Abstract
Introduction
Merkel cell carcinoma (MCC) is a rare, aggressive cutaneous neuroendocrine cancer that typically arises in sun-exposed areas of the skin, especially in the elderly. Significant advances have recently been made regarding skin cancers, but data on cases of MCC are rather limited as these patients are frequently grouped together with other non-melanoma skin cancers (NMSC). Here, we performed an analysis of the clinical profile of patients with MCC in Poland to identify major factors influencing the prognosis.
Methods
Approximately 13,000 pathology and medical records were examined to identify patients with MCC diagnosed between 2010 and 2019. The management and outcomes of patients with histologically confirmed MCC were retrospectively evaluated.
Results
Thirty-one patients diagnosed with MCC were identified. The tumor occurred predominantly in women (61.3%) and in the elderly (mean 75.6 years). Twenty-nine patients had locoregional MCC and two had metastatic MCC at the time of diagnosis. Patients in stage I disease had excellent prognosis. In stages II and III, respectively 22.2% and 50.0% of patients developed metastases. Among patients who received chemotherapy with cisplatin and etoposide, 17% achieved partial remission with progression-free survival (PFS) of 8.0 months, and a further 50% achieved stable disease with PFS of 4.0, 4.5, and 4.5 months respectively. In 6 (19.4%) patients MCC coexisted with chronic lymphocytic leukemia (CLL). In all six cases CLL preceded MCC development.
Conclusions
Female gender, tumor-free resection margins, and local disease were found to be independent prognostic factors in MCC progression. Patients with hematological malignancies, immunosuppression, and those with immune deficiencies should be closely followed up as they are predisposed to develop MCC.
Similar content being viewed by others
Why carry out this study? |
Merkel cell carcinoma (MCC) is a rare but aggressive cutaneous neuroendocrine cancer that typically arises in sun-exposed areas of the skin. The annual incidence rate in Europe is estimated at 0.13 per 100,000 person-years |
Study was conducted to analyze the clinical profile of MCC to identify the main factors influencing the prognosis |
What was learned from the study? |
Female gender, local disease, and tumor-free resection margin were found to be independent prognostic indicators in MCC |
Special care should be given to patients with hematological malignancies and immunosuppression as they may be predisposed to develop MCC |
Introduction
Basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) are the two main types of cutaneous malignancies that account for about 99% of all non-melanoma skin cancers (NMSC) [1, 2]. The remaining 1% includes rare skin tumors such as Merkel cell carcinoma (MCC), apocrine adenocarcinoma, and sebaceous carcinoma. MCC is a rare but aggressive malignancy which, after melanoma, is the second most frequent cause of death due to skin neoplasms [3]. The exact origin of MCC is still a matter of discussion [4]. It was thought that MCC derived from neuroendocrine cells called Merkel cells localized within the dermoepidermal junction [5, 6]. However, other studies indicated that precursor B cells or totipotent stem cells found in the dermis may be connected with the development of MCC [7].
The real incidence rates of MCC are unknown because of the rarity of the cancer. We performed a retrospective study on management and outcomes of patients diagnosed with MCC over a 10-year period assessing the influence of gender, tumor localization, and age on the treatment results and prognosis. In addition, the medical histories of patients with primary MCC were examined to assess relevant factors which could have contributed to the weakening of the immune system and promoting MCC development.
The aim of the study is to analyze the clinical profile of MCC to identify the main factors influencing the prognosis.
Methods
Patients with histopathologically proven MCC diagnosed at the Nicolaus Copernicus Multidisciplinary Centre for Oncology and Traumatology in Poland between 2010 and 2019 were identified within the pathology database of approximately 13,000 records and their medical records were retrospectively reviewed. The following demographic and clinicopathological features were recorded: age, sex, site of the primary tumor, stage, treatment, and clinical outcomes. Patients with MCC were classified according to the 8th American Joint Committee on Cancer (AJCC) staging system [8]. MCC stages I and II were defined as a disease that is limited to the skin at the primary site. Stages III and IV were defined as a disease that involves regional lymph nodes and with metastases beyond regional lymph nodes, respectively. Approval for this study was obtained from the Human Research Ethics Committee of the Medical University of Lodz, Poland (RNN/2019/18/KE). All methods and procedures were conducted in accordance with the relevant guidelines and regulations as well as with the updated Declaration of Helsinki.
Statistical Analysis
All results were analyzed statistically with Statistica 13.0 (Statsoft, Kraków, Poland). Normal distribution of age variable was confirmed with Shapiro–Wilk test, using a right-tailed normal distribution. The Kaplan–Meier overall survival (OS) was calculated from the time of diagnosis to death by any cause or to the last follow-up. Progression-free survival (PFS) was defined as the date from first day of treatment to either progression of the disease or death. The differences between the curves were estimated by the log-rank test and p values of less than 0.05 were considered significant. Cox proportional hazard regression model was used for multivariate analysis. The response of MCC to palliative treatment was evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.
Results
Characteristics of Patients with Merkel Cell Carcinoma
Over the 10-year study period, 31 patients were diagnosed with histologically confirmed MCC. During the analyzed period of time, 4026 cases of NMSC were diagnosed in our Oncology Centre; therefore, MCC constituted 0.77% of all NMSCs. The mean (standard deviation) age at the time of diagnosis was 75.6 (± 9.4) years and ranged from 51 to 93 years. Nineteen patients (61.3%) were female while 12 (38.7%) were male. The primary tumor sites were head (n = 16, 51.6%), lower extremities (n = 10, 32.2%), trunk (n = 3, 9.7%), and upper extremities (n = 2, 6.5%). Patients were diagnosed with local, regional, and metastatic MCC in 19 (61.3%), 10 (32.2%), and 2 (6.5%) cases, respectively. The detailed characteristics of analyzed subjects are presented in Table 1.
Survival Analysis
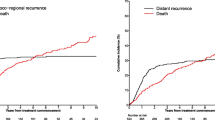
The median follow-up time was 21 months with a range from 2 to 117 months. Twelve patients (38.7%) died during the analyzed period. The median OS for all 31 patients was not reached, while the estimated 2-year OS was 70.8% and 5-year OS was 54.5%. Estimated 5-year OS rates according to clinical parameters are presented in Table 2. Patients in stage I of the disease had excellent prognosis with 5-year OS of 0.89. One patient died of a heart attack, unrelated to the cancer, with no signs of MCC relapse observed during follow-up. All stage IV individuals died within the period of 14 months from the diagnosis (Fig. 1). No significant differences in OS were found between stages II and III patients (Fig. 1). Interestingly, female patients demonstrated significantly better prognosis than male ones (Fig. 2).
Other prognostic factors included location of primary tumor on the face (p = 0.04), no tumor cells at the resection margin (p = 0.02), and local disease only (p = 0.002) (Table 2). Age at diagnosis, size of the primary tumor, concomitant CLL, and the treatment with radiotherapy did not have a significant impact on the patient survival (Table 2). Cox regression model revealed that female gender, tumor-free resection margin, and local disease were independent prognostic indicators (Table 3). Clinicopathological characteristics of all deceased patients are presented in Table S1.
Surgery
Out of 31 patients 19 had a local disease at the time of diagnosis and 10 had a regional disease. All patients with local and regional stage of MCC (n = 29) underwent wide local excision of the primary MCC with or without lymph node management technique. Figure 3 presents the details of the management of patients with lymph node involvement.
Among 23 patients with MCC and no clinical signs of nodal disease undergoing wide local excision, sentinel lymph node biopsy (SLNB) was performed in 14, while nodal observation alone was advised in nine patients. Relapse was observed in two out of nine patients under nodal surveillance (observation), and in seven out of 14 patients who underwent SLNB (four cases with positive SLNB, three cases with negative SLNB). In six out of nine patients under nodal surveillance (observation) the MCC’s tumor dimension was below 1 cm. In one patient with recurrence the dimension of MCC was above 2 cm (T2); however, SLNB was not performed because of the patient’s overall poor condition.
Adjuvant and Palliative Care
Adjuvant radiotherapy was selected on the basis of the pathology report. Patients with inoperable MCC were treated with palliative chemotherapy (PE—cisplatin and etoposide; CAV—cyclophosphamide, doxorubicin, and vincristine; or cyclophosphamide in monotherapy). Among two patients with primary metastatic MCC, one received best supportive care and one underwent palliative chemotherapy. Out of seven patients who were diagnosed with the primary locoregional disease (stage II—two patients, stage III—five patients) and developed metastases, six received palliative chemotherapy. In total, six patients underwent palliative chemotherapy with PE and one transplant patient received cyclophosphamide in monotherapy. Among patients who received PE chemotherapy one patient (17%) had partial remission with PFS of 8.0 months, and three patients (50%) achieved stable disease with PFS of 4.0, 4.5, and 4.5 months, respectively. In two remaining patients (33%) the disease progressed with PFS of 2.0 and 2.5 months, respectively. In one patient who was treated with cyclophosphamide in monotherapy MCC progressed with PFS of 2.0 months. Only two patients who did not respond to PE treatment received second-line CAV chemotherapy. Both patients achieved stable disease with PFS of 3.5 and 4.0 months after four cycles of chemotherapy.
Discussion
MCC is one of the most aggressive skin cancers of which incidence rates are dramatically rising. This skin cancer is characterized by rapid progression, high mortality rates, and challenging treatment. Clinically MCC usually presents as a painless, single, red or purple, rapidly growing cutaneous nodule. The diagnosis of MCC is often not suspected until the histopathology examination report. Early diagnosis is essential to achieve optimal clinical outcomes, considering the aggressive nature of the disease and high risk of recurrence and metastasis. In recent years significant advances in the understanding of the pathophysiology of MCC have been made. Several main risk factors for MCC development have been identified, including exposure to UV radiation, advanced age, fair skin, and immunosuppression [7, 9, 10].
The exact incidence of MCC is difficult to establish, and available epidemiological and survival data are still incomplete or inconsistent. The incidence of MCC varies between 0.1 and 0.88 per 100,000 person-years, depending on the geographical region, which can be associated with both the exposure to UV radiation and the life expectancy in the population of the defined area. Higher incidence rates of MCC were noted in Australia [11] and New Zealand, while the lowest rates were observed in Eastern France [12]. The data from 1995 to 2002 estimate the annual incidence rate in Europe at 0.13 per 100,000 person-years [13]. Our study is, to the best of our knowledge, the largest series of cases of MCC reported in Poland. In our cohort MCC was reported in 0.77% of all cases of NMSC, confirming the rarity of this tumor. Unexpectedly, 19 cases of MCC (61.3%) were found in women. The majority of the previous studies indicated male predominance [14, 15] and even defined male gender as a risk factor for MCC development.
Regarding the location, the MCC was found most frequently on the face, followed by lower extremities. MCC typically occurs in the sun-exposed skin of the head, neck, and extremities of elderly patients. It was suggested that MCC located on the head and neck is linked to a worse prognosis, when compared to MCC from other anatomical regions [16]. Interestingly, in our cohort, location of the tumor on the face was a good prognostic factor. Lesions located on the face, especially in female patients (as in our cohort) may be detected earlier than in other locations. On the other hand, surgical treatment of facial tumors can be difficult as the doctor needs to achieve the required resection margins as well as satisfactory functional and esthetic results. Furthermore, SLNB and lymph node dissection can be challenging to perform taking into account the extremely variable lymphatic drainage system within the head and neck area. It is worth mentioning that one of our male patients developed MCC in a non-sun-exposed area of the gluteal region. Only a few other cases of MCC involving this anatomical site (all male) were reported previously [17,18,19].
In our cohort 61.3% patients were diagnosed at stages I and II and classified as local disease without signs of regional lymph node involvement. In those cases, wide excision with at least 1–2 cm margin and sentinel lymph node biopsy are recommended with possible addition of adjuvant radiotherapy. Not all the patients were able to proceed with further treatment after resection of the primary lesion, mainly as a result of advanced age, poor general condition, or/and significant comorbidities.
More women than men were diagnosed with stage I of the disease. The reported differences between sexes and cancer advancement at the time of diagnosis may be connected with different approach regarding visible skin lesions, as women seek medical advice earlier and men may visit the doctor only when lesions become painful and uncomfortable, e.g., in more advanced stages of MCC. The observed distribution of MCC stages in our patients is very similar to the data presented by Harms et al. [20], where local, regional, and distant disease was presented respectively in 65%, 26%, and 8% of all reported cases.
Our observations regarding the survival and prognosis of MCC are consistent with earlier studies [14, 20]. We noted that the stage of MCC at the time of diagnosis closely correlates with the prognosis and survival rates, which decreased sharply with MCC progression to regional or metastatic disease. Only 50% of patients at stage III survived 2 years after diagnosis compared to 100% 2-year survival in patients at stage I at the time of diagnosis Patients with distant metastatic disease had a survival time of only a few months after diagnosis. Overall, in agreement with previous studies [21, 22], we observed better survival rates in female patients which was probably related to earlier diagnosis and lower stage of the disease. Despite the data indicating that MCC is a chemosensitive cancer, none of our patients showed long-term survival after treatment with different regimens of chemotherapy. Unfortunately, as a result of the lack of funding, none of the patients with metastatic disease were treated with avelumab, an anti-programmed cell death 1 ligand 1 (PD-L1) binding monoclonal antibody [4, 23].
It is confirmed that the patient’s immune status plays a crucial role in MCC development. A higher incidence of MCC is seen in immunosuppressed patients with T cell dysfunction after organ transplantation, patients infected with human immunodeficiency virus, as well as patients with hematological malignancies, e.g., multiple myeloma or CLL. Immunosuppression caused by abnormalities in humoral and cellular immunity observed in CLL as well as during the treatment [24] may explain the association between CLL and higher risk for development of other primary malignancies. We report coexistence of CLL and MCC in six patients. In all six cases skin cancer developed after hematological malignancies were diagnosed, but only in two cases CLL required treatment. We observed one unique case of coexisting CLL with primary MCC in a relatively young male patient. MCC and CLL usually affect older patients and rarely occur in individuals under 50 years of age. In our group the coexistence of MCC and CLL was not correlated with lower survival rate. In analysis presented by Koljonen et al., in the group of 4164 patients with CLL and 172 patients with MCC, both malignancies were found only in six patients [25]. The high incidence of MCC co-occurrence with CLL in our study may be due to the specificity of our center, in which both the hematological and oncological services are located.
Only one transplant-related MCC was found in our cohort. This patient underwent a kidney transplant in 1998 and has been on immunosuppression since then. According to the literature, solid organ transplant patients on immunosuppression have a fivefold increased risk of MCC development [26].
MCC is highly associated with other cancers including cutaneous squamous cell carcinoma and adenocarcinoma of the breast, ovary, or salivary glands [27]. In our cohort of patients there was one case of prostate cancer diagnosis. Four other patients in our group had an earlier history of other NMSCs or developed further skin cancers after the diagnosis of primary MCC. Two of those patients had multiple BCCs and SCCs. These results corroborate the data indicating that the patients who develop skin cancer are predisposed to developing subsequent NMSCs within 5 years [28]. That data underlines the need for frequent follow-ups with whole body examination for all patients diagnosed with skin cancer.
Limitations
As our data come from one center in Poland over the period of 10 years, we suggest that further studies in the whole of Europe are needed to confirm our findings in trends and characteristics of MCC.
Conclusions
Since 2000 we reported an increase in the incidence of MCC, which can only be partially explained by improved detection and histopathological reports of MCC. In contrast to previous data, the present study has shown a higher incidence of MCC in women, in whom MCC was diagnosed at an earlier stage than in men. Facial lesions were also the most frequent site of the cancer in women which in this group was not confirmed to be associated with worse prognosis.
In our group, MCCs diagnosed at an early stage (I or II) appeared less aggressive and less likely to recur or metastasize than those reported in the literature. Palliative chemotherapy showed limited effectiveness in advanced MCC; thus, it is necessary to consider immunotherapy as a valuable alternative. The physicians should be aware of frequent coexistence of MCC with chronic lymphocytic leukemia and other skin cancers.
References
Katalinic A, Kunze U, Schäfer T. Epidemiology of cutaneous melanoma and non-melanoma skin cancer in Schleswig-Holstein, Germany: incidence, clinical subtypes, tumour stages and localization (epidemiology of skin cancer). Br J Dermatol. 2003;149:1200–6.
Malone JP, Fedok FG, Belchis DA, Maloney ME. Basal cell carcinoma metastatic to the parotid: report of a new case and review of the literature. Ear Nose Throat J. 2000;79:518–9.
Harms PW. Update on Merkel cell carcinoma. Clin Lab Med. 2017;37:485–501.
Kwiatkowska D, Reich A. Landscape of current and future therapies of Merkel cell carcinoma. Dermatol Ther. 2020;21:e13281.
Schadendorf D, Lebbé C, Zur Hausen A, et al. Merkel cell carcinoma: epidemiology, prognosis, therapy and unmet medical needs. Eur J Cancer. 2017;71:53–69.
Tetzlaff MT, Nagarajan P. Update on Merkel cell carcinoma. Head Neck Pathol. 2018;12:31–433.
Uchi H. Merkel cell carcinoma: an update and immunotherapy. Front Oncol. 2018;8:48.
Amin MB, Edge S, Greene F, et al. AJCC cancer staging manual. 8th ed. New York: Springer; 2017.
Howard RA, Dores GM, Curtis RE, Anderson WF, Travis LB. Merkel cell carcinoma and multiple primary cancers. Cancer Epidemiol Biomark Prev. 2006;15:1545.
Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–100.
Youlden DR, Soyer HP, Youl PH, Fritschi L, Baade PD. Incidence and survival for Merkel cell carcinoma in Queensland, Australia, 1993–2010. JAMA Dermatol. 2014;150:864–72.
Riou-Gotta MO, Fournier E, Danzon A, et al. Rare skin cancer: a population-based cancer registry descriptive study of 151 consecutive cases diagnosed between 1980 and 2004. Acta Oncol. 2009;48:605–9.
Van Der Zwan JM, Trama A, Otter R, et al. Rare neuroendocrine tumours: results of the surveillance of rare cancers in Europe project. Eur J Cancer. 2013;49:2565–78.
Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49:832–41.
Chang J, Chang YY, Huang YL, et al. Merkel cell carcinoma in Taiwan: a series of 24 cases and literature review. Medicine. 2019;98:e17538.
Morand GB, Madana J, Da Silva SD, Hier MP, Mlynarek AM, Black MJ. Merkel cell carcinoma of the head and neck: poorer prognosis than non-head and neck sites. J Laryngol Otol. 2016;130:393–7.
Turkkan G, Agdogan O, Saynak M, Uygun AC, Ustun F. Recurrent Merkel cell carcinoma of the gluteal region: a case report. Dermatol Ther. 2019;32:e12749.
Perman MJ, King JM, Leithauser LL, Gloster HM. Giant Merkel cell carcinoma masquerading as a benign cyst on the buttock of an African American man. Case Rep Oncol Med. 2011;2011:849767.
Howell RS, Rice JA, Sticco K, et al. An unusual presentation of Merkel cell carcinoma: a case report. J Surg Case Rep. 2018;2018:rjy185.
Harms KL, Healy MA, Nghiem P. Analysis of prognostic factors from 9387 Merkel cell carcinoma cases forms the basis for the new 8th edition AJCC staging system. Ann Surg Oncol. 2016;23:3564–71.
Goessling W, McKee PH, Mayer RJ. Merkel cell carcinoma. J Clin Oncol. 2002;20:588–98.
Tai PT, Yu E, Tonita J, Gilchrist J. Merkel cell carcinoma of the skin. J Cutan Med Surg. 2000;4:186–95.
Kaufman HL, Russell JS, Hamid O, et al. Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after ≥1 year of follow-up: JAVELIN Merkel 200, a phase 2 clinical trial. J Immunother Cancer. 2018;6:7.
Dasanu CA, Alexandrescu DT. Risk for second nonlymphoid neoplasms in chronic lymphocytic leukemia. Med Gen Med. 2007;9:35.
Koljonen V, Kukko H, Pukkala E, et al. Chronic lymphocytic leukaemia patients have a high risk of Merkel-cell polyomavirus DNA-positive Merkel-cell carcinoma. Br J Cancer. 2009;101:1444–7.
Choudhury K, Volkmer B, Greinert R, et al. Effectiveness of skin cancer screening programmes. Br J Dermatol. 2012;167(Suppl 2):94–8.
Brenner B, Sulkes A, Rakowsy E, et al. Second neoplasm in patients with Merkel cell carcinoma. Cancer. 2001;91:1358–62.
Rutkowski P, Owczarek W. Guidelines for diagnostic and therapeutic management: skin carcinomas. Oncol Clin Pract. 2018;14:129–47.
Acknowledgments
Funding
This work was supported by Medical University of Lodz [503/5-064-01/503-1]; and the National Centre of Science [2017/27/B/NZ5/02011]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No Rapid Service Fee was received by the journal for the publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Magdalena Ciążyńska, Katarzyna Szczepaniak, Marta Pabianek, Dariusz Nejc and Małgorzata Ułańska have nothing to disclose. Adam Reich has worked as a Consultant or Speaker for AbbVie, Bioderma, Celgene, Chema Elektromet, Eli Lilly, Galderma, Janssen, Leo Pharma, Medac, Menlo Therapeutics, Novartis, Pierre-Fabre, Trevi, and participated as Principal Investigator or Subinvestigator in clinical trials sponsored by AbbVie, Drug Delivery Solutions Ltd, Galderma, Janssen, Kymab Limited, Leo Pharma, Menlo Therapeutics, MetrioPharm, MSD, Novartis, Trevi. Adam Reich is a member of the journal’s Editorial Board. Witold Owczarek has worked as a Consultant or Speaker and participated as Principal Investigator or Subinvestigator in clinical trials sponsored by AbbVie, Alfasigma, Almirall, Bioderma, Egis, Eli Lilly, Galenica, Galderma, Janssen-Cilag, Leo Pharma, Medac GmbH, Novartis, Pfizer, Pierre-Fabre, Roche, Sandoz, Teva Pharmaceuticals. Małgorzata Skibińska has worked as a Speaker for Novartis, Sanofi, Solverx and Polfarmex. Joanna Narbutt has worked as a Consultant or Speaker for AbbVie, Bioderma, Eli Lilly, Janssen, Leo Pharma, Medac, Novartis, Pierre-Fabre, and participated as Principal Investigator or Subinvestigator in clinical trials sponsored by AbbVie, UCB, Galderma, Janssen, Leo Pharma, Pfizer. Aleksandra Lesiak has worked as a Consultant or Speaker for AbbVie, Bioderma, Eli Lilly, Janssen, Leo Pharma, Medac, Novartis, Pierre-Fabre, and participated as Principal Investigator or Subinvestigator in clinical trials sponsored by AbbVie, UCB, Galderma, Janssen, Leo Pharma, Pfizer.
Compliance with Ethics Guidelines
Approval for this study was obtained from the Human Research Ethics Committee of the Medical University of Lodz, Poland (RNN/2019/18/KE). All methods and procedures were conducted in accordance with the relevant guidelines and regulations as well as with the updated Declaration of Helsinki.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Digital Features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12594308.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ciążyńska, M., Szczepaniak, K., Pabianek, M. et al. Primary Merkel Cell Carcinoma: A Retrospective Analysis of 31 Cases in Poland. Dermatol Ther (Heidelb) 10, 1003–1012 (2020). https://doi.org/10.1007/s13555-020-00424-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-020-00424-5