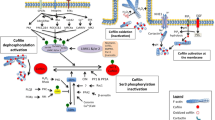
Abstract
Background
Metastasis accounts for the majority of cancer-related deaths. Actin dynamics and actin-based cell migration and invasion are important factors in cancer metastasis. Metastasis is characterized by actin polymerization and depolymerization, which are precisely regulated by molecular changes involving a plethora of actin regulators, including actin-binding proteins (ABPs) and signalling pathways, that enable cancer cell dissemination from the primary tumour. Research on deubiquitinating enzymes (DUBs) has revealed their vital roles in actin dynamics and actin-based migration and invasion during cancer metastasis.
Conclusion
Here, we review how DUBs drive tumour metastasis by participating in actin rearrangement and actin-based migration and invasion. We summarize the well-characterized and essential actin cytoskeleton signalling molecules related to DUBs, including Rho GTPases, Src kinases, and ABPs such as cofilin and cortactin. Other DUBs that modulate actin-based migration signalling pathways are also discussed. Finally, we discuss and address therapeutic opportunities and ongoing challenges related to DUBs with respect to actin dynamics.


Similar content being viewed by others
Data availability
Not applicable.
References
X. Jin, Z. Demere, K. Nair, A. Ali, G.B. Ferraro, T. Natoli, A. Deik, L. Petronio, A.A. Tang, C. Zhu, L. Wang, D. Rosenberg, V. Mangena, J. Roth, K. Chung, R.K. Jain, C.B. Clish, M.G. Vander Heiden, T.R. Golub, A metastasis map of human cancer cell lines. Nature 588, 331–336 (2020)
S. Valastyan, R.A. Weinberg, Tumor metastasis: molecular insights and evolving paradigms. Cell 147, 275–292 (2011)
S. SenGupta, C.A. Parent, J.E. Bear, The principles of directed cell migration. Nat. Rev. Mol. Cell Biol. 22, 529–547 (2021)
L.M. Machesky, H.R. Tang, Actin-based protrusions: promoters or inhibitors of cancer invasion? Cancer Cell 16, 5–7 (2009)
D.A. Cruz Walma, Z. Chen, A.N. Bullock, K.M. Yamada, Ubiquitin ligases: guardians of mammalian development. Nat. Rev. Mol. Cell Biol. 23(5), 350–367 (2022)
C. Pohl, I. Dikic, Cellular quality control by the ubiquitin-proteasome system and autophagy. Science 366, 818–822 (2019)
T.E.T. Mevissen, D. Komander, Mechanisms of deubiquitinase specificity and regulation. Annu. Rev. Biochem. 86, 159–192 (2017)
M.J. Clague, S. Urbé, D. Komander, Breaking the chains: deubiquitylating enzyme specificity begets function. Nat Rev Mol Cell Biol 20, 338–352 (2019)
K.P. Lai, J. Chen, W.K.F. Tse, Role of deubiquitinases in human cancers: potential targeted therapy. Int. J. Mol. Sci. 21 (2020)
Z. Xiao, P. Zhang, L. Ma, The role of deubiquitinases in breast cancer. Cancer Metastasis Rev. 35, 589–600 (2016)
T. Bonacci, M.J. Emanuele, Dissenting degradation: deubiquitinases in cell cycle and cancer. Semin. Cancer Biol. 67, 145–158 (2020)
M.T. Islam, F. Chen, H. Chen, The oncogenic role of ubiquitin specific peptidase (USP8) and its signaling pathways targeting for cancer therapeutics. Arch. Biochem. Biophys. 701, 108811 (2021)
B. Artegiani, L. van Voorthuijsen, R.G.H. Lindeboom, D. Seinstra, I. Heo, P. Tapia, C. López-Iglesias, D. Postrach, T. Dayton, R. Oka, H. Hu, R. van Boxtel, J.H. van Es, J. Offerhaus, P.J. Peters, J. van Rheenen, M. Vermeulen, H. Clevers, Probing the tumor suppressor function of BAP1 in CRISPR-engineered human liver organoids. Cell Stem Cell 24, 927–943.e926 (2019)
M. Murtaza, L.A. Jolly, J. Gecz, S.A. Wood, La FAM fatale: USP9X in development and disease. Cell Mol. Life Sci. 72, 2075–2089 (2015)
L. Masclef, O. Ahmed, B. Estavoyer, B. Larrivée, N. Labrecque, A. Nijnik, E.B. Affar, Roles and mechanisms of BAP1 deubiquitinase in tumor suppression. Cell Death Differ. 28, 606–625 (2021)
J.R. Testa, M. Cheung, J. Pei, J.E. Below, Y. Tan, E. Sementino, N.J. Cox, A.U. Dogan, H.I. Pass, S. Trusa, M. Hesdorffer, M. Nasu, A. Powers, Z. Rivera, S. Comertpay, M. Tanji, G. Gaudino, H. Yang, M. Carbone, Germline BAP1 mutations predispose to malignant mesothelioma. Nat. Genet. 43, 1022–1025 (2011)
G.Z. Qiu, W. Sun, M.Z. Jin, J. Lin, P.G. Lu, W.L. Jin, The bad seed gardener: deubiquitinases in the cancer stem-cell signaling network and therapeutic resistance. Pharmacol. Ther. 172, 127–138 (2017)
M. He, Z. Zhou, G. Wu, Q. Chen, Y. Wan, Emerging role of DUBs in tumor metastasis and apoptosis: therapeutic implication. Pharmacol. Ther. 177, 96–107 (2017)
J. He, H.J. Lee, S. Saha, D. Ruan, H. Guo, C.H. Chan, Inhibition of USP2 eliminates cancer stem cells and enhances TNBC responsiveness to chemotherapy. Cell Death Dis. 10, 285 (2019)
J. Kim, F. Alavi Naini, Y. Sun, L. Ma, Ubiquitin-specific peptidase 2a (USP2a) deubiquitinates and stabilizes β-catenin. Am. J. Cancer Res. 8, 1823–1836 (2018)
W. Huang, X. Liu, Y. Zhang, M. Deng, G. Li, G. Chen, L. Yu, L. Jin, T. Liu, Y. Wang, Y. Chen, USP5 promotes breast cancer cell proliferation and metastasis by stabilizing HIF2α. J. Cell Physiol. 237(4), 2211–2219 (2022)
D. Duan, M. Shang, Y. Han, J. Liu, J. Liu, S.H. Kong, J. Hou, B. Huang, J. Lu, Y. Zhang, EZH2-CCF-cGAS axis promotes breast cancer metastasis. Int. J. Mol. Sci. 23(3), 1788 (2022)
J. Liu, J. Feng, L. Li, L. Lin, J. Ji, C. Lin, L. Liu, N. Zhang, D. Duan, Z. Li, B. Huang, Y. Zhang, J. Lu, Arginine methylation-dependent LSD1 stability promotes invasion and metastasis of breast cancer. EMBO Rep. 21, e48597 (2020)
L. Zhang, F. Zhou, Y. Drabsch, R. Gao, B.E. Snaar-Jagalska, C. Mickanin, H. Huang, K.A. Sheppard, J.A. Porter, C.X. Lu, P. ten Dijke, USP4 is regulated by AKT phosphorylation and directly deubiquitylates TGF-β type I receptor. Nat. Cell Biol. 14, 717–726 (2012)
P.J. Eichhorn, L. Rodón, A. Gonzàlez-Juncà, A. Dirac, M. Gili, E. Martínez-Sáez, C. Aura, I. Barba, V. Peg, A. Prat, I. Cuartas, J. Jimenez, D. García-Dorado, J. Sahuquillo, R. Bernards, J. Baselga, J. Seoane, USP15 stabilizes TGF-β receptor I and promotes oncogenesis through the activation of TGF-β signaling in glioblastoma. Nat. Med. 18, 429–435 (2012)
S. Liu, R. González-Prieto, M. Zhang, P.P. Geurink, R. Kooij, P.V. Iyengar, M. van Dinther, E. Bos, X. Zhang, S.E. Le Dévédec, B. van de Water, R.I. Koning, H.J. Zhu, W.E. Mesker, A.C.O. Vertegaal, H. Ovaa, L. Zhang, J.W.M. Martens, P. Ten Dijke, Deubiquitinase activity profiling identifies UCHL1 as a candidate oncoprotein that promotes TGFβ-induced breast cancer metastasis. Clin. Cancer Res. 26, 1460–1473 (2020)
D. Kim, A. Hong, H.I. Park, W.H. Shin, L. Yoo, S.J. Jeon, K.C. Chung, Deubiquitinating enzyme USP22 positively regulates c-Myc stability and tumorigenic activity in mammalian and breast cancer cells. J. Cell Physiol. 232, 3664–3676 (2017)
J. Qin, Z. Zhou, W. Chen, C. Wang, H. Zhang, G. Ge, M. Shao, D. You, Z. Fan, H. Xia, R. Liu, C. Chen, BAP1 promotes breast cancer cell proliferation and metastasis by deubiquitinating KLF5. Nat. Commun. 6, 8471 (2015)
G. Lambies, M. Miceli, C. Martínez-Guillamon, R. Olivera-Salguero, R. Peña, C.P. Frías, I. Calderón, B.S. Atanassov, S.Y.R. Dent, J. Arribas, A. García de Herreros, V.M. Díaz, TGFβ-activated USP27X deubiquitinase regulates cell migration and chemoresistance via stabilization of Snail1. Cancer Res. 79, 33–46 (2019)
T. Liu, J. Yu, M. Deng, Y. Yin, H. Zhang, K. Luo, B. Qin, Y. Li, C. Wu, T. Ren, Y. Han, P. Yin, J. Kim, S. Lee, J. Lin, L. Zhang, J. Zhang, S. Nowsheen, L. Wang, J. Boughey, M.P. Goetz, J. Yuan, Z. Lou, CDK4/6-dependent activation of DUB3 regulates cancer metastasis through SNAIL1. Nat. Commun. 8, 13923 (2017)
H. Zou, H. Chen, Z. Zhou, Y. Wan, Z. Liu, ATXN3 promotes breast cancer metastasis by deubiquitinating KLF4. Cancer Lett. 467, 19–28 (2019)
L. Zhang, J. Qiang, X. Yang, D. Wang, A.U. Rehman, X. He, W. Chen, D. Sheng, L. Zhou, Y.Z. Jiang, T. Li, Y. Du, J. Feng, X. Hu, J. Zhang, X.C. Hu, Z.M. Shao, S. Liu, IL1R2 blockade suppresses breast tumorigenesis and progression by impairing USP15-dependent BMI1 stability. Adv. Sci. (Weinh.) 7, 1901728 (2020)
Z. Zhang, Y. Fan, F. Xie, H. Zhou, K. Jin, L. Shao, W. Shi, P. Fang, B. Yang, H. van Dam, P. Ten Dijke, X. Zheng, X. Yan, J. Jia, M. Zheng, J. Jin, C. Ding, S. Ye, F. Zhou, L. Zhang, Breast cancer metastasis suppressor OTUD1 deubiquitinates SMAD7. Nat. Commun. 8, 2116 (2017)
Z. Zhang, J. Li, Y. Ou, G. Yang, K. Deng, Q. Wang, Z. Wang, W. Wang, Q. Zhang, H. Wang, W. Sun, P. Sun, S. Yang, CDK4/6 inhibition blocks cancer metastasis through a USP51-ZEB1-dependent deubiquitination mechanism. Signal Transduct. Target. Ther. 5, 25 (2020)
Q. Lu, D. Lu, Z.M. Shao, D.Q. Li, Deubiquitinase ubiquitin-specific protease 9X regulates the stability and function of E3 ubiquitin ligase ring finger protein 115 in breast cancer cells. Cancer Sci. 110, 1268–1278 (2019)
Z. Shang, J. Zhao, Q. Zhang, C. Cao, S. Tian, K. Zhang, L. Liu, L. Shi, N. Yu, S. Yang, USP9X-mediated deubiquitination of B-cell CLL/lymphoma 9 potentiates Wnt signaling and promotes breast carcinogenesis. J. Biol. Chem. 294, 9844–9857 (2019)
A. Ma, M. Tang, L. Zhang, B. Wang, Z. Yang, Y. Liu, G. Xu, L. Wu, T. Jing, X. Xu, S. Yang, Y. Liu, USP1 inhibition destabilizes KPNA2 and suppresses breast cancer metastasis. Oncogene 38, 2405–2419 (2019)
Y. Goto, L. Zeng, C.J. Yeom, Y. Zhu, A. Morinibu, K. Shinomiya, M. Kobayashi, K. Hirota, S. Itasaka, M. Yoshimura, K. Tanimoto, M. Torii, T. Sowa, T. Menju, M. Sonobe, H. Kakeya, M. Toi, H. Date, E.M. Hammond, M. Hiraoka, H. Harada, UCHL1 provides diagnostic and antimetastatic strategies due to its deubiquitinating effect on HIF-1α. Nat. Commun. 6, 6153 (2015)
L. Yuan, Y. Lv, H. Li, H. Gao, S. Song, Y. Zhang, G. Xing, X. Kong, L. Wang, Y. Li, T. Zhou, D. Gao, Z.X. Xiao, Y. Yin, W. Wei, F. He, L. Zhang, Deubiquitylase OTUD3 regulates PTEN stability and suppresses tumorigenesis. Nat. Cell Biol. 17, 1169–1181 (2015)
Z. Zhou, H. Zhou, L. Ponzoni, A. Luo, R. Zhu, M. He, Y. Huang, K.L. Guan, I. Bahar, Z. Liu, Y. Wan, EIF3H orchestrates hippo pathway-mediated oncogenesis via catalytic control of YAP stability. Cancer Res. 80, 2550–2563 (2020)
Z. Zhang, J. Du, S. Wang, L. Shao, K. Jin, F. Li, B. Wei, W. Ding, P. Fu, H. van Dam, A. Wang, J. Jin, C. Ding, B. Yang, M. Zheng, X.H. Feng, K.L. Guan, L. Zhang, OTUB2 promotes cancer metastasis via hippo-independent activation of YAP and TAZ. Mol. Cell 73, 7–21.e27 (2019)
B. Li, Z.P. Qi, D.L. He, Z.H. Chen, J.Y. Liu, M.W. Wong, J.W. Zhang, E.P. Xu, Q. Shi, S.L. Cai, D. Sun, L.Q. Yao, P.H. Zhou, Y.S. Zhong, NLRP7 deubiquitination by USP10 promotes tumor progression and tumor-associated macrophage polarization in colorectal cancer. J. Exp. Clin. Cancer Res. 40, 126 (2021)
C. Xing, X.X. Lu, P.D. Guo, T. Shen, S. Zhang, X.S. He, W.J. Gan, X.M. Li, J.R. Wang, Y.Y. Zhao, H. Wu, J.M. Li, Ubiquitin-specific protease 4-mediated deubiquitination and stabilization of PRL-3 is required for potentiating colorectal oncogenesis. Cancer Res. 76, 83–95 (2016)
Y.Y. Huang, C.M. Zhang, Y.B. Dai, J.G. Lin, N. Lin, Z.X. Huang, T.W. Xu, USP11 facilitates colorectal cancer proliferation and metastasis by regulating IGF2BP3 stability. Am. J. Transl. Res. 13, 480–496 (2021)
H. Sun, B. Ou, S. Zhao, X. Liu, L. Song, X. Liu, R. Wang, Z. Peng, USP11 promotes growth and metastasis of colorectal cancer via PPP1CA-mediated activation of ERK/MAPK signaling pathway. EBioMedicine 48, 236–247 (2019)
D. Miao, Y. Wang, Y. Jia, J. Tong, S. Jiang, L. Liu, ZRANB1 enhances stem-cell-like features and accelerates tumor progression by regulating Sox9-mediated USP22/Wnt/β-catenin pathway in colorectal cancer. Cell. Signal. 90, 110200 (2022)
S.I. Yun, H.K. Hong, S.Y. Yeo, S.H. Kim, Y.B. Cho, K.K. Kim, Ubiquitin-specific protease 21 promotes colorectal cancer metastasis by acting as a Fra-1 deubiquitinase. Cancers 12(1), 207 (2020)
C. Wu, K. Luo, F. Zhao, P. Yin, Y. Song, M. Deng, J. Huang, Y. Chen, L. Li, S. Lee, J. Kim, Q. Zhou, X. Tu, S. Nowsheen, Q. Luo, X. Gao, Z. Lou, Z. Liu, J. Yuan, USP20 positively regulates tumorigenesis and chemoresistance through β-catenin stabilization. Cell Death Differ. 25, 1855–1869 (2018)
J. Zhong, M. Zhao, Y. Ma, Q. Luo, J. Liu, J. Wang, X. Yuan, J. Sang, C. Huang, UCHL1 acts as a colorectal cancer oncogene via activation of the β-catenin/TCF pathway through its deubiquitinating activity. Int.J. Mol. Med. 30, 430–436 (2012)
S. Haq, S. Das, D.H. Kim, A.P. Chandrasekaran, S.H. Hong, K.S. Kim, S. Ramakrishna, The stability and oncogenic function of LIN28A are regulated by USP28. Biochim. Biophys. Acta Mol. Basis Dis. 1865(3), 599–610 (2019)
A.P. Chandrasekaran, B. Suresh, N. Sarodaya, N.R. Ko, S.J. Oh, K.S. Kim, S. Ramakrishna, Ubiquitin specific protease 29 functions as an oncogene promoting tumorigenesis in colorectal carcinoma. Cancers 13(11), 2706 (2021)
W. Xu, B. Chen, D. Ke, X. Chen, DUSP4 directly deubiquitinates and stabilizes Smad4 protein, promoting proliferation and metastasis of colorectal cancer cells. Aging 12, 17634–17646 (2020)
L. Yu, L. Dong, Y. Wang, L. Liu, H. Long, H. Li, J. Li, X. Yang, Z. Liu, G. Duan, X. Dai, Z. Lin, Reversible regulation of SATB1 ubiquitination by USP47 and SMURF2 mediates colon cancer cell proliferation and tumor progression. Cancer Lett. 448, 40–51 (2019)
B.J. Choi, S.A. Park, S.Y. Lee, Y.N. Cha, Y.J. Surh, Hypoxia induces epithelial-mesenchymal transition in colorectal cancer cells through ubiquitin-specific protease 47-mediated stabilization of Snail: a potential role of Sox9. Sci. Rep. 7, 15918 (2017)
J.M. Fraile, D. Campos-Iglesias, F. Rodríguez, Y. Español, J.M. Freije, The deubiquitinase USP54 is overexpressed in colorectal cancer stem cells and promotes intestinal tumorigenesis. Oncotarget 7, 74427–74434 (2016)
X. Chen, W. Wang, Y. Li, Y. Huo, H. Zhang, F. Feng, W. Xi, T. Zhang, J. Gao, F. Yang, S. Chen, A. Yang, T. Wang, MYSM1 inhibits human colorectal cancer tumorigenesis by activating miR-200 family members/CDH1 and blocking PI3K/AKT signaling. J. Exp. Clin. Cancer Res. 40, 341 (2021)
K. Watanabe, S. Yokoyama, N. Kaneto, T. Hori, Y. Iwakami, S. Kato, Y. Hayakawa, H. Sakurai, J. Fukuoka, I. Saiki, COP9 signalosome subunit 5 regulates cancer metastasis by deubiquitinating SNAIL. Oncotarget 9, 20670–20680 (2018)
Q. Li, Q. Chao, Y. Liu, J. Fang, J. Xie, J. Zhen, Y. Ding, B. Fu, Y. Ke, F. Xiao, H. Wu, Z. Huang, H. Hao, D. Huang, Deubiquitinase ZRANB1 drives hepatocellular carcinoma progression through SP1-LOXL2 axis. Am. J. Cancer Res. 11, 4807–4825 (2021)
Y. Liao, Z. Shao, Y. Liu, X. Xia, Y. Deng, C. Yu, W. Sun, W. Kong, X. He, F. Liu, Z. Guo, G. Chen, D. Tang, H. Gan, J. Liu, H. Huang, USP1-dependent RPS16 protein stability drives growth and metastasis of human hepatocellular carcinoma cells. J. Exp. Clin. Cancer Res. 40, 201 (2021)
Y. Li, Y. Xu, C. Gao, Y. Sun, K. Zhou, P. Wang, J. Cheng, W. Guo, C. Ya, J. Fan, X. Yang, USP1 maintains the survival of liver circulating tumor cells by deubiquitinating and stabilizing TBLR1. Front. Oncol. 10, 554809 (2020)
B. Xiong, J. Huang, Y. Liu, M. Zou, Z. Zhao, J. Gong, X. Wu, C. Qiu, Ubiquitin-specific protease 2a promotes hepatocellular carcinoma progression via deubiquitination and stabilization of RAB1A. Cell Oncol. (Dordr.) 44, 329–343 (2021)
C. Qiu, Y. Liu, Y. Mei, M. Zou, Z. Zhao, M. Ye, X. Wu, Ubiquitin-specific protease 4 promotes metastasis of hepatocellular carcinoma by increasing TGF-β signaling-induced epithelial-mesenchymal transition. Aging 10, 2783–2799 (2018)
T. Li, B. Yan, Y. Ma, J. Weng, S. Yang, N. Zhao, X. Wang, X. Sun, Ubiquitin-specific protease 4 promotes hepatocellular carcinoma progression via cyclophilin A stabilization and deubiquitination. Cell Death Dis. 9, 148 (2018)
M.J. Kim, B. Choi, J.Y. Kim, Y. Min, D.H. Kwon, J. Son, J.S. Lee, J.S. Lee, E. Chun, K.Y. Lee, USP8 regulates liver cancer progression via the inhibition of TRAF6-mediated signal for NF-κB activation and autophagy induction by TLR4. Transl. Oncol. 15, 101250 (2022)
T. Yuan, Z. Chen, F. Yan, M. Qian, H. Luo, S. Ye, J. Cao, M. Ying, X. Dai, R. Gai, B. Yang, Q. He, H. Zhu, Deubiquitinating enzyme USP10 promotes hepatocellular carcinoma metastasis through deubiquitinating and stabilizing Smad4 protein. Mol. Oncol. 14, 197–210 (2020)
H. Zhu, F. Yan, T. Yuan, M. Qian, T. Zhou, X. Dai, J. Cao, M. Ying, X. Dong, Q. He, B. Yang, USP10 promotes proliferation of hepatocellular carcinoma by deubiquitinating and stabilizing YAP/TAZ. Cancer Res. 80, 2204–2216 (2020)
L. Qiao, Q. Zhang, Z. Sun, Q. Liu, Z. Wu, W. Hu, S. Bao, Q. Yang, L. Liu, The E2F1/USP11 positive feedback loop promotes hepatocellular carcinoma metastasis and inhibits autophagy by activating ERK/mTOR pathway. Cancer Lett. 514, 63–78 (2021)
C. Zhang, C. Xie, X. Wang, Y. Huang, S. Gao, J. Lu, Y. Lu, S. Zhang, Aberrant USP11 expression regulates NF90 to promote proliferation and metastasis in hepatocellular carcinoma. Am. J. Cancer Res. 10, 1416–1428 (2020)
S. Gao, T. Chen, L. Li, X. Liu, Y. Liu, J. Zhao, Q. Lu, Z. Zeng, Q. Xu, D. Huang, K. Tu, Hypoxia-inducible ubiquitin specific peptidase 13 contributes to tumor growth and metastasis via enhancing the toll-like receptor 4/myeloid differentiation primary response gene 88/nuclear factor-κB pathway in hepatocellular carcinoma. Front. Cell Dev. Biol. 8, 587389 (2020)
Y. Zhang, J. Jia, W. Jin, J. Cao, T. Fu, D. Ma, Y. Zhang, Lidocaine inhibits the proliferation and invasion of hepatocellular carcinoma by downregulating USP14 induced PI3K/Akt pathway. Pathol. Res. Pract. 216, 152963 (2020)
Y. Qiu, D. Huang, Y. Sheng, J. Huang, N. Li, S. Zhang, Z. Hong, X. Yin, J. Yan, Deubiquitinating enzyme USP46 suppresses the progression of hepatocellular carcinoma by stabilizing MST1. Exp. Cell. Res. 405, 112646 (2021)
M. Huang, H. Xiong, D. Luo, B. Xu, H. Liu, CSN5 upregulates glycolysis to promote hepatocellular carcinoma metastasis via stabilizing the HK2 protein. Exp. Cell. Res. 388, 111876 (2020)
Q. Ni, J. Chen, X. Li, X. Xu, N. Zhang, A. Zhou, B. Zhou, Q. Lu, Z. Chen, Expression of OTUB1 in hepatocellular carcinoma and its effects on HCC cell migration and invasion. Acta Biochim. Biophys. Sin. 49, 680–688 (2017)
P. Xie, Y. Chen, H. Zhang, G. Zhou, Q. Chao, J. Wang, Y. Liu, J. Fang, J. Xie, J. Zhen, Z. Wang, L. Hao, D. Huang, The deubiquitinase OTUD3 stabilizes ACTN4 to drive growth and metastasis of hepatocellular carcinoma. Aging 13, 19317–19338 (2021)
C. Qiu, K. Liu, S. Zhang, S. Gao, W. Chen, D. Li, Y. Huang, Bisdemethoxycurcumin inhibits hepatocellular carcinoma proliferation through Akt inactivation via CYLD-mediated deubiquitination. Drug Des. Devel. Ther. 14, 993–1001 (2020)
J. Lv, S. Zhang, H. Wu, J. Lu, Y. Lu, F. Wang, W. Zhao, P. Zhan, J. Lu, Q. Fang, C. Xie, Z. Yin, Deubiquitinase PSMD14 enhances hepatocellular carcinoma growth and metastasis by stabilizing GRB2. Cancer Lett. 469, 22–34 (2020)
B. Wang, X. Xu, Z. Yang, L. Zhang, Y. Liu, A. Ma, G. Xu, M. Tang, T. Jing, L. Wu, Y. Liu, POH1 contributes to hyperactivation of TGF-β signaling and facilitates hepatocellular carcinoma metastasis through deubiquitinating TGF-β receptors and caveolin-1. EBioMedicine 41, 320–332 (2019)
Y. Zhu, C. Qu, X. Hong, Y. Jia, M. Lin, Y. Luo, F. Lin, X. Xie, X. Xie, J. Huang, Q. Wu, X. Qiu, D. Piao, Y. Xing, T. Yu, Y. Lu, Q. Huang, C. Yu, J. Jin, Z. Zhang, Trabid inhibits hepatocellular carcinoma growth and metastasis by cleaving RNF8-induced K63 ubiquitination of Twist1. Cell Death Differ. 26, 306–320 (2019)
J. Li, X. Xiao, H. Wang, W. Wang, Y. Ou, Z. Wang, H. Jiang, Y. Liu, Z. Zhang, S. Yang, CDK4/6-USP51 axis regulates lung adenocarcinoma metastasis through ZEB1. Cancer Gene Ther. 29(8–9), 1181–1192 (2022)
J. Cai, M. Li, X. Wang, L. Li, Q. Li, Z. Hou, H. Jia, S. Liu, USP37 promotes lung cancer cell migration by stabilizing snail protein via deubiquitination. Front. Genet. 10, 1324 (2019)
J. Yuan, G. Zhang, X. Li, Q. Ma, W. Cheng, W. Wang, B. Zhang, T. Hu, G. Song, Knocking down USP39 inhibits the growth and metastasis of non-small-cell lung cancer cells through activating the p53 pathway. Int. J. Mol. Sci. 21(23), 8949 (2020)
J. Ji, S. Yang, L. Zu, Y. Li, Y. Li, Deubiquitinating enzyme USP41 promotes lung cancer cell proliferation and migration. Thorac. Cancer 12, 1041–1047 (2021)
B. Zhang, C. Yang, R. Wang, J. Wu, Y. Zhang, D. Liu, X. Sun, X. Li, H. Ren, S. Qin, OTUD7B suppresses Smac mimetic-induced lung cancer cell invasion and migration via deubiquitinating TRAF3. J. Exp. Clin. Cancer Res. 39, 244 (2020)
F. Li, Q. Hu, T. He, J. Xu, Y. Yi, S. Xie, L. Ding, M. Fu, R. Guo, Z.J. Xiao, M. Niu, The deubiquitinase USP4 stabilizes Twist1 protein to promote lung cancer cell stemness. Cancers 12(6), 1582 (2020)
E.J. Jang, J.Y. Sung, H.E. Yoo, H. Jang, J. Shim, E.S. Oh, S.H. Goh, Y.N. Kim, FAM188B downregulation sensitizes lung cancer cells to anoikis via EGFR downregulation and inhibits tumor metastasis in vivo. Cancers 13(2), 247 (2021)
X.Y. Kang, J. Zhang, L. Tang, L. Huang, J. Tong, Q. Fu, OTU deubiquitinase 5 inhibits the progression of non-small cell lung cancer via regulating p53 and PDCD5. Chem. Biol. Drug Des. 96, 790–800 (2020)
R. Yang, N. Liu, L. Chen, Y. Jiang, Y. Shi, C. Mao, Y. Liu, M. Wang, W. Lai, H. Tang, M. Gao, D. Xiao, X. Wang, H. Zhou, C.E. Tang, W. Liu, F. Yu, Y. Cao, Q. Yan, S. Liu, Y. Tao, GIAT4RA functions as a tumor suppressor in non-small cell lung cancer by counteracting Uchl3-mediated deubiquitination of LSH. Oncogene 38, 7133–7145 (2019)
T. Wang, B. Jing, B. Sun, Y. Liao, H. Song, D. Xu, W. Guo, K. Li, M. Hu, S. Liu, J. Ling, Y. Kuang, Y. Feng, B.P. Zhou, J. Deng, Stabilization of PTGES by deubiquitinase USP9X promotes metastatic features of lung cancer via PGE(2) signaling. Am. J. Cancer Res. 9, 1145–1160 (2019)
J. Li, D. Cheng, M. Zhu, H. Yu, Z. Pan, L. Liu, Q. Geng, H. Pan, M. Yan, M. Yao, OTUB2 stabilizes U2AF2 to promote the Warburg effect and tumorigenesis via the AKT/mTOR signaling pathway in non-small cell lung cancer. Theranostics 9, 179–195 (2019)
Y. Wang, S. Zhang, H. Bao, S. Mu, B. Zhang, H. Ma, S. Ma, MicroRNA-365 promotes lung carcinogenesis by downregulating the USP33/SLIT2/ROBO1 signalling pathway. Cancer Cell Int. 18, 64 (2018)
Q. Luo, X. Wu, Y. Nan, W. Chang, P. Zhao, Y. Zhang, D. Su, Z. Liu, TRIM32/USP11 balances ARID1A stability and the oncogenic/tumor-suppressive status of squamous cell carcinoma. Cell Rep. 30, 98–111.e115 (2020)
T. Zhang, J. Zheng, L. Qiao, W. Zhao, Deubiquitinase USP13 promotes the epithelial-mesenchymal transition and metastasis in gastric cancer by maintaining Snail protein. Pathol. Res. Pract. 229, 153705 (2022)
N. Li, L. Wu, X. Zuo, H. Luo, Y. Sheng, J. Yan, USP1 promotes GC metastasis via stabilizing ID2. Dis. Markers 2021, 3771990 (2021)
Z. Zhang, X. Hu, J. Kuang, J. Liao, Q. Yuan, LncRNA DRAIC inhibits proliferation and metastasis of gastric cancer cells through interfering with NFRKB deubiquitination mediated by UCHL5. Cell. Mol. Biol. Lett. 25, 29 (2020)
X. Wu, M. Liu, H. Zhu, J. Wang, W. Dai, J. Li, D. Zhu, W. Tang, Y. Xiao, J. Lin, W. Zhang, Y. Sun, Y. Zhang, Y. Chen, G. Li, A. Li, L. Xiang, S. Liu, J. Wang, Ubiquitin-specific protease 3 promotes cell migration and invasion by interacting with and deubiquitinating SUZ12 in gastric cancer. J. Exp. Clin. Cancer Res. 38, 277 (2019)
Y. Xia, L. Wang, Z. Xu, R. Kong, F. Wang, K. Yin, J. Xu, B. Li, Z. He, L. Wang, H. Xu, D. Zhang, L. Yang, J.Y. Wu, Z. Xu, Reduced USP33 expression in gastric cancer decreases inhibitory effects of Slit2-Robo1 signalling on cell migration and EMT. Cell Prolif. 52, e12606 (2019)
L.J. Zhao, T. Zhang, X.J. Feng, J. Chang, F.Z. Suo, J.L. Ma, Y.J. Liu, Y. Liu, Y.C. Zheng, H.M. Liu, USP28 contributes to the proliferation and metastasis of gastric cancer. J. Cell Biochem. 120(5), 7657–7666 (2018)
K. Hou, Z. Zhu, Y. Wang, C. Zhang, S. Yu, Q. Zhu, B. Yan, Overexpression and biological function of ubiquitin-specific protease 42 in gastric cancer. PLoS ONE 11, e0152997 (2016)
Y.Y. Gu, M. Yang, M. Zhao, Q. Luo, L. Yang, H. Peng, J. Wang, S.K. Huang, Z.X. Zheng, X.H. Yuan, P. Liu, C.Z. Huang, The de-ubiquitinase UCHL1 promotes gastric cancer metastasis via the Akt and Erk1/2 pathways. Tumour Biol. 36, 8379–8387 (2015)
K. Shi, J.Z. Zhang, L. Yang, N.N. Li, Y. Yue, X.H. Du, X.Z. Zhang, Y.C. Lu, D. Guo, Protein deubiquitylase USP3 stabilizes Aurora A to promote proliferation and metastasis of esophageal squamous cell carcinoma. BMC Cancer 21, 1196 (2021)
C. Jing, X. Li, M. Zhou, S. Zhang, Q. Lai, D. Liu, B. Ye, L. Li, Y. Wu, H. Li, K. Yue, P. Chen, X. Yao, Y. Wu, Y. Duan, X. Wang, The PSMD14 inhibitor Thiolutin as a novel therapeutic approach for esophageal squamous cell carcinoma through facilitating SNAIL degradation. Theranostics 11, 5847–5862 (2021)
L. Li, H. Zhou, R. Zhu, Z. Liu, USP26 promotes esophageal squamous cell carcinoma metastasis through stabilizing Snail. Cancer Lett. 448, 52–60 (2019)
X. Guo, R. Zhu, A. Luo, H. Zhou, F. Ding, H. Yang, Z. Liu, EIF3H promotes aggressiveness of esophageal squamous cell carcinoma by modulating Snail stability. J. Exp. Clin. Cancer Res. 39, 175 (2020)
C. Song, J. Peng, Y. Wei, J. Shao, X. Chen, X. Zhang, J. Xu, USP18 promotes tumor metastasis in esophageal squamous cell carcinomas via deubiquitinating ZEB1. Exp. Cell. Res. 409, 112884 (2021)
M. Tian, R. Zhu, F. Ding, Z. Liu, Ubiquitin-specific peptidase 46 promotes tumor metastasis through stabilizing ENO1 in human esophageal squamous cell carcinoma. Exp. Cell. Res. 395, 112188 (2020)
J. Sun, Y. Deng, J. Shi, W. Yang, MicroRNA-542-3p represses OTUB1 expression to inhibit migration and invasion of esophageal cancer cells. Mol. Med. Rep. 21, 35–42 (2020)
W. Wang, J. Wang, H. Yan, K. Zhang, Y. Liu, Upregulation of USP11 promotes epithelial-to-mesenchymal transition by deubiquitinating Snail in ovarian cancer. Oncol. Rep. 41, 1739–1748 (2019)
R. Zhu, Y. Liu, H. Zhou, L. Li, Y. Li, F. Ding, X. Cao, Z. Liu, Deubiquitinating enzyme PSMD14 promotes tumor metastasis through stabilizing SNAIL in human esophageal squamous cell carcinoma. Cancer Lett. 418, 125–134 (2018)
X. Lei, X. Li, H. Chen, Z. Liu, USP48 sustains chemoresistance and metastasis in ovarian cancer. Curr. Cancer Drug Targets 20, 689–699 (2020)
K. Hong, L. Hu, X. Liu, J.M. Simon, T.S. Ptacek, X. Zheng, C. Liao, A.S. Baldwin, Q. Zhang, USP37 promotes deubiquitination of HIF2α in kidney cancer. Proc. Natl. Acad. Sci. U. S. A. 117, 13023–13032 (2020)
X. Meng, Z. Xiong, W. Xiao, C. Yuan, C. Wang, Y. Huang, J. Tong, J. Shi, Z. Chen, C. Liu, K. Xie, H. Xiong, K. Chen, H. Yang, X. Zhang, Downregulation of ubiquitin-specific protease 2 possesses prognostic and diagnostic value and promotes the clear cell renal cell carcinoma progression. Ann. Transl. Med. 8, 319 (2020)
D. Gui, Z. Dong, W. Peng, W. Jiang, G. Huang, G. Liu, Z. Ye, Y. Wang, Z. Xu, J. Fu, S. Luo, Y. Zhao, Ubiquitin-specific peptidase 53 inhibits the occurrence and development of clear cell renal cell carcinoma through NF-κB pathway inactivation. Cancer Med. 10, 3674–3688 (2021)
E. Kobayashi, D. Hwang, A. Bheda-Malge, C.B. Whitehurst, A.V. Kabanov, S. Kondo, M. Aga, T. Yoshizaki, J.S. Pagano, M. Sokolsky, J. Shakelford, Inhibition of UCH-L1 deubiquitinating activity with two forms of LDN-57444 has anti-invasive effects in metastatic carcinoma cells. Int. J. Mol. Sci. 20(15), 3733 (2019)
L. Feng, J. Zhang, M. Sun, F. Qiu, W. Chen, W. Qiu, Tumor suppressor LINC02487 inhibits oral squamous cell carcinoma cell migration and invasion through the USP17-SNAI1 axis. Front. Oncol. 10, 559808 (2020)
W.L. Ge, J.F. Xu, J. Hu, Regulation of oral squamous cell carcinoma proliferation through crosstalk between SMAD7 and CYLD. Cell Physiol. Biochem. 38, 1209–1217 (2016)
S. Yokoyama, Y. Iwakami, Z. Hang, R. Kin, Y. Zhou, Y. Yasuta, A. Takahashi, Y. Hayakawa, H. Sakurai, Targeting PSMD14 inhibits melanoma growth through SMAD3 stabilization. Sci. Rep. 10, 19214 (2020)
Y. Iwakami, S. Yokoyama, K. Watanabe, Y. Hayakawa, STAM-binding protein regulates melanoma metastasis through SLUG stabilization. Biochem. Biophys. Res. Commun. 507, 484–488 (2018)
W. Guo, J. Ma, T. Pei, T. Zhao, S. Guo, X. Yi, Y. Liu, S. Wang, G. Zhu, Z. Jian, T. Gao, C. Li, W. Liao, Q. Shi, Up-regulated deubiquitinase USP4 plays an oncogenic role in melanoma. J. Cell Mol. Med. 22, 2944–2954 (2018)
M. Zhan, X. Sun, J. Liu, Y. Li, Y. Li, X. He, Z. Zhou, L. Lu, Usp7 promotes medulloblastoma cell survival and metastasis by activating Shh pathway. Biochem. Biophys. Res. Commun. 484, 429–434 (2017)
H. Ke, C.K. Augustine, V.D. Gandham, J.Y. Jin, D.S. Tyler, S.K. Akiyama, R.P. Hall, J.Y. Zhang, CYLD inhibits melanoma growth and progression through suppression of the JNK/AP-1 and β1-integrin signaling pathways. J. Invest. Dermatol. 133, 221–229 (2013)
R. Massoumi, S. Kuphal, C. Hellerbrand, B. Haas, P. Wild, T. Spruss, A. Pfeifer, R. Fässler, A.K. Bosserhoff, Down-regulation of CYLD expression by Snail promotes tumor progression in malignant melanoma. J. Exp. Med. 206, 221–232 (2009)
H.J. Kim, V. Magesh, J.J. Lee, S. Kim, U.G. Knaus, K.J. Lee, Ubiquitin C-terminal hydrolase-L1 increases cancer cell invasion by modulating hydrogen peroxide generated via NADPH oxidase 4. Oncotarget 6, 16287–16303 (2015)
H.T. Wu, Y.C. Kuo, J.J. Hung, C.H. Huang, W.Y. Chen, T.Y. Chou, Y. Chen, Y.J. Chen, Y.J. Chen, W.C. Cheng, S.C. Teng, K.J. Wu, K63-polyubiquitinated HAUSP deubiquitinates HIF-1α and dictates H3K56 acetylation promoting hypoxia-induced tumour progression. Nat. Commun. 7, 13644 (2016)
J.E. Lee, C.M. Park, J.H. Kim, USP7 deubiquitinates and stabilizes EZH2 in prostate cancer cells. Genet. Mol. Biol. 43, e20190338 (2020)
Y. Wang, Z. Ou, Y. Sun, S. Yeh, X. Wang, J. Long, C. Chang, Androgen receptor promotes melanoma metastasis via altering the miRNA-539-3p/USP13/MITF/AXL signals. Oncogene 36, 1644–1654 (2017)
H.M. Song, J.E. Lee, J.H. Kim, Ubiquitin C-terminal hydrolase-L3 regulates EMT process and cancer metastasis in prostate cell lines. Biochem. Biophys. Res. Commun. 452, 722–727 (2014)
S. Lei, Z. He, T. Chen, X. Guo, Z. Zeng, Y. Shen, J. Jiang, Long noncoding RNA 00976 promotes pancreatic cancer progression through OTUD7B by sponging miR-137 involving EGFR/MAPK pathway. J. Exp. Clin. Cancer Res. 38, 470 (2019)
F. Ma, H. Wang, K. Liu, Z. Wang, S. Chen, CSN6 inhibition suppresses pancreatic adenocarcinoma metastasis via destabilizing the c-Fos protein. Exp. Cell. Res. 391, 112004 (2020)
J. Li, H. Li, W. Zhu, B. Zhou, J. Ying, J. Wu, H. Zhang, H. Sun, S. Gao, Deubiquitinase inhibitor degrasyn suppresses metastasis by targeting USP5-WT1-E-cadherin signalling pathway in pancreatic ductal adenocarcinoma. J. Cell Mol. Med. 24, 1370–1382 (2020)
J. Lian, C. Liu, X. Guan, B. Wang, Y. Yao, D. Su, Y. Ma, L. Fang, Y. Zhang, Ubiquitin specific peptidase 5 enhances STAT3 signaling and promotes migration and invasion in Pancreatic Cancer. J. Cancer 11, 6802–6811 (2020)
X. Liu, B. Chen, J. Chen, Z. Su, S. Sun, Deubiquitinase USP10 maintains Cyr61 expression via YAP1 to augment immune escape and metastasis of PAAD. Cancer Sci. 113(5), 1868–1879 (2022)
Y. Li, J. Zhou, USP5 promotes uterine corpus endometrial carcinoma cell growth and migration via mTOR/4EBP1 activation. Cancer Manag. Res. 13, 3913–3924 (2021)
P.P. Han, G.Q. Zhang, L. Li, L. Yue, Downregulation of USP33 inhibits Slit/Robo signaling pathway and is associated with poor patient survival of glioma. J. Neurosurg. Sci. (2020)
S.A. Vilchez Mercedes, F. Bocci, H. Levine, J.N. Onuchic, M.K. Jolly, P.K. Wong, Decoding leader cells in collective cancer invasion. Nat. Rev. Cancer 21, 592–604 (2021)
A. Lardennois, G. Pásti, T. Ferraro, F. Llense, P. Mahou, J. Pontabry, D. Rodriguez, S. Kim, S. Ono, E. Beaurepaire, C. Gally, M. Labouesse, An actin-based viscoplastic lock ensures progressive body-axis elongation. Nature 573, 266–270 (2019)
J. Gao, F. Nakamura, Actin-associated proteins and small molecules targeting the actin cytoskeleton. Int. J. Mol. Sci. 23(4), 2118 (2022)
C. Mondal, J.S. Di Martino, J.J. Bravo-Cordero, Actin dynamics during tumor cell dissemination. Int. Rev. Cell Mol. Biol. 360, 65–98 (2021)
A.J. Ridley, Life at the leading edge. Cell 145, 1012–1022 (2011)
A. Callan-Jones, Self-organization in amoeboid motility. Front. Cell Dev. Biol. 10, 1000071 (2022)
Y.J. Liu, M. Le Berre, F. Lautenschlaeger, P. Maiuri, A. Callan-Jones, M. Heuzé, T. Takaki, R. Voituriez, M. Piel, Confinement and low adhesion induce fast amoeboid migration of slow mesenchymal cells. Cell 160, 659–672 (2015)
M. Lintz, A. Muñoz, C.A. Reinhart-King, The mechanics of single cell and collective migration of tumor cells. J. Biomech. Eng. 139, 0210051–0210059 (2017)
R. Mayor, S. Etienne-Manneville, The front and rear of collective cell migration. Nat. Rev. Mol. Cell Biol. 17, 97–109 (2016)
K. Skruber, T.A. Read, E.A. Vitriol, Reconsidering an active role for G-actin in cytoskeletal regulation. J. Cell Sci. 131(1), jcs203760 (2018)
A. Gaertner, A. Wegner, Mechanism of the insertion of actin monomers between the barbed ends of actin filaments and barbed end-bound insertin. J. Muscle Res. Cell. Motil. 12, 27–36 (1991)
R. Dominguez, K.C. Holmes, Actin structure and function. Annu. Rev. Biophys. 40, 169–186 (2011)
C.A. Schoenenberger, H.G. Mannherz, B.M. Jockusch, Actin: from structural plasticity to functional diversity. Eur. J. Cell Biol. 90, 797–804 (2011)
E.D. Korn, M.F. Carlier, D. Pantaloni, Actin polymerization and ATP hydrolysis. Science 238, 638–644 (1987)
C.G. Dos Remedios, D. Chhabra, M. Kekic, I.V. Dedova, M. Tsubakihara, D.A. Berry, N.J. Nosworthy, Actin binding proteins: regulation of cytoskeletal microfilaments. Physiol Rev. 83, 433–473 (2003)
T.M. Svitkina, G.G. Borisy, Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J. Cell Biol. 145, 1009–1026 (1999)
D. Pantaloni, R. Boujemaa, D. Didry, P. Gounon, M.F. Carlier, The Arp2/3 complex branches filament barbed ends: functional antagonism with capping proteins. Nat. Cell Biol. 2, 385–391 (2000)
A.W. Kinley, S.A. Weed, A.M. Weaver, A.V. Karginov, E. Bissonette, J.A. Cooper, J.T. Parsons, Cortactin interacts with WIP in regulating Arp2/3 activation and membrane protrusion. Curr. Biol. 13, 384–393 (2003)
A. Bisaria, A. Hayer, D. Garbett, D. Cohen, T. Meyer, Membrane-proximal F-actin restricts local membrane protrusions and directs cell migration. Science 368, 1205–1210 (2020)
C. Chan, C.C. Beltzner, T.D. Pollard, Cofilin dissociates Arp2/3 complex and branches from actin filaments. Curr. Biol. 19, 537–545 (2009)
Q. Chen, T.D. Pollard, Actin filament severing by cofilin dismantles actin patches and produces mother filaments for new patches. Curr. Biol. 23, 1154–1162 (2013)
Z. Ostrowska, J. Moraczewska, Cofilin - a protein controlling dynamics of actin filaments. Postepy Hig. Med. Dosw. (Online) 71, 339–351 (2017)
V. DesMarais, M. Ghosh, R. Eddy, J. Condeelis, Cofilin takes the lead. J. Cell Sci. 118, 19–26 (2005)
S. Seetharaman, S. Etienne-Manneville, Cytoskeletal crosstalk in cell migration. Trends Cell Biol. 30, 720–735 (2020)
Y. Moshfegh, J.J. Bravo-Cordero, V. Miskolci, J. Condeelis, L. Hodgson, A Trio-Rac1-Pak1 signalling axis drives invadopodia disassembly. Nat. Cell Biol. 16, 574–586 (2014)
M. Krause, A. Gautreau, Steering cell migration: lamellipodium dynamics and the regulation of directional persistence. Nat. Rev. Mol. Cell Biol. 15, 577–590 (2014)
K.M. Alblazi, C.H. Siar, Cellular protrusions–lamellipodia, filopodia, invadopodia and podosomes–and their roles in progression of orofacial tumours: current understanding. Asian Pac. J Cancer Prev. 16, 2187–2191 (2015)
A.J. Ridley, Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 16, 522–529 (2006)
E. Foxall, A. Pipili, G.E. Jones, C.M. Wells, Significance of kinase activity in the dynamic invadosome. Eur. J. Cell Biol. 95, 483–492 (2016)
S. Etienne-Manneville, A. Hall, Rho GTPases in cell biology. Nature 420, 629–635 (2002)
Y. Yoo, H.J. Ho, C. Wang, J.L. Guan, Tyrosine phosphorylation of cofilin at Y68 by v-Src leads to its degradation through ubiquitin-proteasome pathway. Oncogene 29, 263–272 (2010)
A. Deglincerti, Y. Liu, D. Colak, U. Hengst, G. Xu, S.R. Jaffrey, Coupled local translation and degradation regulate growth cone collapse. Nat. Commun. 6, 6888 (2015)
X. Ma, Y. Dang, X. Shao, X. Chen, F. Wu, Y. Li, Ubiquitination and long non-coding RNAs regulate actin cytoskeleton regulators in cancer progression. Int. J. Mol. Sci. 20(12), 2997 (2019)
S. Hussain, Y. Zhang, P.J. Galardy, DUBs and cancer: the role of deubiquitinating enzymes as oncogenes, non-oncogenes and tumor suppressors. Cell Cycle 8, 1688–1697 (2009)
R. Pfoh, I.K. Lacdao, V. Saridakis, Deubiquitinases and the new therapeutic opportunities offered to cancer. Endocr.-Relat. Cancer 22, T35–54 (2015)
Y. Xue, M. Li, J. Hu, Y. Song, W. Guo, C. Miao, D. Ge, Y. Hou, X. Wang, X. Huang, T. Liu, X. Zhang, Q. Huang, Ca(v)2.2-NFAT2-USP43 axis promotes invadopodia formation and breast cancer metastasis through cortactin stabilization. Cell Death Dis. 13, 812 (2022)
K. Itoh, K. Yoshioka, H. Akedo, M. Uehata, T. Ishizaki, S. Narumiya, An essential part for Rho-associated kinase in the transcellular invasion of tumor cells. Nat. Med. 5, 221–225 (1999)
R.G. Hodge, A.J. Ridley, Regulating Rho GTPases and their regulators. Nat. Rev. Mol. Cell Biol. 17, 496–510 (2016)
J. Wei, R.K. Mialki, S. Dong, A. Khoo, R.K. Mallampalli, Y. Zhao, J. Zhao, A new mechanism of RhoA ubiquitination and degradation: roles of SCF(FBXL19) E3 ligase and Erk2. Biochim. Biophys. Acta 1833, 2757–2764 (2013)
T.K. Oberoi, T. Dogan, J.C. Hocking, R.P. Scholz, J. Mooz, C.L. Anderson, C. Karreman, D. Meyer Zu Heringdorf, G. Schmidt, M. Ruonala, K. Namikawa, G.S. Harms, A. Carpy, B. Macek, R.W. Köster, K. Rajalingam, IAPs regulate the plasticity of cell migration by directly targeting Rac1 for degradation. EMBO J. 31, 14–28 (2012)
J. Zhao, R.K. Mialki, J. Wei, T.A. Coon, C. Zou, B.B. Chen, R.K. Mallampalli, Y. Zhao, SCF E3 ligase F-box protein complex SCF(FBXL19) regulates cell migration by mediating Rac1 ubiquitination and degradation. FASEB J. 27, 2611–2619 (2013)
A. Murali, J. Shin, H. Yurugi, A. Krishnan, M. Akutsu, A. Carpy, B. Macek, K. Rajalingam, Ubiquitin-dependent regulation of Cdc42 by XIAP. Cell Death Dis. 8, e2900 (2017)
C. McFarlane, A.A. Kelvin, M. de la Vega, U. Govender, C.J. Scott, J.F. Burrows, J.A. Johnston, The deubiquitinating enzyme USP17 is highly expressed in tumor biopsies, is cell cycle regulated, and is required for G1-S progression. Cancer Res. 70, 3329–3339 (2010)
M. de la Vega, A.A. Kelvin, D.J. Dunican, C. McFarlane, J.F. Burrows, J. Jaworski, N.J. Stevenson, K. Dib, J.Z. Rappoport, C.J. Scott, A. Long, J.A. Johnston, The deubiquitinating enzyme USP17 is essential for GTPase subcellular localization and cell motility. Nat. Commun. 2, 259 (2011)
R. Massoumi, CYLD: a deubiquitination enzyme with multiple roles in cancer. Future Oncol. 7, 285–297 (2011)
J. Guo, S. Shinriki, Y. Su, T. Nakamura, M. Hayashi, Y. Tsuda, Y. Murakami, M. Tasaki, T. Hide, T. Takezaki, J. Kuratsu, S. Yamashita, M. Ueda, J.D. Li, Y. Ando, H. Jono, Hypoxia suppresses cylindromatosis (CYLD) expression to promote inflammation in glioblastoma: possible link to acquired resistance to anti-VEGF therapy. Oncotarget 5, 6353–6364 (2014)
E. Ghadami, N. Nikbakhsh, S. Fattahi, M. Kosari-Monfared, M. Ranaee, H. Taheri, F. Amjadi-Moheb, G. Godazandeh, S. Shafaei, A. Nosrati, M. Pilehchian Langroudi, A.A. Samadani, G. Amirbozorgi, V. Mirnia, H. Akhavan-Niaki, Epigenetic alterations of CYLD promoter modulate its expression in gastric adenocarcinoma: a footprint of infections. J. Cell Physiol. 234, 4115–4124 (2019)
J. Font-Burgada, E. Seki, M. Karin, CYLD and HCC: when being too sensitive to your dirty neighbors results in self-destruction. Cancer Cell 21, 711–712 (2012)
T. Orfanidou, K. Xanthopoulos, D. Dafou, A. Pseftogas, P. Hadweh, C. Psyllaki, E. Hatzivassiliou, G. Mosialos, Down-regulation of the tumor suppressor CYLD enhances the transformed phenotype of human breast cancer cells. Anticancer Res. 37, 3493–3503 (2017)
Z. Cui, H. Kang, J.R. Grandis, D.E. Johnson, CYLD alterations in the tumorigenesis and progression of human papillomavirus-associated head and neck cancers. Mol. Cancer Res. 19, 14–24 (2021)
Y. Ishikawa, K. Tsunoda, M. Shibazaki, K. Takahashi, T. Akasaka, T. Masuda, C. Maesawa, Downregulation of cylindromatosis gene, CYLD, confers a growth advantage on malignant melanoma cells while negatively regulating their migration activity. Int. J. Oncol. 41, 53–60 (2012)
J. Majolée, F. Podieh, P.L. Hordijk, I. Kovačević, The interplay of Rac1 activity, ubiquitination and GDI binding and its consequences for endothelial cell spreading. PLoS ONE 16, e0254386 (2021)
J. Gao, L. Sun, L. Huo, M. Liu, D. Li, J. Zhou, CYLD regulates angiogenesis by mediating vascular endothelial cell migration. Blood 115, 4130–4137 (2010)
Y. Yang, L. Sun, Tala, J. Gao, D. Li, J. Zhou, M. Liu, CYLD regulates RhoA activity by modulating LARG ubiquitination. PLoS ONE 8, e55833 (2013)
N.Z. Ghanem, M.L. Matter, J.W. Ramos, Regulation of leukaemia associated Rho GEF (LARG/ARHGEF12. Small GTPases 1–9 (2021)
D. Li, J. Gao, Y. Yang, L. Sun, S. Suo, Y. Luo, W. Shui, J. Zhou, M. Liu, CYLD coordinates with EB1 to regulate microtubule dynamics and cell migration. Cell Cycle 13, 974–983 (2014)
J.L. Henty-Ridilla, A. Rankova, J.A. Eskin, K. Kenny, B.L. Goode, Accelerated actin filament polymerization from microtubule plus ends. Science 352, 1004–1009 (2016)
B.J. Mathis, Y. Lai, C. Qu, J.S. Janicki, T. Cui, CYLD-mediated signaling and diseases. Curr. Drug Targets 16, 284–294 (2015)
M. Saldana, K. VanderVorst, A.L. Berg, H. Lee, K.L. Carraway, Otubain 1: a non-canonical deubiquitinase with an emerging role in cancer. Endocr.-Relat. Cancer 26, R1–r14 (2019)
P. Chen, H. Wang, W. Zhang, Y. Chen, Y. Lv, D. Wu, M. Guo, H. Deng, Loss of BAP1 results in growth inhibition and enhances mesenchymal-epithelial transition in kidney tumor cells. Mol. Cell. Proteomics 18, 1320–1329 (2019)
L.C. Kim, L. Song, E.B. Haura, Src kinases as therapeutic targets for cancer. Nat. Rev. Clin. Oncol. 6, 587–595 (2009)
K. Minoguchi, H. Kihara, H. Nishikata, M.M. Hamawy, R.P. Siraganian, Src family tyrosine kinase Lyn binds several proteins including paxillin in rat basophilic leukemia cells. Mol. Immunol. 31, 519–529 (1994)
D. Gianni, N. Taulet, C. DerMardirossian, G.M. Bokoch, c-Src-mediated phosphorylation of NoxA1 and Tks4 induces the reactive oxygen species (ROS)-dependent formation of functional invadopodia in human colon cancer cells. Mol. Biol. Cell 21, 4287–4298 (2010)
S.A. Courtneidge, E.F. Azucena, I. Pass, D.F. Seals, L. Tesfay, The SRC substrate Tks5, podosomes (invadopodia), and cancer cell invasion. Cold Spring Harb. Symp. Quant. Biol. 70, 167–171 (2005)
J.V. Evans, A.G. Ammer, J.E. Jett, C.A. Bolcato, J.C. Breaux, K.H. Martin, M.V. Culp, P.M. Gannett, S.A. Weed, Src binds cortactin through an SH2 domain cystine-mediated linkage. J. Cell Sci. 125, 6185–6197 (2012)
N. Martinez-Quiles, H.Y. Ho, M.W. Kirschner, N. Ramesh, R.S. Geha, Erk/Src phosphorylation of cortactin acts as a switch on-switch off mechanism that controls its ability to activate N-WASP. Mol. Cell Biol. 24, 5269–5280 (2004)
S. Tehrani, N. Tomasevic, S. Weed, R. Sakowicz, J.A. Cooper, Src phosphorylation of cortactin enhances actin assembly. Proc. Natl. Acad. Sci. U. S. A. 104, 11933–11938 (2007)
S. Huveneers, E.H. Danen, Adhesion signaling - crosstalk between integrins, Src and Rho. J. Cell Sci. 122, 1059–1069 (2009)
R. Roskoski Jr., Src kinase regulation by phosphorylation and dephosphorylation. Biochem. Biophys. Res. Commun. 331, 1–14 (2005)
M. Okada, Regulation of the SRC family kinases by Csk. Int. J. Biol. Sci. 8, 1385–1397 (2012)
Y.P. Chong, T.D. Mulhern, H.C. Cheng, C-terminal Src kinase (CSK) and CSK-homologous kinase (CHK)–endogenous negative regulators of Src-family protein kinases. Growth Factors 23, 233–244 (2005)
L. Moro, D. Simoneschi, E. Kurz, A.A. Arbini, S. Jang, N. Guaragnella, S. Giannattasio, W. Wang, Y.A. Chen, G. Pires, A. Dang, E. Hernandez, P. Kapur, A. Mishra, A. Tsirigos, G. Miller, J.T. Hsieh, M. Pagano, Epigenetic silencing of the ubiquitin ligase subunit FBXL7 impairs c-SRC degradation and promotes epithelial-to-mesenchymal transition and metastasis. Nat. Cell Biol. 22, 1130–1142 (2020)
A.P. McCann, P. Smyth, F. Cogo, W.J. McDaid, L. Jiang, J. Lin, E. Evergren, R.E. Burden, S. Van Schaeybroeck, C.J. Scott, J.F. Burrows, USP17 is required for trafficking and oncogenic signaling of mutant EGFR in NSCLC cells. Cell Commun. Signal. 16, 77 (2018)
R. Al-Mahdi, N. Babteen, K. Thillai, M. Holt, B. Johansen, H.L. Wetting, O.M. Seternes, C.M. Wells, A novel role for atypical MAPK kinase ERK3 in regulating breast cancer cell morphology and migration. Cell Adhes. Migr. 9, 483–494 (2015)
S. Vallabhaneni, J. Liu, M. Morel, J. Wang, F.J. DeMayo, W. Long, Conditional ERK3 overexpression cooperates with PTEN deletion to promote lung adenocarcinoma formation in mice. Mol. Oncol. 16(5), 1184–1199 (2021)
S. Mathien, P. Déléris, M. Soulez, L. Voisin, S. Meloche, Deubiquitinating enzyme USP20 regulates extracellular signal-regulated kinase 3 stability and biological activity. Mol. Cell Biol. 37, e00432-16 (2017)
N. Stöhr, M. Köhn, M. Lederer, M. Glass, C. Reinke, R.H. Singer, S. Hüttelmaier, IGF2BP1 promotes cell migration by regulating MK5 and PTEN signaling. Genes Dev. 26, 176–189 (2012)
O.M. Seternes, T. Mikalsen, B. Johansen, E. Michaelsen, C.G. Armstrong, N.A. Morrice, B. Turgeon, S. Meloche, U. Moens, S.M. Keyse, Activation of MK5/PRAK by the atypical MAP kinase ERK3 defines a novel signal transduction pathway. EMBO J. 23, 4780–4791 (2004)
L. Elkhadragy, H. Alsaran, W. Long, The C-terminus tail regulates ERK3 kinase activity and its ability in promoting cancer cell migration and invasion. Int. J. Mol. Sci. 21, 4044 (2020)
R. El-Merahbi, J.T. Viera, A.L. Valdes, K. Kolczynska, S. Reuter, M.C. Löffler, M. Erk, C.P. Ade, T. Karwen, A.E. Mayer, M. Eilers, G. Sumara, The adrenergic-induced ERK3 pathway drives lipolysis and suppresses energy dissipation. Genes Dev. 34, 495–510 (2020)
S. Schumacher, K. Laass, S. Kant, Y. Shi, A. Visel, A.D. Gruber, A. Kotlyarov, M. Gaestel, Scaffolding by ERK3 regulates MK5 in development. EMBO J. 23, 4770–4779 (2004)
W. Li, M. Shen, Y.Z. Jiang, R. Zhang, H. Zheng, Y. Wei, Z.M. Shao, Y. Kang, Deubiquitinase USP20 promotes breast cancer metastasis by stabilizing SNAI2. Genes Dev. 34, 1310–1315 (2020)
M. Zhu, H. Zhao, J. Liao, X. Xu, HERC2/USP20 coordinates CHK1 activation by modulating CLASPIN stability. Nucleic Acids Res. 42, 13074–13081 (2014)
I. Shanmugam, M. Abbas, F. Ayoub, S. Mirabal, M. Bsaili, E.K. Caulder, D.M. Weinstock, A.E. Tomkinson, R. Hromas, M. Shaheen, Ubiquitin-specific peptidase 20 regulates Rad17 stability, checkpoint kinase 1 phosphorylation and DNA repair by homologous recombination. J. Biol. Chem. 289, 22739–22748 (2014)
H. Badmos, N. Cobbe, A. Campbell, R. Jackson, D. Bennett, Drosophila USP22/nonstop polarizes the actin cytoskeleton during collective border cell migration. J. Cell Biol. 220, e202007005 (2021)
T.J. Stanek, V.J. Gennaro, M.A. Tracewell, D. Di Marcantonio, K.L. Pauley, S. Butt, C. McNair, F. Wang, A.V. Kossenkov, K.E. Knudsen, T. Butt, S.M. Sykes, S.B. McMahon, The SAGA complex regulates early steps in transcription via its deubiquitylase module subunit USP22. EMBO J. 40, e102509 (2021)
Y. Xie, M. Avello, M. Schirle, E. McWhinnie, Y. Feng, E. Bric-Furlong, C. Wilson, R. Nathans, J. Zhang, M.W. Kirschner, S.M. Huang, F. Cong, Deubiquitinase FAM/USP9X interacts with the E3 ubiquitin ligase SMURF1 protein and protects it from ligase activity-dependent self-degradation. J. Biol. Chem. 288, 2976–2985 (2013)
J.M. Lamar, P. Stern, H. Liu, J.W. Schindler, Z.G. Jiang, R.O. Hynes, The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc. Natl. Acad. Sci. U. S. A. 109, E2441–E2450 (2012)
H.J. Lee, M.F. Diaz, K.M. Price, J.A. Ozuna, S. Zhang, E.M. Sevick-Muraca, J.P. Hagan, P.L. Wenzel, Fluid shear stress activates YAP1 to promote cancer cell motility. Nat. Commun. 8, 14122 (2017)
Y. Qiao, J. Chen, Y.B. Lim, M.L. Finch-Edmondson, V.P. Seshachalam, L. Qin, T. Jiang, B.C. Low, H. Singh, C.T. Lim, M. Sudol, YAP regulates actin dynamics through ARHGAP29 and promotes metastasis. Cell Rep. 19, 1495–1502 (2017)
A. Prasad, V. Paruchuri, A. Preet, F. Latif, R.K. Ganju, Slit-2 induces a tumor-suppressive effect by regulating beta-catenin in breast cancer cells. J. Biol. Chem. 283, 26624–26633 (2008)
T.S. Chiang, M.C. Lin, M.C. Tsai, C.H. Chen, L.T. Jang, F.S. Lee, ADP-ribosylation factor-like 4A interacts with Robo1 to promote cell migration by regulating Cdc42 activation. Mol. Biol. Cell 30, 69–81 (2019)
Y. Wang, H.L. Teng, Z.H. Huang, Repulsive migration of Schwann cells induced by Slit-2 through Ca2+-dependent RhoA-myosin signaling. Glia 61, 710–723 (2013)
A.R. Anand, H. Zhao, T. Nagaraja, L.A. Robinson, R.K. Ganju, N-terminal Slit2 inhibits HIV-1 replication by regulating the actin cytoskeleton. Retrovirology 10, 2 (2013)
A. Prasad, P.M. Kuzontkoski, A. Shrivastava, W. Zhu, D.Y. Li, J.E. Groopman, Slit2N/Robo1 inhibit HIV-gp120-induced migration and podosome formation in immature dendritic cells by sequestering LSP1 and WASp. PLoS ONE 7, e48854 (2012)
H. Sheldon, M. Andre, J.A. Legg, P. Heal, J.M. Herbert, R. Sainson, A.S. Sharma, J.K. Kitajewski, V.L. Heath, R. Bicknell, Active involvement of Robo1 and Robo4 in filopodia formation and endothelial cell motility mediated via WASP and other actin nucleation-promoting factors. FASEB J. 23, 513–522 (2009)
J. Yuasa-Kawada, M. Kinoshita-Kawada, Y. Rao, J.Y. Wu, Deubiquitinating enzyme USP33/VDU1 is required for Slit signaling in inhibiting breast cancer cell migration. Proc. Natl. Acad. Sci. U. S. A. 106, 14530–14535 (2009)
T. Mgrditchian, G. Sakalauskaite, T. Müller, C. Hoffmann, C. Thomas, The multiple roles of actin-binding proteins at invadopodia. Int. Rev. Cell Mol. Biol. 360, 99–132 (2021)
W. Wang, R. Eddy, J. Condeelis, The cofilin pathway in breast cancer invasion and metastasis. Nat. Rev. Cancer 7, 429–440 (2007)
G.L. Zhou, H. Zhang, H. Wu, P. Ghai, J. Field, Phosphorylation of the cytoskeletal protein CAP1 controls its association with cofilin and actin. J. Cell Sci. 127, 5052–5065 (2014)
K. Moriyama, K. Iida, I. Yahara, Phosphorylation of Ser-3 of cofilin regulates its essential function on actin. Genes Cells 1, 73–86 (1996)
J.M. Cameron, M. Gabrielsen, Y.H. Chim, J. Munro, E.J. McGhee, D. Sumpton, P. Eaton, K.I. Anderson, H. Yin, M.F. Olson, Polarized cell motility induces hydrogen peroxide to inhibit cofilin via cysteine oxidation. Curr. Biol. 25, 1520–1525 (2015)
F. Larbret, P. Biber, N. Dubois, S. Ivanov, L. Lafanechere, S. Tartare-Deckert, M. Deckert, Deubiquitinase inhibitors impair leukemic cell migration through cofilin oxidation and alteration of actin reorganization. Front. Pharmacol. 12, 778216 (2021)
M. Schnoor, T.E. Stradal, K. Rottner, Cortactin: cell functions of A multifaceted actin-binding protein. Trends Cell Biol. 28, 79–98 (2018)
T. Uruno, J. Liu, P. Zhang, Y. Fan, C. Egile, R. Li, S.C. Mueller, X. Zhan, Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat. Cell Biol. 3, 259–266 (2001)
J.J. Tyler, E.G. Allwood, K.R. Ayscough, WASP family proteins, more than Arp2/3 activators. Biochem. Soc. Trans. 44, 1339–1345 (2016)
J.R. Kowalski, C. Egile, S. Gil, S.B. Snapper, R. Li, S.M. Thomas, Cortactin regulates cell migration through activation of N-WASP. J. Cell Sci. 118, 79–87 (2005)
L.A. Helgeson, B.J. Nolen, Mechanism of synergistic activation of Arp2/3 complex by cortactin and N-WASP. eLife 2, e00884 (2013)
M. Oser, H. Yamaguchi, C.C. Mader, J.J. Bravo-Cordero, M. Arias, X. Chen, V. Desmarais, J. van Rheenen, A.J. Koleske, J. Condeelis, Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J. Cell Biol. 186, 571–587 (2009)
L.C. Kelley, S.A. Weed, Cortactin is a substrate of activated Cdc42-associated kinase 1 (ACK1) during ligand-induced epidermal growth factor receptor downregulation. PLoS ONE 7, e44363 (2012)
J.Y. Kim, H.G. Hwang, J.Y. Lee, M. Kim, J.Y. Kim, Cortactin deacetylation by HDAC6 and SIRT2 regulates neuronal migration and dendrite morphogenesis during cerebral cortex development. Mol. Brain 13, 105 (2020)
X. Zhang, Z. Yuan, Y. Zhang, S. Yong, A. Salas-Burgos, J. Koomen, N. Olashaw, J.T. Parsons, X.J. Yang, S.R. Dent, T.P. Yao, W.S. Lane, E. Seto, HDAC6 modulates cell motility by altering the acetylation level of cortactin. Mol. Cell 27, 197–213 (2007)
J. Zhao, J. Wei, R. Mialki, C. Zou, R.K. Mallampalli, Y. Zhao, Extracellular signal-regulated kinase (ERK) regulates cortactin ubiquitination and degradation in lung epithelial cells. J. Biol. Chem. 287, 19105–19114 (2012)
W.D. Wu, M. Wang, H.H. Ding, Z.J. Qiu, FBXL5 attenuates RhoGDI2-induced cisplatin resistance in gastric cancer cells. Eur. Rev. Med. Pharmacol. Sci. 20, 2551–2557 (2016)
Y. Zhao, Y. Lei, S.W. He, Y.Q. Li, Y.Q. Wang, X.H. Hong, Y.L. Liang, J.Y. Li, Y. Chen, W.J. Luo, P.P. Zhang, X.J. Yang, Q.M. He, J. Ma, N. Liu, L.L. Tang, Hypermethylation of UCHL1 promotes metastasis of nasopharyngeal carcinoma by suppressing degradation of cortactin (CTTN). Cells 9(3), 559 (2020)
S.H. Lee, R. Dominguez, Regulation of actin cytoskeleton dynamics in cells. Mol. Cells 29, 311–325 (2010)
M.H. Lee, J.K. Kundu, J.I. Chae, J.H. Shim, Targeting ROCK/LIMK/cofilin signaling pathway in cancer. Arch. Pharm. Res. 42, 481–491 (2019)
L.L. Lohmer, L.C. Kelley, E.J. Hagedorn, D.R. Sherwood, Invadopodia and basement membrane invasion in vivo. Cell Adhes. Migr. 8, 246–255 (2014)
R.J. Eddy, M.D. Weidmann, V.P. Sharma, J.S. Condeelis, Tumor cell invadopodia: invasive protrusions that orchestrate metastasis. Trends Cell Biol. 27, 595–607 (2017)
T. Ideker, V. Thorsson, J.A. Ranish, R. Christmas, J. Buhler, J.K. Eng, R. Bumgarner, D.R. Goodlett, R. Aebersold, L. Hood, Integrated genomic and proteomic analyses of a systematically perturbed metabolic network. Science 292, 929–934 (2001)
K.P. Lai, J. Chen, W.K.F. Tse, Role of deubiquitinases in human cancers: potential targeted therapy. Int. J. Mol. Sci. 21(7), 2548 (2020)
US National Library of Medicine (2021), ClinicalTrials.gov. https://www.clinicaltrials.gov/study/NCT05240898
S.M. Nijman, T.T. Huang, A.M. Dirac, T.R. Brummelkamp, R.M. Kerkhoven, A.D. D’Andrea, R. Bernards, The deubiquitinating enzyme USP1 regulates the Fanconi anemia pathway. Mol. Cell 17, 331–339 (2005)
A. Simoneau, J.L. Engel, M. Bandi, K. Lazarides, S. Liu, S.R. Meier, A.H. Choi, H. Zhang, B. Shen, L. Martires, D. Gotur, T.V. Pham, F. Li, L. Gu, S. Gong, M. Zhang, E. Wilker, X. Pan, D.A. Whittington, S. Throner, J.P. Maxwell, Y. Chen, Y. Yu, A. Huang, J.N. Andersen, T. Feng, Ubiquitinated PCNA drives USP1 synthetic lethality in cancer. Mol. Cancer Ther. 22, 215–226 (2023)
A.P. Turnbull, S. Ioannidis, W.W. Krajewski, A. Pinto-Fernandez, C. Heride, A.C.L. Martin, L.M. Tonkin, E.C. Townsend, S.M. Buker, D.R. Lancia, J.A. Caravella, A.V. Toms, T.M. Charlton, J. Lahdenranta, E. Wilker, B.C. Follows, N.J. Evans, L. Stead, C. Alli, V.V. Zarayskiy, A.C. Talbot, A.J. Buckmelter, M. Wang, C.L. McKinnon, F. Saab, J.F. McGouran, H. Century, M. Gersch, M.S. Pittman, C.G. Marshall, T.M. Raynham, M. Simcox, L.M.D. Stewart, S.B. McLoughlin, J.A. Escobedo, K.W. Bair, C.J. Dinsmore, T.R. Hammonds, S. Kim, S. Urbé, M.J. Clague, B.M. Kessler, D. Komander, Molecular basis of USP7 inhibition by selective small-molecule inhibitors. Nature 550, 481–486 (2017)
S.M. Lange, L.A. Armstrong, Y. Kulathu, Deubiquitinases: from mechanisms to their inhibition by small molecules. Mol. Cell 82, 15–29 (2022)
N.J. Henning, L. Boike, J.N. Spradlin, C.C. Ward, G. Liu, E. Zhang, B.P. Belcher, S.M. Brittain, M.J. Hesse, D. Dovala, L.M. McGregor, R. Valdez Misiolek, L.W. Plasschaert, D.J. Rowlands, F. Wang, A.O. Frank, D. Fuller, A.R. Estes, K.L. Randal, A. Panidapu, J.M. McKenna, J.A. Tallarico, M. Schirle, D.K. Nomura, Deubiquitinase-targeting chimeras for targeted protein stabilization. Nat. Chem. Biol. 18, 412–421 (2022)
J. Paulk, Lysosome-targeting chimeras evolve. Nat. Chem. Biol. 17, 931–933 (2021)
N. Poondla, A.P. Chandrasekaran, K.S. Kim, S. Ramakrishna, Deubiquitinating enzymes as cancer biomarkers: new therapeutic opportunities? BMB Rep. 52, 181–189 (2019)
D. Ren, Y. Sun, D. Li, H. Wu, X. Jin, USP22-mediated deubiquitination of PTEN inhibits pancreatic cancer progression by inducing p21 expression. Mol. Oncol. 16, 1200–1217 (2022)
F. Pareja, D.A. Ferraro, C. Rubin, H. Cohen-Dvashi, F. Zhang, S. Aulmann, N. Ben-Chetrit, G. Pines, R. Navon, N. Crosetto, W. Köstler, S. Carvalho, S. Lavi, F. Schmitt, I. Dikic, Z. Yakhini, P. Sinn, G.B. Mills, Y. Yarden, Deubiquitination of EGFR by Cezanne-1 contributes to cancer progression. Oncogene 31, 4599–4608 (2012)
H.N. Du, Transcription, DNA damage and beyond: the roles of histone ubiquitination and deubiquitination. Curr. Protein Pept. Sci. 13, 447–466 (2012)
T. Sun, Z. Liu, Q. Yang, The role of ubiquitination and deubiquitination in cancer metabolism. Mol. Cancer 19, 146 (2020)
D. Mennerich, K. Kubaichuk, T. Kietzmann, DUBs, hypoxia, and cancer. Trends Cancer 5, 632–653 (2019)
Funding
This review was supported by Key Research and Development Plan of Shandong Province (2022CXGC020501), Taishan Scholar Foundation of Shandong Province (tsqnz20230632) and National Natural Science Foundation of China (8230101506).
Author information
Authors and Affiliations
Contributions
Y.X writed the review article. W.S edited the review. C.X constructed the figure. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors consent on publication of this review article.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xue, Y., Xue, C. & Song, W. Emerging roles of deubiquitinating enzymes in actin cytoskeleton and tumor metastasis. Cell Oncol. (2024). https://doi.org/10.1007/s13402-024-00923-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s13402-024-00923-z