Abstract
The number of melanocytic naevi is a major risk factor for melanoma. The divergent pathway hypothesis proposes that the propensity for naevus proliferation and malignant transformation may differ by body site and exposure to ultraviolet (UV) radiation. This scoping review aimed to summarise the evidence on the number and distribution of naevi (≥ 2 mm) on the body overall and by individual anatomical sites in Caucasian adults, and to assess whether studies used the International Agency for Research on Cancer (IARC) protocol to guide naevus counting processes. Systematic searches of Embase and PubMed identified 661 potentially relevant studies, and 12 remained eligible after full-text review. Studies varied widely in their counting protocols, reporting of naevus counts overall and by body sites, and used counting personnel with differing qualifications. Only one study used the IARC protocol. Studies reported that the highest number of naevi was on the trunk in males and on the arms in females. Body sites which receive intermittent exposure to UV radiation had higher density of naevi. Larger naevi (≥ 5 mm) were detected mostly on body sites intermittently exposed to UV radiation, and smaller naevi (< 5 mm) on chronically exposed sites. Studies reported that environmental and behavioural aspects related to UV radiation exposure, as well as genetic factors, all impact body site and size distribution of naevi. This review found that to overcome limitations of the current evidence, future studies should use consistent naevus counting protocols. Skin surface imaging could improve the reliability of findings. An updated IARC protocol is required that integrates these emerging standards and technologies to guide reliable and reproducible naevus counting in the future.
Similar content being viewed by others
Melanocytic naevi are benign pigmented lesions resulting from melanocytic proliferation. Having many naevi indicates melanoma risk profile, and up to 30% of melanomas may directly arise from a naevus. |
It is important to study the body site-specific distribution of naevi to better understand the apparent regional variation in their susceptibility for malignant transformation. Hence, body site distribution of naevi has been a subject of research in dermatology for many years, and is summarised in this review. |
The existing studies on site distribution of melanocytic naevi vary widely in their naevus counting and reporting methodology, and how they aggregated body sites and naevus sizes for analysis and reporting. |
Research shows that environmental and behavioural aspects related to ultraviolet (UV) radiation exposure, as well as genetic factors, all impact the number, site distribution and size of melanocytic naevi. |
Given the important role that naevi play in the development and understanding of melanoma, future studies should use large population-based samples and standardised naevus counting protocols. Comparable studies using non-white populations and people of colour are lacking. |
Introduction
Melanocytic naevi are benign pigmented skin lesions that start to appear on the skin in childhood. Besides skin, hair and eye colour, the number of common and atypical naevi constitutes the most important phenotypic risk factor for cutaneous melanoma [1]. Naevi were considered to be an obligatory precursor for the majority of cutaneous melanomas [2,3,4]. However, more recent studies found low associations between site-specific naevus distribution and naevus-related melanomas (ratio on face 1:6, back 1:2) [5], suggesting that the proportion of melanomas arising from melanocytic naevi was likely overestimated [2, 6, 7]. Remnants of naevus tissue are only found in approximately 30% of melanoma histopathology specimens [8], and studies suggest that melanomas arising from naevi and other melanomas follow divergent body site distribution and pathways [9].
Several studies reported that the density of melanocytes differs by anatomical location [10,11,12,13]. This supports the hypothesis that different body sites may differ in their susceptibility for melanocytic proliferation and malignant transformation [14, 15]. The location of a particular melanocyte may also affect its likelihood of being exposed to ultraviolet (UV) radiation [5]. Whiteman et al. proposed the divergent pathway hypothesis [16] that acute and chronic UV radiation exposure give rise to melanoma in different ways. This hypothesis suggests that people with inherently low propensity for melanocytic proliferation (and therefore often low naevus counts) require chronic UV exposure to stimulate the development of melanoma. In contrast, individuals with an inherently high propensity for melanocytic proliferation (and often high naevus count) only need a low dose of UV radiation exposure to promote the onset of melanoma. For these people, melanoma more often occurs at a younger age, and in body areas most covered by clothing such as the back [16, 17]. Given their important role in melanoma risk prediction, multiple studies recommended detailed assessment of naevus counts and body site distribution [1, 18,19,20].
In 1990, the International Agency for Research on Cancer (IARC) developed a standard protocol for identifying, counting and reporting naevi [21]. This protocol provides guidelines for differentiating a naevus from other skin lesions, definitions and boundaries of anatomical body sites, and how to categorise anatomical sites for naevus reporting. Despite this, it is unknown how many studies have followed the IARC protocol, what other naevus identification and counting methods were used, and what influence this may have on estimated naevus counts by anatomical site [22].
This scoping review summarises the evidence on the distribution of melanocytic naevi (referred to as naevi henceforth) by anatomical sites in the general population of Caucasian adults, reports on factors associated with body site distribution, and identifies whether studies used the IARC protocol to guide their naevus counting processes.
Methods
This review followed guidelines by Arksey and O’Malley [23] and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses—Extension for Scoping Reviews (PRISMA-ScR) [24], and is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Eligibility Criteria
Studies reporting the prevalence of naevi ≥ 2 mm in diameter by specific body sites in population-based samples of the adult general population (majority of participants ≥ 18 years) from any country, written in English, were eligible. Cross-sectional or longitudinal population-based studies were included, as well as control groups from melanoma case-control studies. Clinical studies were only eligible if conducted outside dermatology settings, as people attending dermatology clinics may have higher risk for melanoma or skin cancer. Reviews [25], abstracts and pooled analyses [14] were excluded. Studies concerned solely with naevus-related syndromes (e.g. familial dysplastic naevus syndrome, giant congenital melanocytic naevi), considered only a specific type of naevus (e.g. halo naevus, blue naevus), reported self-counted naevi or counted naevi on only a specific part of the body (e.g. arm) or focussed on children or adolescents were also excluded.
Information Sources and Search Strategy
PubMed and Embase databases were searched in May 2021 using a combination of keywords and Medical Subject Heading (MeSH) terms (Appendix 1). Additional studies were identified by manually screening the references and citations of the search results.
Selection of Sources of Evidence
The web-based Covidence application [26] was used for screening of the studies. Duplicates in the combined search results were identified and removed. Relevance of the studies was assessed from the title and abstract, and the full text of potentially relevant studies were reviewed for the consideration of inclusion by one reviewer (D.J.), and conflicts were resolved by discussing with a second reviewer (M.J.).
Data Extraction and Synthesis
From each article, we extracted the following: first author, year of publication, country, study design, study objective, sample size, age range of participants, inclusion or exclusion criteria, sizes of naevi considered, who conducted the counts and their qualifications, and average total naevus count (Table 1). We further extracted whether naevus counts or naevus density (e.g. naevus count per m2 or naevus counts per body site), or both (where reported) were reported; body sites excluded from naevus counting; categorisation of body sites used; body surface area calculations; body site distribution of naevi and associated factors; and whether there were measures taken to address inter- and intra-observer variation (Table 2). Due to the major inconsistencies in how studies reported results, the naevus counts per body site were not able to be quantitatively summarised. Overview results are reported, with detailed results from each individual study provided in Table S1. Patterns of body site distribution of naevi were summarised across studies, in relation to participant characteristics such as sex, age and phenotype.
Results
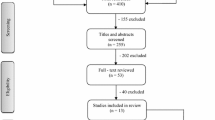
The PRISMA flow diagram (Fig. 1) summarises the search and screening results. A total of 661 articles were identified. After removal of duplicates (n = 75), and screening of titles and abstracts, 115 articles remained for full text review. According to the eligibility criteria, 18 articles [19, 27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43] were potentially suitable to be included. However, duplication of study samples was identified in nine studies and required further exclusions. If two studies reported on the same sample, the study focusing on body site distribution of naevi was selected for inclusion (e.g. [28] was used, while [19 and 40] were excluded). This resulted in exclusion of five publications [19, 39,40,41,42]. If a study sample was used to answer two relevant research questions and the results did not overlap, both publications were included [34, 44]. One study [43] was excluded because the majority of the participants in the sample (55%) were younger than 18 years. Therefore, a total of 12 studies remained for review.
Characteristics of Sources of Evidence
One study was from the USA [28], one from Australia [35], and 10 were from Europe [27, 29,30,31,32,33,34, 36,37,38] (Table 1). Study designs included one randomised control trial [37], five case-control studies [27, 28, 35, 36, 38], and five cross-sectional studies [29,30,31,32,33], while one study was a heritability and genome-wide association study using twins [34]. The 12 studies reported data from 9593 participants, whose age ranged from 15 to 92 years. Ten (91%) studies included only Caucasian participants [28,29,30,31,32,33,34,35,36,37]. Out of the five case-control studies, four matched the controls to cases with regards to sex and age [28, 35, 36, 38], one study [29] used visitors to patients in hospital wards, and three [28, 35, 36] used data from patients in a hospital setting other than dermatology clinics as the sample. One study [33] which recruited participants from patients in a hospital setting, included 31 people (16%) with psoriasis. Only three studies [27, 30, 32] used a general population-based sample.
Naevus Counting and Reporting
One study [33] was published before the IARC protocol was available, and of the remaining 11 studies, only one [36] explicitly mentioned that it followed the IARC protocol. The reporting of naevus size differed between studies (Table 1). For example, Richard et al. [37] used naevus categories of 2–4.99 mm and ≥ 5 mm, while Randi et al. [36] used 2–5.99 mm and ≥ 6 mm. The experience and qualification of personnel differed widely. Five studies [29, 32, 33, 36, 37] reported that counting was done only by dermatologists, two studies [28, 35] by dermatologists and other trained clinical staff, one study [27] by a doctor (not further specified), and four studies [30, 31, 34, 38] by nurses trained by dermatologists (Table 1). Four studies [27, 29, 30, 33] reported how they reduced inter- and intra-observer variation, and two studies quantified the inter-observer correlation of total naevus counts, which was r = 0.88 in [35] and r > 0.75 for intra and inter-observer correlation [36], respectively.
Most studies (n = 10) reported overall naevus counts, two studies [32, 37] reported naevus density by body site, based on body surface area calculations proposed by Lund and Browder [45], while one study [35] reported only naevus count categories (Table 2). Only one study [32] did not exclude any part of the body, while most studies [28, 30, 31, 33, 35, 37, 38] did not count on genitalia and breasts in females, and four studies [28, 31, 35, 36] did not include the scalp in the counting protocols. One study [27] used dermoscopy to discriminate naevi from non naevi (Table 2).
Naevus Counts
Studies reported a mean total-body naevus count ranging between 12 and 58 naevi [27,28,29,30,31, 33, 35, 36].
Naevus Counts and Distribution by Sex
Males had on average 9–36 naevi, while females had 14–58 naevi [27, 29, 30, 33, 36] (Table 1). Ten studies reported that naevus counts by body sites varied with sex [27,28,29,30,31,32,33,34,35,36].
Most studies (n = 9) reported naevus counts by specific body sites for males and females separately. Five studies reported that males had the highest number of naevi on the trunk (one study on the upper back [28], one on thorax [29], two studies on the posterior trunk [35, 36], one on trunk [27]) and one study on upper limbs [33]. In comparison, only one study reported that females had the highest naevus counts on the trunk [27], six on the arms (four on arms [29, 30, 33, 36]; two on upper arms [28, 31]) and one on legs [35].
One study reported a higher mean number of naevi in every anatomical location in males compared with females, although statistical significance was not shown [27]. According to two studies, males had more naevi on the following body sites than females: buttocks, chest and back (one study, p < 0.01) [32], and on the trunk (one study, p < 0.001) [33]. According to three studies, females had more naevi than males on the face (one study, p < 0.01) [32] and lower limbs or parts of lower limbs (three studies, thigh: p = 0.05 [29]; anterior surface of the thigh: p < 0.01 [32] and lower limbs: p < 0.01 [32, 33]).
Naevus Density
Only two studies [32, 37] reported the overall distribution of naevi in participants without grouping them according to sex. Males and females both had the highest density per m2 on the lateral arms, followed by the back [32] and the neck [37]. The lowest naevus density was reported on scalp, hands, soles [32] and buttocks [37].
Age
Only one study reported on the differences in naevus counts on different body sites between age groups [33], and reported that the mean naevus counts on sites with extensive number of naevi decreased in the older age groups.
Phenotype
Differences in body site distribution of naevi by participants’ phenotypes were reported in two studies [32, 37]. One study found no significant difference comparing people with skin types I–II and III–IV [32]. In an age–sex–phenotype controlled study [37], the highest mean density of naevi 2–5 mm were on the face and neck in people with the ‘darker phenotype’ (brown or black hair, dark complexion on the inner part of the arm, absence of freckles and easy tanning without burning), while the highest naevus density was on the neck, exterior side of forearms and dorsum of the hand in people with the ‘red phenotype’ (red or red–blonde hair, white complexion on the inner part of the arm and inability to tan).
Exposure to Sunlight
Four studies [30, 32, 37, 38] aggregated body sites as chronically, intermittently or rarely exposed to sun. One study reported the highest density of naevi on intermittently exposed sites, followed by chronically exposed sites and rarely exposed sites [32]. When the size of naevi was considered, the same pattern was observed for ≥ 5 mm naevi [37], while for small (< 5 mm) naevi, the mean density was higher in chronically exposed sites than intermittently or rarely exposed sites (p < 0.001). In the same study, it was reported that the number of small naevi (< 5 mm) was significantly higher on the outer, more sun exposed arm than the inner arm (p < 0.0001).
Other studies showed a positive relationship between intermittently holiday sun exposed exposure sites (trunk and upper legs) and increased naevus counts [30, 38]. Fewer naevi were reported by one study in sites chronically exposed to sun [38].
Correlations between Body Site Specific and Total Naevus Count
Four studies reported correlations between site-specific and total naevus counts in different ways, as detailed in Table 2.
Two studies reported that the best predictor for total naevus count was the naevus count on the thigh [anterior thigh (r = 0.85) [32], thigh (r = 0.88 for females) [29]], while three studies reported that the best correlation with total naevus count was with the number of naevi on arms [upper arm (r not given) [33], upper right arm (r = 0.83) [31], arms (r = 0.88) [29], right arm (r = 0.86) [31]] and one study the back (only males considered r = 0.84) [31].
The UK twin study reported that people with more than 7 or 11 naevi on the arms are more likely to also have more than 50 or 100 total naevi, respectively [31].
Correlations between Body Sites
Naevus counts were reported to correlate across body sites, with correlation coefficients ranging from 0.30 to 0.70. For example, the strongest correlations were seen between upper limbs and trunk (r = 0.62–0.71), lower limbs and trunk (r = 0.49–0.61), and upper and lower limbs (r = 0.55–0.71) [30, 33].
Heritability
One study used classic additive genetic (A), common (C) and individual‐specific environment (E) (ACE) twin models to estimate the contribution of genes on the body site distribution of naevi in males and females [34]. In females, it was estimated that naevus counts on the lower limbs were strongly under genetic influence (69%), while the influence of genetic disposition on naevi on the trunk was lower (26%). In males, there was only environmental effect and no genetic effect for lower limbs, while more than 67% of naevus count variation in other sites were estimated to be due to genetic influential factors [34].
Discussion
This review included 12 studies that provided detailed information on the body site distribution of naevi in Caucasian adults. A meta-analysis could not be performed because the 12 studies differed widely in their naevus counting and reporting methodology. The studies also differed in how they aggregated body sites for analysis and reporting and in their naevus size categories, and only one study reported the use of the IARC protocol. Despite this, some findings were relatively consistent across most studies.
In most of the studies, males had more naevi on the trunk than females [32,33,34,35], while females had more naevi on the lower limbs than males [29, 32, 33, 35]. Females had the highest number of naevi on the arms compared with other parts of the body in most [28,29,30,31, 33, 36], but not all studies [27, 35]. This is in contrast to studies in high-risk cohorts and children, in which higher density of naevi were reported on the face and neck [46,47,48].
Irrespective of sun exposure in later life, genetic effects related to gender play an important role in site-specific naevus development [49]. Sex differences in body site distribution of naevi can be observed as early as 7–8 years of age [47]. In a study using Canadian Hutterite children [50], whose religious clothing protects females from UV exposure, sex differences in site distribution of naevi were observed despite low exposure to UV. Hence, the apparent differences in site distribution of naevi reported in the studies reviewed here are likely due to a combination of genetic and environmental factors [34, 49, 51, 52].
There is sufficient epidemiological evidence that exposure to UV radiation is a strong modifiable risk factor for the development of naevi [53, 54], with intermittent rather than chronic sun exposure associated with a higher density of naevi [32, 37]. One proposed reason for this suggests that chronic sun exposure may have a protective effect against the development of naevi, as observed in people who have higher average weekend sun exposure [38]. A second hypothesis postulates that intermittent UV exposure has strong ‘naevogenic’ effect on melanocytes [32], as observed in people with higher holiday exposure and increased naevus counts in intermittently exposed body sites compared with chronically exposed body sites [30, 38]. However, the correlation between total body and body site-specific naevus counts was high (range: 0.83–0.88), regardless of whether intermittent or chronically exposed body sites were considered.
When the size of naevi was considered, some studies found that small naevi (< 5 mm, [37] or < 2 mm [47, 55] which are often considered as lentigines) are more common on chronically exposed sites of the body than on intermittently exposed sites. Autier et al. proposed that small and large naevi are both independent risk factors for melanoma, reflecting two different biological pathways under genetic and environmental influence involved in melanoma development [49]. In summary, this review shows that the relationship between sun exposure and naevus development and growth is very complex and requires further investigation.
This review focused on adult general population-based samples only, and the results do not reflect the more often used high-risk cohorts. Further, there was a lack of naevus studies reporting site distribution in the recent past, and the majority of the studies included in the review were more than 20 years old. The results may therefore not reflect contemporary cohorts and sun exposure patterns, or changes in practices and definitions among dermatologists regarding what constitutes a naevus. One of the other main limitations of the review is due to our narrow inclusion criteria and lack of naevus studies in non-white and people of colour; our review mainly consists of information of Caucasian adults. Even though we applied stringent eligibility criterion to select studies for the review, summarising the results was difficult due to the varied methods of aggregating anatomical sites and reporting naevus prevalence. For example, while English (1988) [33] considered both legs together as ‘lower limbs’ to report the number of naevi, Augustsson (1992) [32] reported details for the anterior and posterior aspect of the thigh.
Future studies could benefit from using a standardised method of aggregating body sites. To best address the differences in sun exposure and sun damage due to clothing [56], naevus counts ≥ 2 mm and ≥ 5 mm should be reported separately for head and neck, upper anterior trunk, lower anterior trunk, upper posterior trunk, lower posterior trunk, upper arms, lower arms, upper legs and lower legs. It is also recommended that all summary measures of naevus distribution are reported stratified by sex due to the evidence for genetic and environmental differences in naevus development.
Novel technologies such as 3D total-body imaging could improve the precision in naevus counts and tracking in the future [57, 58]. Figure 2 shows an example of how such information could be presented as a skin cancer education and awareness tool. An interactive dashboard would visualise the number and density of naevi people have on different body sites; could allow filtering by a person’s characteristics as shown in Fig. 2, or by naevus size, colour and other naevus features; and provide comparisons with the wider population.
Images from an interactive dashboard using 3D total-body imaging data to visualize body site distribution of naevi and associated characteristics. (i) Body site distribution of naevi filtered for ‘male’ participants in the age range 50–59 years. (ii) Body site distribution of naevi and characteristics of participant ‘MYM10188’. (iii) Comparison of the body site distribution of naevi of participant ‘MYM10188’ with participants in the sample with the same age and sex category
Before new technologies such as 3D imaging are widely rolled out to achieve automated naevus counts, a number of scientific hurdles still need to be overcome. Firstly, IARC protocol [21], which was developed more than 30 years ago, needs to be updated and should provide guidance for how state-of-the-art skin imaging technologies and emerging artificial intelligence algorithms should be integrated. Automated naevus counts are likely a better alternative for naevus counting [59], as they could overcome issues of intra- and inter-observer reliability, as well as observer training and experience [60], but this needs to be confirmed in future studies. Secondly, currently available automated naevus identification algorithms still have limitations such as low accuracy for people with severely photodamaged skin and those with many seborrheic keratoses [59], and datasets used for training the algorithms are mainly comprised of images from Caucasian adults. Hence these algorithms need to be trained with larger and unbiased datasets [61], but are promising to improve the accuracy of this complex naevus identification and counting task.
Conclusion
In conclusion, environmental and behavioural aspects related to UV radiation exposure, as well as genetic factors impact the number, site distribution and size of melanocytic naevi. Given the important role that naevi play in the development and understanding of melanoma, reproducible studies using large unbiased population-based samples need be undertaken to further assess these relationships.
References
Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. Eur J Cancer. 2005;41(1):28–44.
Marks R, Dorevitch AP, Mason G. Do all melanomas come from “moles”? A study of the histological association between melanocytic naevi and melanoma. Australas J Dermatol. 1990;31(2):77–80.
Allen AC. Melanocarcinoma. In: Anderson WAD, editor. Pathology. 2nd ed. St Louis: CV Cosby Company; 1953. p. 1167.
Smith JL. Malignant melanoma. In: Graeme JH, Johnson WC, Helwig EB, editors. Dermal pathology. Maryland: Harper and Row; 1972. p. 490–1.
Green A. A theory of site distribution of melanomas: Queensland, Australia. Cancer Causes Control. 1992;3(6):513–6.
McGovern VJ. Epidemiological aspects of melanoma: a review. Pathology. 1977;9(3):233–41.
Kaddu S, Smolle J, Zenahlik P, Hofmann-Wellenhof R, Kerl H. Melanoma with benign melanocytic naevus components: reappraisal of clinicopathological features and prognosis. Melanoma Res. 2002;12(3):271–8.
Pampena R, Kyrgidis A, Lallas A, et al. A meta-analysis of nevus-associated melanoma: prevalence and practical implications. J Am Acad Dermatol. 2017;77(5):938-45.e4.
Dessinioti C, Geller AC, Stergiopoulou A, et al. A multicentre study of naevus-associated melanoma vs. de novo melanoma, tumour thickness and body site differences. Br J Dermatol. 2021;185(1):101–9.
Szabo G. The number of melanocytes in human epidermis. Br Med J. 1954;1(4869):1016–7.
Quevedo WC Jr, Szabo G, Virks J, Sinesi SJ. Melanocyte populations in UV-irradiated human skin. J Invest Dermatol. 1965;45(4):295–8.
Whiteman DC, Parsons PG, Green AC. Determinants of melanocyte density in adult human skin. Arch Dermatol Res. 1999;291(9):511–6.
Whiteman DC, Brown RM, Purdie DM, Hughes MC. Prevalence and anatomical distribution of naevi in young Queensland children. Int J Cancer. 2003;106(6):930–3.
Olsen CM, Zens MS, Stukel TA, et al. Nevus density and melanoma risk in women: a pooled analysis to test the divergent pathway hypothesis. Int J Cancer. 2009;124(4):937–44.
Siskind V, Whiteman DC, Aitken JF, Martin NG, Green AC. An analysis of risk factors for cutaneous melanoma by anatomical site (Australia). Cancer Causes Control. 2005;16(3):193–9.
Whiteman DC, Watt P, Purdie DM, et al. Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. J Natl Cancer Inst. 2003;95(11):806–12.
Whiteman D, Green A. Epidemiology of malignant melanoma. In: Dummer R, editor. Skin cancer—a world-wide perspective. Berlin Heidelberg: Springer-Verlag; 2011. p. 13–26.
Swerdlow AJ, English J, MacKie RM, et al. Benign melanocytic naevi as a risk factor for malignant melanoma. Br Med J (Clin Res Ed). 1986;292(6535):1555–9.
Holly EA, Kelly JW, Shpall SN, Chiu SH. Number of melanocytic nevi as a major risk factor for malignant-melanoma. J Am Acad Dermatol. 1987;17(3):459–68.
Rieger E, Soyer HP, Garbe C, et al. Overall and site-specific risk of malignant melanoma associated with nevus counts at different body sites: a multicenter case-control study of the German Central Malignant-Melanoma Registry. Int J Cancer. 1995;62(4):393–7.
English DR, Rivers J, Kelly JW, Armstrong BK. Epidemiological studies on melanocytic naevi: protocol for identifying and recording naevi. Lyon France: International Agency for Research on Cancer; 1990. Contract No.: 002.
Gallagher RP, McLean DI, Yang CP, et al. Anatomic distribution of acquired melanocytic nevi in white children. A comparison with melanoma: the Vancouver Mole Study. Arch Dermatol. 1990;126(4):466–71.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32.
Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73.
Gallagher RP, McLean DI. The epidemiology of acquired melanocytic nevi. A brief review. Dermatol Clin. 1995;13(3):595–603.
Veritas Health Innovation M, Australia. Covidence systematic review software. 2021.
Dabkowski J, Omulecki A, Zalewska A. Identification of melanoma risk factors in the Polish population. Dermatol Surg. 1997;23(11):1039–42.
Holly EA, Kelly JW, Ahn DK, Shpall SN, Rosen JI. Risk of cutaneous melanoma by number of melanocytic nevi and correlation of nevi by anatomic site. In: Gallagher RP, Elwood JM, editors. Epidemiological aspects of cutaneous malignant melanoma. Boston, MA: Springer, US; 1994. p. 159–72.
Farinas-Alvarez C, Rodenas JM, Herranz MT, Delgado-Rodriguez M. The naevus count on the arms as a predictor of the number of melanocytic naevi on the whole body. Br J Dermatol. 1999;140(3):457–62.
Silva IS, Higgins CD, Abramsky T, et al. Overseas sun exposure, nevus counts, and premature skin aging in young English women: a population-based survey. J Invest Dermatol. 2009;129(1):50–9.
Ribero S, Zugna D, Osella-Abate S, et al. Prediction of high naevus count in a healthy U.K. population to estimate melanoma risk. Br J Dermatol. 2016;174(2):312–8.
Augustsson A, Stierner U, Rosdahl I, Suurkula M. Regional distribution of melanocytic naevi in relation to sun exposure, and site-specific counts predicting total number of naevi. Acta Derm Venereol. 1992;72(2):123–7.
English JS, Swerdlow AJ, Mackie RM, et al. Site-specific melanocytic naevus counts as predictors of whole body naevi. Br J Dermatol. 1988;118(5):641–4.
Visconti A, Ribero S, Sanna M, et al. Body site-specific genetic effects influence naevus count distribution in women. Pigment Cell Melanoma Res. 2020;33(2):326–33.
Grulich AE, Bataille V, Swerdlow AJ, et al. Naevi and pigmentary characteristics as risk factors for melanoma in a high-risk population: a case–control study in New South Wales, Australia. Int J Cancer. 1996;67(4):485–91.
Randi G, Naldi L, Gallus S, Di Landro A, La Vecchia C. Number of nevi at a specific anatomical site and its relation to cutaneous malignant melanoma. J Invest Dermatol. 2006;126(9):2106–10.
Richard MA, Grob J-J, Gouvernet J, et al. Role of sun exposure on nevus: first study in age-sex phenotype-controlled populations. Arch Dermatol. 1993;129(10):1280–5.
Newton-Bishop JA, Chang YM, Iles MM, et al. Melanocytic nevi, nevus genes, and melanoma risk in a large case-control study in the United Kingdom. Cancer Epidemiol Biomarkers Prev. 2010;19(8):2043–54.
Rodenas JM, DelgadoRodriguez M, FarinasAlvarez C, Herranz MT, Serrano S. Melanocytic nevi and risk of cutaneous malignant melanoma in southern Spain. Am J Epidemiol. 1997;145(11):1020–9.
Kelly JW, Holly EA, Shpall SN, Ahn DK. The distribution of melanocytic naevi in melanoma patients and control subjects. Australas J Dermatol. 1989;30(1):1–8.
Stierner U, Augustsson A, Rosdahl I, Suurkula M. Regional distribution of common and dysplastic naevi in relation to melanoma site and sun exposure. A case-control study. Melanoma Res. 1992;1(5–6):367–75.
Naldi L, Imberti GL, Parazzini F, et al. Pigmentary traits, modalities of sun reaction, history of sunburns, and melanocytic nevi as risk factors for cutaneous malignant melanoma in the Italian population—results of a collaborative case-control study. Cancer. 2000;88(12):2703–10.
Nicholls EM. Development and elimination of pigmented moles, and the anatomical distribution of primary malignant melanoma. Cancer. 1973;32(1):191–5.
Ribero S, Osella-Abate S, Reyes-Garcia D, Glass D, Bataille V. Effects of sex on naevus body distribution and melanoma risk in two melanoma case–control studies at different latitudes. Br J Dermatol. 2017;176(4):1093–4.
Lund C. The estimation of areas of burns. Surg Gynecol Obstetr. 1944;79:352–8.
Valiukeviciene S, Gollnick H, Stang A. Body-site distribution of common acquired melanocytic nevi associated with severe sunburns among children in Lithuania. Int J Dermatol. 2007;46(12):1242–9.
Dodd AT, Morelli J, Mokrohisky ST, et al. Melanocytic nevi and sun exposure in a cohort of Colorado children: anatomic distribution and site-specific sunburn. Cancer Epidemiol Biomarkers Prev. 2007;16(10):2136–43.
Walter S, Ashbolt R, Dwyer T, Marrett L. Do larger people have more naevi? Naevus frequency versus naevus density. Int J Epidemiol. 2000;29(6):1025–30.
Autier P, Boniol M, Severi G, et al. Sex differences in numbers of nevi on body sites of young European children: implications for the etiology of cutaneous melanoma. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2003–5.
Kwan TY, Belke TW, Enta T. Sex differences in the anatomical distribution of melanocytic nevi in Canadian Hutterite children. J Cutan Med Surg. 2000;4(2):58–62.
Autier P, Boniol M, Severi G, et al. The body site distribution of melanocytic naevi in 6–7 year old European children. Melanoma Res. 2001;11(2):123–31.
Severi G, Cattaruzza MS, Baglietto L, et al. Sun exposure and sun protection in young European children: an EORTC multicentric study. Eur J Cancer. 2002;38(6):820–6.
Armstrong BK, Kricker A. How much melanoma is caused by sun exposure? Proc IARC Monogr Eval Carcinog Risks Hum. 1993;13(6):395–401.
Fears TR, Bird CC, Guerry Iv D, et al. Average midrange ultraviolet radiation flux and time outdoors predict melanoma risk. Can Res. 2002;62(14):3992–6.
Harrison SL, Buettner PG, MacLennan R. Body-site distribution of melanocytic nevi in young Australian children. Arch Dermatol. 1999;135(1):47–52.
Betz-Stablein B, Llewellyn S, Bearzi P, et al. High variability in anatomic patterns of cutaneous photodamage: a population-based study. J Eur Acad Dermatol Venereol. 2021;35:1896–903.
Koh U, Janda M, Aitken JF, et al. “Mind your Moles” study: protocol of a prospective cohort study of melanocytic naevi. BMJ Open. 2018;8(9): e025857.
Rayner JE, Laino AM, Nufer KL, et al. Clinical perspective of 3D total body photography for early detection and screening of melanoma. Front Med (Lausanne). 2018;5:152.
Betz-Stablein B, D’Alessandro B, Koh U, et al. Reproducible naevus counts using 3D total body photography and convolutional neural networks. Dermatology. 2021;238:4–11.
Walter SD, Marrett LD, Hertzman C. Reliability of interviewer and subject assessments of nevus counts in a study of melanoma. J Clin Epidemiol. 1991;44(7):633–40.
Adamson AS, Smith A. Machine learning and health care disparities in dermatology. JAMA Dermatol. 2018;154(11):1247–8.
Acknowledgements
Funding
This research was supported by the Centre of Research Excellence for the Study of Naevi funded by the National Health and Medical Research Council, Australia (NHMRC; Grant ID: APP1099021) and Princess Alexandra Hospital foundation. H. Peter Soyer holds an NHMRC MRFF Next Generation Clinical Researchers Program Practitioner Fellowship (APP1137127). Dilki Jayasinghe is funded by a research training program (RTP) PhD scholarship.
Author Contributions
Conceptualization: Dilki Jayasinghe, Monika Janda and Kaitlin L. Nufer; Methodology: Dilki Jayasinghe and Monika Janda; Literature search, screening and synthesis: Dilki Jayasinghe; Interpretation of results: All authors; Writing—Original draft preparation: Dilki Jayasinghe; Writing—Review and editing: All authors; Funding acquisition: Monika Janda and H. Peter Soyer; Supervision: Monika Janda, Brigid Betz-Stablein and H. Peter Soyer; Project Administration: Dilki Jayasinghe. All authors have read and approved the final manuscript.
Disclosures
The presented work is original research that has not been published and is not under consideration for publication elsewhere. H. Peter Soyer is a shareholder of MoleMap NZ Limited and e‐derm-consult GmbH, and undertakes regular tele-dermatological reporting for both companies. H. Peter Soyer is also a Medical Consultant for Canfield Scientific Inc., MoleMap Australia Pty Ltd, Blaze Bioscience Inc, Revenio Research Oy and a Medical Advisor for First Derm. Dilki Jayasinghe, Monika Janda, Brigid Betz-Stablein and Kaitlin L. Nufer declare that they have no competing interests.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Jayasinghe, D., Nufer, K.L., Betz-Stablein, B. et al. Body Site Distribution of Acquired Melanocytic Naevi and Associated Characteristics in the General Population of Caucasian Adults: A Scoping Review. Dermatol Ther (Heidelb) 12, 2453–2488 (2022). https://doi.org/10.1007/s13555-022-00806-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-022-00806-x