Abstract
Objective
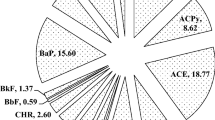
Asphalt is known as an important source of exposure to polycyclic aromatic hydrocarbons (PAHs). PAHs are one of the major concerns of the workplace and the scientific community, and have high stability and various toxicity effects.
Methods
Since asphalt workers are exposed to PAHs, this study was designed to evaluate the exposure of asphalt workers to PAHs and oxidative stress in neutrophils isolated from the workers.
Results
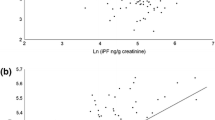
Results showed that exposure of asphalt workers with different PAHs is lower than the standard set. The level of reactive oxygen species (ROS), lipid peroxidation (LPO), and glutathione disulfide (GSSG) in neutrophils isolated from the blood of asphalt workers were significantly higher than the normal group. Glutathione (GSH) levels and GSH/GSSG ratio in neutrophils isolated from the blood of asphalt workers were significantly lower than in the normal group.
Conclusion
Results suggest that due to the lower level of exposure to PAHs compared to the standard level, the level of oxidative stress in these workers is high. Based on the toxic effects and the lack of a standard level for some PAHs, the use of personal protective equipment and the use of antioxidants is recommended.





Similar content being viewed by others
References
Brudi LC, Adolfo FR, do Nascimento PC, Cargnin RS, Bohrer D, de Carvalho LM et al (2020) Emission and collection of polycyclic aromatic hydrocarbons from raw asphalt samples heated at 130 °C. Energy Fuels 34:11248–11257
Chong D, Wang Y, Zhao K, Wang D, Oeser M (2018) Asphalt fume exposures by pavement construction workers: current status and project cases. J Constr Eng Manag 144:05018002
Song M, Lee K, Oh S-H, Bae M-S (2020) Impact of polycyclic aromatic Hydrocarbons (PAHs) from an asphalt mix plant in a suburban residential area. Appl Sci 10:4632
Unwin J, Cocker J, Scobbie E, Chambers H (2006) An assessment of occupational exposure to polycyclic aromatic hydrocarbons in the UK. Ann Occup Hyg 50:395–403. https://doi.org/10.1093/annhyg/mel010
Yu Y, Peng M, Liu Y, Ma J, Wang N, Ma S et al (2021) Co-exposure to polycyclic aromatic hydrocarbons and phthalates and their associations with oxidative stress damage in school children from South China. J Hazard Mater 401:123390. https://doi.org/10.1016/j.jhazmat.2020.123390
Deng Q, Dai X, Feng W, Huang S, Yuan Y, Xiao Y et al (2019) Co-exposure to metals and polycyclic aromatic hydrocarbons, microRNA expression, and early health damage in coke oven workers. Environ Int 122:369–380. https://doi.org/10.1016/j.envint.2018.11.056
Jain RB (2020) Contributions of dietary, demographic, disease, lifestyle and other factors in explaining variabilities in concentrations of selected monohydroxylated polycyclic aromatic hydrocarbons in urine: Data for US children, adolescents, and adults. Environ Pollut 266:115178. https://doi.org/10.1016/j.envpol.2020.115178
Wang Q, Xu X, Zeng Z, Zheng X, Ye K, Huo X (2020) Antioxidant alterations link polycyclic aromatic hydrocarbons to blood pressure in children. Sci Total Environ 732:138944. https://doi.org/10.1016/j.scitotenv.2020.138944
Kuang D, Zhang W, Deng Q, Zhang X, Huang K, Guan L et al (2013) Dose-response relationships of polycyclic aromatic hydrocarbons exposure and oxidative damage to DNA and lipid in coke oven workers. Environ Sci Technol 47:7446–7456. https://doi.org/10.1021/es401639x
Oliveira M, Costa S, Vaz J, Fernandes A, Slezakova K, Delerue-Matos C et al (2020) Firefighters exposure to fire emissions: Impact on levels of biomarkers of exposure to polycyclic aromatic hydrocarbons and genotoxic/oxidative-effects. J Hazard Mater 383:121179. https://doi.org/10.1016/j.jhazmat.2019.121179
Guan X, Fu W, Wei W, Li G, Wu X, Bai Y et al (2020) Mediation of the association between polycyclic aromatic hydrocarbons exposure and telomere attrition by oxidative stress: a prospective cohort study. J Hazard Mater 399:123058. https://doi.org/10.1016/j.jhazmat.2020.123058
Miglani K, Kumar S, Yadav A, Aggarwal N, Ahmad I, Gupta R (2019) A multibiomarker approach to evaluate the effect of polyaromatic hydrocarbon exposure on oxidative and genotoxic damage in tandoor workers. Toxicol Ind Health 35:486–496. https://doi.org/10.1177/0748233719862728
Peng M, Lu S, Yu Y, Liu S, Zhao Y, Li C et al (2020) Urinary monohydroxylated polycyclic aromatic hydrocarbons in primiparas from Shenzhen, South China: Levels, risk factors, and oxidative stress. Environ Pollut 259:113854. https://doi.org/10.1016/j.envpol.2019.113854
Rapisarda V, Carnazza ML, Caltabiano C, Loreto C, Musumeci G, Valentino M et al (2009) Bitumen products induce skin cell apoptosis in chronically exposed road pavers. J Cutan Pathol 36:781–787. https://doi.org/10.1111/j.1600-0560.2008.01140.x
Yazdani M (2020) Comparative toxicity of selected PAHs in rainbow trout hepatocytes: genotoxicity, oxidative stress and cytotoxicity. Drug Chem Toxicol 43:71–78. https://doi.org/10.1080/01480545.2018.1497054
Ekici E, Güney M, Nazıroğlu M (2020) Protective effect of cabergoline on mitochondrial oxidative stress-induced apoptosis is mediated by modulations of TRPM2 in neutrophils of patients with endometriosis. J Bioenerg Biomembr 52:131–142. https://doi.org/10.1007/s10863-020-09830-y
Marí-Alexandre J, Carcelén AP, Agababyan C, Moreno-Manuel A, García-Oms J, Calabuig-Fariñas S et al (2019) Interplay between microRNAs and oxidative stress in ovarian conditions with a focus on ovarian cancer and endometriosis. Int J Mol Sci. https://doi.org/10.3390/ijms20215322
Dogru A, Nazıroglu M, Cig B (2019) Modulator role of infliximab and methotrexate through the transient receptor potential melastatin 2 (TRPM2) channel in neutrophils of patients with rheumatoid arthritis: a pilot study. Arch Med Sci 15:1415–1424. https://doi.org/10.5114/aoms.2018.79485
Köse SA, Nazıroğlu M (2014) Selenium reduces oxidative stress and calcium entry through TRPV1 channels in the neutrophils of patients with polycystic ovary syndrome. Biol Trace Elem Res 158:136–142. https://doi.org/10.1007/s12011-014-9929-3
Vitte J, Michel BF, Bongrand P, Gastaut JL (2004) Oxidative stress level in circulating neutrophils is linked to neurodegenerative diseases. J Clin Immunol 24:683–692. https://doi.org/10.1007/s10875-004-6243-4
Chen Q, Wang Q, Zhu J, Xiao Q, Zhang L (2018) Reactive oxygen species: key regulators in vascular health and diseases. Br J Pharmacol 175:1279–1292
Collin F (2019) Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases. Int J Mol Sci 20:2407
Reczek CR, Chandel NS (2018) ROS promotes cancer cell survival through calcium signaling. Cancer Cell 33:949–951
Gaikwad AS, Mahmood R, Ravichandran B, Kondhalkar S (2020) Evaluation of telomere length and genotoxicity among asphalt associated workers. Mutat Res Genet Toxicol Environ Mutagen 858:503255
Fostinelli J, Madeo E, Toraldo E, Sarnico M, Luzzana G, Tomasi C et al (2018) Environmental and biological monitoring of occupational exposure to polynuclear aromatic hydrocarbons during highway pavement construction in Italy. Toxicol Lett 298:134–140
Andrisic L, Dudzik D, Barbas C, Milkovic L, Grune T, Zarkovic N (2018) Short overview on metabolomics approach to study pathophysiology of oxidative stress in cancer. Redox Biol 14:47–58
Wei Z, Li X, Li X, Liu Q, Cheng Y (2018) Oxidative stress in Parkinson’s disease: a systematic review and meta-analysis. Front Mol Neurosci 11:236
Banerjee S, Ghosh S, Mandal A, Ghosh N, Sil PC (2020) ROS-associated immune response and metabolism: a mechanistic approach with implication of various diseases. Arch Toxicol 94:2293–2317
Sies H, Jones DP (2020) Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol 21:363–383
Chowdhury AR, Zielonka J, Kalyanaraman B, Hartley RC, Murphy MP, Avadhani NG (2020) Mitochondria-targeted paraquat and metformin mediate ROS production to induce multiple pathways of retrograde signaling: a dose-dependent phenomenon. Redox Biol 36:101606
Mailloux RJ (2020) An update on mitochondrial reactive oxygen species production. Antioxidants 9:472
Paris L, Roussel M, Pereira B, Delbac F, Diogon M (2017) Disruption of oxidative balance in the gut of the western honeybee Apis mellifera exposed to the intracellular parasite Nosema ceranae and to the insecticide fipronil. Microb Biotechnol 10:1702–1717
Angelova PR, Esteras N, Abramov AY (2021) Mitochondria and lipid peroxidation in the mechanism of neurodegeneration: finding ways for prevention. Med Res Rev 41:770–784
Jambunathan N (2010) In: Plant stress tolerance, pp 291–297. Springer
Zhang S, He Y, Sen B, Wang G (2020) Reactive oxygen species and their applications toward enhanced lipid accumulation in oleaginous microorganisms. Bioresour Technol 307:123234
Er R, Aydın B, Şekeroğlu V, Atlı Şekeroğlu Z (2020) Protective effect of Argan oil on mitochondrial function and oxidative stress against acrylamide-induced liver and kidney injury in rats. Biomarkers 25:458–467
Zhang L, Yang L, Zhou Q, Zhang X, Xing W, Wei Y et al (2020) Size distribution of particulate polycyclic aromatic hydrocarbons in fresh combustion smoke and ambient air: a review. J Environ Sci (China) 88:370–384. https://doi.org/10.1016/j.jes.2019.09.007
Zitka O, Skalickova S, Gumulec J, Masarik M, Adam V, Hubalek J et al (2012) Redox status expressed as GSH: GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol Lett 4:1247–1253
Owen JB, Butterfield DA (2010) Measurement of oxidized/reduced glutathione ratio. Methods Mol Biol 648:269–277. https://doi.org/10.1007/978-1-60761-756-3_18
Bansal A, Simon MC (2018) Glutathione metabolism in cancer progression and treatment resistance. J Cell Biol 217:2291–2298. https://doi.org/10.1083/jcb.201804161
Kim SJ, Jung HJ, Hyun DH, Park EH, Kim YM, Lim CJ (2010) Glutathione reductase plays an anti-apoptotic role against oxidative stress in human hepatoma cells. Biochimie 92:927–932. https://doi.org/10.1016/j.biochi.2010.03.007
Zhu Y, Wu J, Wang K, Xu H, Qu M, Gao Z et al (2021) Facile and sensitive measurement of GSH/GSSG in cells by surface-enhanced Raman spectroscopy. Talanta 224:121852. https://doi.org/10.1016/j.talanta.2020.121852
Kennedy L, Sandhu JK, Harper ME, Cuperlovic-Culf M (2020) Role of glutathione in cancer: from mechanisms to therapies. Biomolecules. https://doi.org/10.3390/biom10101429
Oh H, Siano B, Diamond S (2008) Neutrophil isolation protocol. J Vis Exp. https://doi.org/10.3791/745
Seydi E, Salimi A, Rasekh HR, Mohsenifar Z, Pourahmad J (2018) Selective cytotoxicity of luteolin and kaempferol on cancerous hepatocytes obtained from rat model of hepatocellular carcinoma: involvement of ROS-mediated mitochondrial targeting. Nutr Cancer 70:594–604
Beach DC, Giroux E (1992) Inhibition of lipid peroxidation promoted by iron (III) and ascorbate. Arch Biochem Biophys 297:258–264
Hissin PJ, Hilf R (1976) A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem 74:214–226
Acknowledgements
This study was supported by the Alborz University of Medical Sciences, Karaj, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Asghar Ghahri, Pouria Seydi, Fatemeh Khademi, Hannaneh Zakersani, Enayatollah Seydi declare that they have no conflict of interest.
Ethical approval
The experimental protocols involved in the study were approved by the University of Alborz (Karaj, Iran) Ethical Committee with approval number: IR.ABZUMS.REC.1399.059.
Rights and permissions
About this article
Cite this article
Ghahri, A., Seydi, P., Khademi, F. et al. The polycyclic aromatic hydrocarbons (PAHs)-induced toxicity in asphalt workers neutrophils through induction of oxidative stress. Toxicol. Environ. Health Sci. 13, 389–396 (2021). https://doi.org/10.1007/s13530-021-00106-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13530-021-00106-5