and patient satisfaction with dlPDT [3, 13]. The other available lesion-directed or field-directed therapies for AK, including cryosurgery, laser treatment, and topical drugs, vary widely in factors such as duration, efficacy, cosmetic outcomes, and cost, and direct comparison of the different modalities is restricted due to few “head-to-head” studies [1, 3, 10]. One study has suggested that the patient’s blood serum profile may affect treatment efficacy; Moreno et al. reported that a poorer response of AK to MAL-PDT may occur with a deficient vitamin D status [14]. Thus, the choice of treatment is guided by patient-, lesion-/field-, and treatment-specific factors prioritizing personalized AK care [1, 3].
Conventional PDT versus Daylight PDT Using MAL
The combination of high efficacy and excellent cosmetic outcomes achieved with cPDT using ALA or MAL in both lesion- and field-directed treatment has positioned these as recommended treatments for AK in Europe, and in Australia by expert panel consensus only [1, 3, 15]. Although cPDT is generally well tolerated by patients, common adverse effects such as pain, burning, and stinging can occur; utilizing dlPDT or reduced incubation/illumination times may alleviate these events [10, 15].
Following lesion preparation, MAL-cPDT requires application and occlusion of MAL for 3 h and, after removal of MAL, illumination with a red light source to achieve a dose of approximately 37 J/cm2. In contrast, MAL-dlPDT requires widespread application of sunscreen for 15 min, lesion preparation, and 30 min MAL application without occlusion, prior to daylight exposure for 2 h [10, 16]. Of note, for MAL-dlPDT an organic sunscreen should be applied to all light-exposed areas to prevent additional solar damage to non-treated areas, while allowing PDT-effective light to penetrate the AK treatment areas [7, 16].
A meta-analysis compared the efficacy between MAL-dlPDT and MAL-cPDT, and while no significant difference was observed (p = 0.07; 79.5% and 83.2%, respectively) [17] in the complete response (CR; defined as clearance of all lesions per patient or randomized field of treatment [3]), on the basis of pooled results for 5556 AK lesions, the authors highlight that the Olsen grade of AK may affect the CR of MAL-dlPDT compared with MAL-cPDT [17]. There was no significant difference (p = 0.41) in CR between the two treatments for Olsen grade I–II AK, yet for grade I–III AK lesions, CR was significantly lower (p < 0.001) in the dlPDT group compared with cPDT [17]. Of note, within this meta-analysis, Rubel et al. presented a non-inferior CR with MAL-dlPDT compared with MAL-cPDT at 12 weeks, with an average of 96% of the lesions remaining in CR at 24 weeks for both treatments [18].
It is hypothesized that in cPDT, the occlusion of MAL for 3 h prior to red light exposure allows synthesized PpIX to accumulate in malignant keratinocytes, and to maintain cell homeostasis, PpIX are then actively transported into the extracellular space. These excreted PpIX are incorporated by free nerve endings in the epidermis, and upon photosensitization, result in neuropathic and capsaicin-induced pain due to a high concentration of reactive oxygen species. Thus for dlPDT, MAL incubation without occlusion allows for continuous photosensitization of PpIX synthesized in the target cells, without stimulating free nerve endings [19].
Studies to determine the feasibility of home-based MAL-dlPDT [20, 21] have shown that, after lesion preparation by a physician and a single home-based treatment session, 62% of overall lesions achieved CR at 3 months [21]. Similarly, in a fully home-based study, CR was observed in 65.9% of lesions (n = 199) at 12 months (after one or two sessions) [20]. In addition to efficacy, both studies demonstrated low pain levels and high patient satisfaction with regard to overall outcome. Nevertheless, with no comparative treatment, it is difficult to ascertain the true significance of this treatment method [20, 21].
Artificial Daylight PDT versus Conventional/Daylight PDT with MAL
Artificial dlPDT follows the same treatment protocol as dlPDT except that an artificial broad-band white light source replaces daylight exposure. This facilitates a year-round, standardized treatment option for regions where dlPDT is not possible [12, 22].
A study comparing MAL-adlPDT with MAL-cPDT showed similar efficacy (p = 0.51) between the treatments at 3-month follow-up post-treatment, with both significantly reducing (p < 0.0001) the total number of AKs compared with baseline [12]. Patients rated both treatments equally effective (p > 0.05) and 70.7% (n = 29/41) of patients preferred MAL-adlPDT over MAL-cPDT (p < 0.001). MAL-adlPDT was also associated with significantly less pain (p < 0.0001) and less severe inflammation (p < 0.0001) [12].
When comparing MAL-adlPDT with MAL-dlPDT, O’Gorman et al. again found no observable differences in efficacy, pain scores, and tolerability between the two treatments at 1-, 3-, 6-, or 9-month follow-up, and both treatments demonstrated significant reductions in AKs compared with baseline. Patients were equally satisfied with both adlPDT and dlPDT treatments. Furthermore, patients in this study who had previously experienced treatment with cPDT reported preferential treatment with either adl- or dlPDT over cPDT [22].
Emerging Protocols for Shorter Incubation/Illumination Times
Studies investigating shorter MAL incubation/illumination times were conducted with the aim of reducing treatment-related pain without impacting efficacy. For these data to be comparable, it is important to consider the effective light dose [7, 23, 24]. One study demonstrated that reduction in red light exposure (cPDT) from 8 min to 4 min (light dose 37 J/cm2 and 18.5 J/cm2, respectively) resulted in no significant difference in overall response at 3 months or 6 months (p = 0.573 and p = 0.433, respectively), but led to a significant reduction in pain (p < 0.05) during treatment [24]. For dlPDT, a reduction in light exposure from 2.5 h to 1.5 h (light dose 10.2 J/cm2 and 8.6 J/cm2, respectively) showed no significant difference in CR (p = 0.96) or maximal pain score (p = 0.94) between groups [7]. For adlPDT, a reduction in MAL incubation from 30 min [22] to 10 min, as well as a reduction in artificial light exposure from 2.5 h (light dose 20.4 J/cm2) to 1 h (7.98 J/cm2), resulted in 13 patients (N = 30) requiring a second treatment session to clear remaining AK lesions at the 3-month follow-up, after which clearance of AK lesions at 6 months was 93% [23]. Thus, it is necessary to consider the effective light dose when reducing the incubation/illumination protocol for adlPDT to avoid the need for a second treatment session.
It is important to note that these emerging protocols are investigational studies and require further evaluation. Nevertheless, they show adequate treatment efficacy with shorter incubation/illumination protocols for MAL-cPDT/dlPDT with one treatment session using lower effective light doses of 18.5 J/cm2 and 8.6 J/cm2, respectively, enabling flexibility in MAL-PDT delivery to suit the patient’s needs.
Modalities to Increase Response to Treatment
Sequential/combination therapy protocols with the use of physical and/or chemical pre-treatments have been suggested to enhance the efficacy of PDT, with physical methods removing hyperkeratosis and increasing uptake of MAL/ALA, and chemical agents potentially interacting with the heme biosynthetic pathway to increase PpIX formation [25].
A long-term, split-side study investigated the effect of daily pre-treatment with calcipotriol for 15 days prior to MAL-cPDT on multiple AK clearance of the scalp compared with MAL-cPDT. Here, overall AK clearance was significantly greater with calcipotriol-MAL-cPDT compared with MAL-cPDT at 3 months, 6 months, and 12 months (p < 0.001 for all). The response rates at 3 months were reported to be similar for both sides (p = 0.055) for Olsen grade I AKs, whereas grade II AKs showed significantly greater response rates with calcipotriol-MAL-cPDT compared with MAL-cPDT (p < 0.001) [25]. Similar findings were observed at 3 months by Piaserico et al. for multiple AKs of the upper extremities treated with calcitriol-MAL-dlPDT: response rates were significantly higher with calcitriol-MAL-dlPDT for grade II/III AKs compared with placebo-MAL-dlPDT (p = 0.038), with no significant differences between the groups for grade I AKs (p = 0.891) [26]. In both studies, patients experienced more intense local skin reactions when treated with calcipotriol/calcitriol [25, 26], but there was no significant difference in median maximal pain intensities between calcitriol and placebo pre-treatments (score of 0 for both) [26]. Taken together, these data suggest that calcipotriol/calcitriol pre-treatment provides greater efficacy, particularly for thicker AKs, than MAL-c/dlPDT alone. This may be attributed to calcipotriol/calcitriol-enhanced PpIX formation via increased MAL uptake in differentiated cells; increased expression of porphyrin synthesis enzyme and decreased ferrochelatase; and the potent induction of an antitumor immunity response [25, 26].
A pre-treatment study with 5-fluorouracil (5-FU) showed increased efficacy with sequential chemical pre-treatment with MAL-dlPDT compared with MAL-dlPDT alone [11]. Pre-treatment with 5-FU twice-daily for 7 days prior to MAL-dlPDT resulted in a significantly higher overall CR (p = 0.0011) than with MAL-dlPDT alone. There was no significant difference in median maximum pain score between the two groups (p = 1.0), and no reports of additional pain or discomfort during the 7-day pre-treatment period. Furthermore, significantly more new AKs developed after MAL-dlPDT alone than after 5-FU-MAL-dlPDT [11].
Physical pre-treatments such as microneedling, fractional ablative laser (AFXL), and microdermabrasion (MD) may assist drug delivery of MAL during PDT. However, in a study by Bento et al. investigating these three pre-treatments in conjunction with MAL-dlPDT compared with MAL-dlPDT alone, only the AFXL-MAL-dlPDT group showed a significant improvement in AK clearance after 1 month and 3 months (p = 0.002 and p = 0.034, respectively) compared with the other groups. Overall, better clinical and histologic results were observed with physical pre-treatments in conjunction with MAL-dlPDT compared with MAL-dlPDT alone [27]. Another study comparing AFXL and MD pre-treatments with MAL-dlPDT demonstrated similar results, with significantly higher mean CR and AK clearance at 3 months with AFXL-MAL-dlPDT compared with MD-MAL-dlPDT (p < 0.001 and p = 0.006, respectively) [28].
Other sequential/combination therapies have also proven to be more efficacious than MAL-PDT alone; Serra-Guillén et al. showed that overall clinical histologic and clinicopathologic responses with MAL-cPDT followed by imiquimod 5% application (three times a week on alternate nights for 4 weeks) were significantly superior to MAL-cPDT alone (p = 0.004, p = 0.008, and p= 0.011, respectively), with no significant differences in tolerance [29].
These studies demonstrate higher treatment efficacies with combination therapies than with MAL-PDT alone [11, 25,26,27], potentially due to operating via different mechanisms of action, and suggest that combination therapies may offer scope for additional improvements in treatment response. Nevertheless, to be an attractive treatment option, it is important that combination therapies should not noticeably increase treatment duration, number of consultations, or adverse events compared with monotherapy [11].
Prevention of Field Cancerization and Photorejuvenation
Field cancerization refers to an area with multiple AKs surrounded by evident UV-induced skin damage [3]. One AK study comparing lesion-directed MAL-cPDT with field MAL-cPDT demonstrated that, after a single treatment session, both therapies resulted in a significant reduction of lesions at 3 months, 6 months, and 9 months (p = 0.009). However, at 9 months, the number of new AKs was significantly lower with field therapy compared with lesion-directed therapy (p = 0.014) [30]. Another study comparing multiple full-face MAL-dlPDT sessions with cryosurgery [31] demonstrated no significant difference in AK clearance (p = 0.154) or number of new AKs (p = 0.542) between the therapies over 24 months. However, this study did show significant reductions in 6 of 9 parameters of photoaging with MAL-dlPDT compared with cryosurgery: fine lines (p < 0.001), mottled pigmentation (p = 0.007), tactile roughness (p < 0.001), skin color (p = 0.016), facial erythema (p < 0.001), and sebaceous gland hyperplasia (p = 0.017). Earlier mentioned studies investigating AFXL pre-treatment to MAL-PDT showed a significant increase in type I collagen fibers (p = 0.028) compared with microneedling, MD, or MAL-dlPDT alone. These findings imply rejuvenation of the skin [27] as well as significantly enhanced photodamage scores (p = 0.001), with particular improvements in skin texture and dyspigmentation (p = 0.001 and p = 0.003, respectively) compared with MD-MAL-dlPDT [28]. Furthermore, skin cosmesis was rated “excellent” by both patients (p = 0.035) and physicians (p = 0.003) after AFXL-MAL-dlPDT as opposed to “good” after MD-MAL-dlPDT.
Taken together, these studies suggest field therapy may delay development of new AKs and have photo-rejuvenating effects [31]. However, a combination of lesion-directed and field therapy may have additional cosmetic advantages to the patient [27, 28], consequently improving treatment adherence.
Organ Transplant Recipients Receiving MAL-PDT
Organ transplant recipients (OTRs) are at high risk for the development of AKs due to systemic immunosuppression [32, 33], and AKs arising in the immunosuppressed are 65–250 times more likely to progress to KCs, particularly SCCs, compared with AKs in the general population [13, 32, 33]. Systematic reviews and/or meta-analyses emphasize the limited evidence available for AK treatment in OTRs, yet provide evidence demonstrating higher CRs with MAL-PDT than with imiquimod, diclofenac, and 5-FU treatments [32], and significant prevention of progression (p = 0.039) to SCC compared with no treatment [33].
In a split-side study comparing MAL-PDT with untreated control in OTRs, MAL-cPDT resulted in significantly fewer AKs compared with the control (p < 0.01) and a significant delay in appearance of new AKs (40 months versus 28 months, p = 0.047) [34]. In another split-side study, Bernad et al. evaluated the efficacy of repeated MAL-dlPDT sessions for the treatment of field cancerization in OTRs compared with lesion-directed cryotherapy. Here, double sessions of MAL-dlPDT were administered 15 days apart at baseline, 3 months, and 9 months, versus one session of lesion-directed cryotherapy at the same timepoints. Repeated MAL-dlPDT resulted in significantly fewer new AKs compared with cryosurgery at 3 months, 9 months, and 15 months (p < 0.001, p = 0.04, and p = 0.02, respectively), and patients reported significantly less pain (p < 0.001 for both) and generally higher satisfaction scores (p = 0.009 and p = 0.02) at 3 months and 9 months [13].
An earlier study by Togsverd-Bo et al. reported that OTRs with field cancerization and grade II/III AKs who were pre-treated with AFXL before MAL-dlPDT showed significantly higher median CR at 3 months than when treated with MAL-dlPDT, MAL-cPDT, or AFXL alone (p = 0.026, p = 0.042, and p = 0.004, respectively) [35], consistent with findings presented earlier in immunocompetent patients [27]. Significantly lower pain intensities were observed during AFXL-MAL-dlPDT and MAL-dlPDT compared with MAL-cPDT (p < 0.001). Moreover, despite AFXL-MAL-dlPDT resulting in significantly more erythema and crusting than MAL-dlPDT or MAL-cPDT (p = 0.026 and p = 0.012, respectively), the cosmetic outcome of AFXL-MAL-dlPDT was more favorable compared with the other three groups (p < 0.01), thus suggesting a safe and efficacious treatment for difficult-to-treat AKs in this patient cohort [35].
Long-Term Efficacy and Patient Satisfaction with MAL-PDT
Long-term efficacy data exist for MAL-c/dlPDT and can be considered alongside patient satisfaction with treatment. A 12-month follow-up study to assess lesion recurrence and clearance rates after a single c- or dlPDT [36] also demonstrated no significant difference in recurrence rate (p = 0.16). Although this study showed a significantly higher 12-month clearance rate (p < 0.01) for MAL-cPDT compared with MAL-dlPDT [36], Sotiriou et al. demonstrated similar CR rates at 3 months and 12 months [37].
However, reported recurrence rates of AK after PDT vary widely. Some studies report recurrence rates of 28% between 6 months and 12 months [38], and others of 53–64% at 12 months depending on the photosensitizing agent [4]. These rates may be attributed to the use of a single PDT session, with studies suggesting that repeat treatments may result in lower recurrence rates in both immunocompetent and OTR populations [13, 38]. Nevertheless, a limitation to reporting recurrence rates exists, as it remains uncertain if the number of AKs at follow-up are relapses or new lesions.
In terms of patient satisfaction, Lacour et al. showed that MAL-dlPDT was better tolerated, as demonstrated by a significantly lower (p < 0.001) maximal pain score and significantly fewer (p < 0.001) adverse events (AEs) [17], and was associated with greater overall satisfaction and increased convenience, compared with MAL-cPDT [5]. Additionally, both therapies demonstrated “excellent” or “good” cosmetic outcomes 12 weeks after the treatment session (98% and 99.7%, respectively) [5]. AK disease chronicity warrants repeated cycles of treatment. The desirable advantages of MAL-PDT, namely high patient and physician satisfaction with cosmetic outcomes, improved tolerability (i.e., less pain), and reduced in-clinic treatment time with MAL-dlPDT, may enhance the overall quality of life and long-term treatment adherence of patients with multiple AKs and field cancerization [4, 5, 37, 39,40,41]. Furthermore, the additional flexibility of various MAL-PDT protocols enable the treatment to be tailored to the patient.
Discussion
There is a known risk of AK progression to KC in the general population, which is anticipated to be 65–250 times higher in immunosuppressed patients; a wait-and-watch approach should be viewed critically and treatment of all AKs is recommended [1,2,3, 13, 32, 33]. There are numerous, expert-recommended options available for the treatment of AK, but as these treatments have not been investigated in a “head-to-head” setting, it is difficult to directly compare them [3].
MAL-cPDT is recommended for single or multiple Olsen grade I–II AKs and for field cancerization due to high CR and good cosmesis [1, 3, 10], yet pain, stinging, and burning sensations are common with this therapy and can lead to premature termination of treatment [15, 19]. Additional logistical disadvantages such as availability of the red light source, need for dedicated staff, and prolonged time spent by the patient in the clinic [12] can also all play a part in influencing the choice of treatment.
With this in mind, MAL-dlPDT is a convenient and desired alternative treatment that provides multiple options to achieve higher patient satisfaction with respect to efficacy, pain, and cosmetic outcomes when compared with MAL-cPDT [5, 10, 12, 17, 22, 39, 40]. MAL-dlPDT still has its limitations, namely in thicker, difficult-to-treat AKs [10, 17], and with respect to variations in light dose owing to geographical latitude, season, and time of day [12]. Studies have shown that indirect sunlight exposure or exposure under a shadow is as effective as exposure to direct sunlight [7]. Thus, with the caveat that heavy clouds and adverse weather conditions may affect patient comfort [12], MAL-dlPDT requires only a minimum temperature of 10 °C and daylight dose of 8 J/cm2 for adequate PpIX synthesis, activation, and effective treatment [10]. It is also important to note that daylight exposure during sunny weather has been associated with a higher pain score than cloudy weather [7]. Furthermore, the advent of home-based MAL-dlPDT protocols empowers patients to take control of their treatment for AK in a safe and effective manner [20, 21]. The aforementioned temperature and light dose requirements for MAL-dlPDT may allow for year-round treatment at certain latitudes, and for regions where this is not possible, MAL-adlPDT is a suitable alternative with comparable efficacy and tolerability [22]. Additionally, the reported trend of a more sustained remission for MAL-adlPDT for patients with significant field cancerization compared with MAL-dlPDT [22] may make it a preferred option for physicians and patients alike.
This targeted review touches upon a few of the available chemical and physical combination therapies that can increase efficacy and clearance rates and reduce recurrence rates with MAL-PDT. However, with limited comparative studies [11, 25, 27, 29] and varying attributes, the direct comparison of these modalities is restricted [1, 3]. A patient-centric decision will undeniably be the predominant factor guiding treatment choice in real-world clinical practice [41, 42], and with the ability to vary the traditional treatment protocol, be it with alternative light sources or pre-treatments, MAL-PDT remains a well-established option for the treatment of AK.
Conclusions
MAL-PDT is widely approved and recommended for the treatment of AK and field cancerization to prevent the likelihood of progression to KC. Importantly, MAL-PDT represents a flexible and effective option to achieve optimal adherence to repeated treatment cycles, while meeting the treatment goals and lifestyle of the individual patient. Daylight exposure (either in the clinic or at home), artificial daylight (for year-round consistency in light exposure), or addition of an appropriate combination therapy offer choices that support the patient-centric, personalized management of AK.
References
Calzavara-Pinton P, Hædersdal M, Barber K, et al. Structured expert consensus on actinic keratosis: treatment algorithm focusing on daylight PDT. J Cutan Med Surg. 2017;21:3s–16s.
Mpourazanis G, Konschake W, Vogiatzis R, et al. The role and effectiveness of photodynamic therapy on patients with actinic keratosis: a systematic review and meta-analysis. Cureus. 2022;14: e26390.
Heppt MV, Leiter U, Steeb T, et al. S3 guideline for actinic keratosis and cutaneous squamous cell carcinoma—short version, part 1: diagnosis, interventions for actinic keratoses, care structures and quality-of-care indicators. J Dtsch Dermatol Ges. 2020;18:275–94.
Dirschka T, Gupta G, Micali G, et al. Real-world approach to actinic keratosis management: practical treatment algorithm for office-based dermatology. J Dermatol Treat. 2017;28:431–42.
Lacour JP, Ulrich C, Gilaberte Y, et al. Daylight photodynamic therapy with methyl aminolevulinate cream is effective and nearly painless in treating actinic keratoses: a randomised, investigator-blinded, controlled, phase III study throughout Europe. J Eur Acad Dermatol Venereol. 2015;29:2342–8.
Villani A, Potestio L, Fabbrocini G, et al. New emerging treatment options for advanced basal cell carcinoma and squamous cell carcinoma. Adv Ther. 2022;39:1164–78.
Wiegell SR, Fabricius S, Stender IM, et al. A randomized, multicentre study of directed daylight exposure times of 1½ vs. 2½ h in daylight-mediated photodynamic therapy with methyl aminolaevulinate in patients with multiple thin actinic keratoses of the face and scalp. Br J Dermatol. 2011;164:1083–90.
Chetty P, Choi F, Mitchell T. Primary care review of actinic keratosis and its therapeutic options: a global perspective. Dermatol Ther (Heidelb). 2015;5:19–35.
Steeb T, Wessely A, von Bubnoff D, et al. Treatment motivations and expectations in patients with actinic keratosis: a German-wide multicenter, cross-sectional trial. J Clin Med. 2020;9:1438.
Morton CA, Szeimies RM, Basset-Seguin N, et al. European Dermatology Forum guidelines on topical photodynamic therapy 2019 Part 1: treatment delivery and established indications—actinic keratoses, Bowen’s disease and basal cell carcinomas. J Eur Acad Dermatol Venereol. 2019;33:2225–38.
Nissen CV, Heerfordt IM, Wiegell SR, et al. Pretreatment with 5-fluorouracil cream enhances the efficacy of daylight-mediated photodynamic therapy for actinic keratosis. Acta Derm Venereol. 2017;97:617–21.
Arisi M, Rossi MT, Spiazzi L, et al. A randomized split-face clinical trial of conventional vs indoor-daylight photodynamic therapy for the treatment of multiple actinic keratosis of the face and scalp and photoaging. J Dermatol Treat. 2022;33:2250–6.
Bernad I, Aguado L, Núñez-Córdoba JM, et al. Daylight photodynamic therapy for prevention of new actinic keratosis and keratinocyte carcinomas in organ transplants. A cryotherapy-controlled randomized clinical trial. J Eur Acad Dermatol Venereol. 2020;34:1464–70.
Moreno R, Nájera L, Mascaraque M, et al. Influence of serum vitamin D level in the response of actinic keratosis to photodynamic therapy with methylaminolevulinate. J Clin Med. 2020;9:398.
See JA, Shumack S, Murrell DF, et al. Consensus recommendations on the use of daylight photodynamic therapy with methyl aminolevulinate cream for actinic keratoses in Australia. Australas J Dermatol. 2016;57:167–74.
Galderma UK. Metvix 160 mg/g cream summary of product characteristics. 2022.
Mei X, Wang L, Zhang R, et al. Daylight versus conventional photodynamic therapy for the treatment of actinic keratosis: a meta-analysis of randomized controlled trials. Photodiagn Photodyn Ther. 2019;25:23–8.
Rubel DM, Spelman L, Murrell DF, et al. Daylight photodynamic therapy with methyl aminolevulinate cream as a convenient, similarly effective, nearly painless alternative to conventional photodynamic therapy in actinic keratosis treatment: a randomized controlled trial. Br J Dermatol. 2014;171:1164–71.
Szeimïes RM. Pain perception during photodynamic therapy: why is daylight PDT with methyl aminolevulinate almost pain-free? A review on the underlying mechanisms, clinical reflections and resulting opportunities. G Ital Dermatol Venereol. 2018;153:793–9.
García-Gil MF, Gracia-Cazaña T, Cerro-Muñoz P, et al. Fully home-based methyl aminolevulinate daylight photodynamic therapy for actinic keratosis of the face or scalp: a real life open study. Dermatol Ther. 2022;35: e15879.
Karrer S, Aschoff RAG, Dominicus R, et al. Methyl aminolevulinate daylight photodynamic therapy applied at home for non-hyperkeratotic actinic keratosis of the face or scalp: an open, interventional study conducted in Germany. J Eur Acad Dermatol Venereol. 2019;33:661–6.
O’Gorman SM, Clowry J, Manley M, et al. Artificial white light vs daylight photodynamic therapy for actinic keratoses: a randomized clinical trial. JAMA Dermatol. 2016;152:638–44.
Creusot M, Mordon S. Clinical evaluation of a short illumination duration (1 hour) when performing photodynamic therapy of actinic keratosis using the Dermaris light source. Photodiagn Photodyn Ther. 2021;36: 102618.
Fernández-Guarino M, Fonda Pascual P, Lizuain Gomez P, et al. Split-face study comparing conventional MAL photodynamic therapy in multiple actinic keratosis with complete time vs. half-time red light LED conventional illumination. J Eur Acad Dermatol Venereol. 2019;33:1529–34.
Torezan L, Grinblat B, Haedersdal M, et al. A 12-month follow-up split-scalp study comparing calcipotriol-assisted MAL-PDT with conventional MAL-PDT for the treatment of actinic keratosis: a randomized controlled trial. Eur J Dermatol. 2021;31:638–44.
Piaserico S, Piccioni A, Gutiérrez Garcìa-Rodrigo C, et al. Sequential treatment with calcitriol and methyl aminolevulinate-daylight photodynamic therapy for patients with multiple actinic keratoses of the upper extremities. Photodiagn Photodyn Ther. 2021;34: 102325.
Bento CO, Pantaleão L, de Souza MB, et al. Comparison of clinical and histologic findings in daylight photodynamic therapy for skin field cancerization: a randomized controlled four-arm study on physical methods-assisted delivery of methyl aminolevulinate. Photodiagn Photodyn Ther. 2021;35: 102404.
Wenande E, Phothong W, Bay C, et al. Efficacy and safety of daylight photodynamic therapy after tailored pretreatment with ablative fractional laser or microdermabrasion: a randomized, side-by-side, single-blind trial in patients with actinic keratosis and large-area field cancerization. Br J Dermatol. 2019;180:756–64.
Serra-Guillén C, Nagore E, Hueso L, et al. A randomized pilot comparative study of topical methyl aminolevulinate photodynamic therapy versus imiquimod 5% versus sequential application of both therapies in immunocompetent patients with actinic keratosis: clinical and histologic outcomes. J Am Acad Dermatol. 2012;66:e131–7.
Seubring I, Groenewoud JMM, Gerritsen MP. Comparison of “lesion-by-lesion” and field photodynamic therapy in the prevention of actinic keratoses: a randomized, split-face, single-blind pilot study. Dermatology. 2016;232:708–14.
Karrer S, Szeimies RM, Philipp-Dormston WG, et al. Repetitive daylight photodynamic therapy versus cryosurgery for prevention of actinic keratoses in photodamaged facial skin: a prospective, randomized controlled multicentre two-armed study. Acta Derm Venereol. 2021;101:00355.
Heppt MV, Steeb T, Niesert AC, et al. Local interventions for actinic keratosis in organ transplant recipients: a systematic review. Br J Dermatol. 2019;180:43–50.
Liew YCC, De Souza NNA, Sultana RG, et al. Photodynamic therapy for the prevention and treatment of actinic keratosis/squamous cell carcinoma in solid organ transplant recipients: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2020;34:251–9.
Togsverd-Bo K, Sandberg C, Helsing P, et al. Cyclic photodynamic therapy delays first onset of actinic keratoses in renal transplant recipients: a 5-year randomized controlled trial with 12-month follow-up. J Eur Acad Dermatol Venereol. 2022;36:e946–8.
Togsverd-Bo K, Lei U, Erlendsson AM, et al. Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients—a randomized controlled trial. Br J Dermatol. 2015;172:467–74.
Fargnoli MC, Piccioni A, Neri L, et al. Long-term efficacy and safety of daylight photodynamic therapy with methyl amninolevulinate for actinic keratosis of the face and scalp. Eur J Dermatol. 2017;27:89–91.
Sotiriou E, Evangelou G, Papadavid E, et al. Conventional vs. daylight photodynamic therapy for patients with actinic keratosis on face and scalp: 12-month follow-up results of a randomized, intra-individual comparative analysis. J Eur Acad Dermatol Venereol. 2018;32:595–600.
Osiecka BJ, Nockowski P, Szepietowski JC. Treatment of actinic keratosis with photodynamic therapy using red or green light: a comparative study. Acta Derm Venereol. 2018;98:689–93.
Fargnoli MC, Ibbotson SH, Hunger RE, et al. Patient and physician satisfaction in an observational study with methyl aminolevulinate daylight photodynamic therapy in the treatment of multiple actinic keratoses of the face and scalp in six European countries. J Eur Acad Dermatol Venereol. 2018;32:757–62.
See JA, Gebauer K, Wu JK, et al. High patient satisfaction with daylight-activated methyl aminolevulinate cream in the treatment of multiple actinic keratoses: results of an observational study in Australia. Dermatol Ther (Heidelb). 2017;7:525–33.
García-Malinis AJ, Gracia-Cazaña TYGI. Self-expressed patient preferences for the treatment of actinic keratosis: results from a non-interventional study based on a real-life setting in Spain. Eur J Dermatol. 2018;28:113–5.
Szeimïes RM, on behalf of PAKT, expert panel. Personalising Actinic Keratosis Treatment (PAKT): Expert recommendations to support patient-centred management of AK (Abstract 729). In: Presented at EADV on Saturday 10 September, 2022. Milan, Italy.
Acknowledgements
Funding
Sponsorship for this study and Rapid Service Fee were funded by Galderma SA.
Medical Writing
Medical writing was provided by Dr. Gopika Nithianandarajah from Ogilvy Health UK. Support for this assistance was funded by Galderma SA.
Author Contributions
All authors were responsible for the concept or design of the work, interpretation of data, revising it critically for important intellectual content, final approval of the version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosures
Rolf-Markus Szeimies has acted as an advisory board member for AbbVie, Almirall, Dr. Wolff Group, Galderma, Janssen, LEO Pharma, photonamic, and Pierre-Fabre, has received grants and/or honoraria from ALK, Almirall, Beiersdorf, Galderma, Janssen, Knappschaft, Novartis, and has acted as an investigator for the Dr. Wolff Group, Galderma, LEO Pharma, and photonamic. Piergiacomo Calzavara-Pinton has acted as an advisory board member for Abbvie, Almirall, Boehringer Ingelheim, Cantabria, Galderma, Janssen, LEO Pharma, Molteni, Novartis, and Sanofi, has received grants for talks from Abbvie, Almirall, LEO Pharma, Novartis, and Sanofi, and has acted as an investigator for Amgen, Biogen, Clinuvel, Galderma, LEO Pharma, Mitsubishi, Novartis, Pierre-Fabre, Regeneron, Sanofi, and SI Health. Thomas Dirschka has acted as an advisory board member for Almirall, Biofrontera, Dr. Pfleger, Galderma, GlaxoSmithKline, Janssen‐Cilag, LEO Pharma, Meda, Neracare, Novartis, and Scibase, has received grants/research support from Almirall, Biofrontera, Galderma, Meda, and Schulze & Böhm, and has acted as a lecturer for Almirall, Biofrontera, Galderma, GlaxoSmithKline, infectopharm, Janssen‐Cilag, LEO Pharma, Meda, Neracare, Novartis, and Riemser. Maria Concetta Fargnoli has served as an advisory board member, received honoraria for lectures and research grants from Abbvie, Almirall, AMGEN, BMS, Galderma, Janssen, Kyowa Kyrin, Lilly, LEO Pharma, MSD, Novartis, Pfizer, Pierre Fabre, Sanofi-Regeneron, and Sun Pharma. Yolanda Gilaberte has acted as consultant and/or researcher for Abbvie, Almirall, Galderma, IFC Cantabria, Isdin, Lilly, Pfizer, Roche Posay, and Sanofi. Merete Hædersdal has received research grants from LEO Pharma, Lutronic, Mirai Medical, Studies&Me, Venus Concept, has acted as a lecturer for Galderma Nordic and Sanofi, and has received equipment from Cherry Imaging, Cynosure, Lutronic, miraDry, Mirai Medical, PerfAction Technologies, and Venus Concept. Rajeev Chavda is an employee of Galderma.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Szeimies, RM., Dirschka, T., Fargnoli, M.C. et al. A Review of MAL-PDT for the Treatment Strategy of Actinic Keratosis: Broader Clinical Perspectives Beyond the Data and Guideline Recommendations. Dermatol Ther (Heidelb) 13, 1409–1421 (2023). https://doi.org/10.1007/s13555-023-00936-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-023-00936-w