Abstract
Excessive fibrosis is a predominant feature of pancreatic stroma and plays a crucial role in the development and progression of pancreatic ductal adenocarcinoma (PDAC) and chronic pancreatitis (CP). Emerging evidence showed diversity and heterogeneity of fibroblasts play crucial and somewhat contradictory roles, the interactions between fibroblasts and pancreatic cells or infiltrating immune cells are of great importance during PDAC and CP progression, with some promising therapeutic strategies being tested. Therefore, in this review, we describe the classification of fibroblasts and their functions in PDAC and pancreatitis, the mechanisms by which fibroblasts mediate the development and progression of PDAC and CP through direct or indirect interaction between fibroblast and pancreatic parenchymal cells, or by remodeling the pancreatic immune microenvironment mediates the development and progression of PDAC and CP. Finally, we summarized the current therapeutic strategies and agents that directly target subtypes of fibroblasts or interfere with their essential functions.

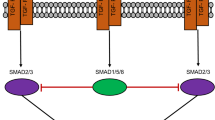
Figure created with BioRender.com

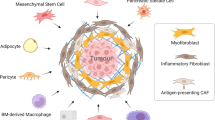
Figure created with BioRender.com

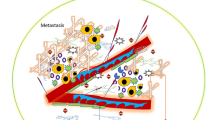
Figure created with BioRender.com
Similar content being viewed by others
Data Availability
Not applicable.
References
P.A.M.J. Phillips, S. Park et al., Rat pancreatic stellate cells secrete matrix metalloproteinases implications for extracellular matrix turnover. Gut 52, 275–282 (2003)
R.J. McAnulty, Fibroblasts and myofibroblasts: their source, function and role in disease. Int. J. Biochem. Cell. Biol. 39, 666–671 (2007)
I.A. Darby, B. Laverdet, F. Bonte et al., Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig Dermatol. 7, 301–311 (2014)
A. Arina, C. Idel, E.M. Hyjek et al., Tumor-associated fibroblasts predominantly come from local and not circulating precursors. Proc. Natl. Acad. Sci. U S A 113, 7551–7556 (2016)
E. Sahai, I. Astsaturov, E. Cukierman et al., A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer. 20, 174–186 (2020)
M. Erkan, G. Adler, M.V. Apte, ea. StellaTUM: current consensus and discussion on pancreatic stellate cell research. Gut 2012
A. Vonlaufen, P.A. Phillips, Z. Xu et al., Pancreatic stellate cells and pancreatic cancer cells: an unholy alliance. Cancer Res. 68, 7707–7710 (2008)
M.G. Bachem, M. Schunemann, M. Ramadani et al., Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 128, 907–921 (2005)
M.V. Apte, J.S. Wilson, A. Lugea et al., A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology. 144, 1210–1219 (2013)
E.J. Helms, M.W. Berry, R.C. Chaw et al., Mesenchymal lineage heterogeneity underlies nonredundant functions of pancreatic Cancer-Associated fibroblasts. Cancer Discov. 12, 484–501 (2022)
G. Sparmann, M.L. Kruse, N. Hofmeister-Mielke et al., Bone marrow-derived pancreatic stellate cells in rats. Cell. Res. 20, 288–298 (2010)
Y. Miyazaki, T. Oda, N. Mori et al., Adipose-derived mesenchymal stem cells differentiate into pancreatic cancer‐associated fibroblasts in vitro. FEBS Open. Bio. 10, 2268–2281 (2020)
M. Quante, S.P. Tu, H. Tomita et al., Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 19, 257–272 (2011)
E.M. Zeisberg, S. Potenta, L. Xie et al., Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 67, 10123–10128 (2007)
X. Huang, C. He, X. Hua et al., Oxidative stress induces monocyte-to-myofibroblast transdifferentiation through p38 in pancreatic ductal adenocarcinoma. Clin. Transl Med. 10, e41 (2020)
F. Marrache, S. Pendyala, G. Bhagat, K.S. Betz, Z. Song, T.C. Wang, Role of bone marrow-derived Cells in Experimental Chronic Pancreatitis. Gut 57, 1113–1120 (2008)
Y. Sunami, J. Häußler, J. Kleeff, Cellular Heterogeneity of pancreatic stellate cells, mesenchymal stem cells, and Cancer-Associated fibroblasts in pancreatic Cancer. Cancers. 12, 3770 (2020)
E. Helms, M.K. Onate, M.H. Sherman, Fibroblast heterogeneity in the pancreatic Tumor Microenvironment. Cancer Discov. 10, 648–656 (2020)
D. Ohlund, A. Handly-Santana, G. Biffi et al., Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 214, 579–596 (2017)
G. Biffi, T.E. Oni, B. Spielman et al., IL1-Induced JAK/STAT signaling is antagonized by TGFbeta to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov. 9, 282–301 (2019)
E. Elyada, M. Bolisetty, P. Laise et al., Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals Antigen-Presenting Cancer-Associated fibroblasts. Cancer Discov. 9, 1102–1123 (2019)
Y. Wang, Y. Liang, H. Xu et al., Single-cell analysis of pancreatic ductal adenocarcinoma identifies a novel fibroblast subtype associated with poor prognosis but better immunotherapy response. Cell. Discov. 7, 36 (2021)
W. Ge, M. Yue, R. Lin et al., PLA2G2A(+) cancer-associated fibroblasts mediate pancreatic cancer immune escape via impeding antitumor immune response of CD8(+) cytotoxic T cells. Cancer Lett. 558, 216095 (2023)
C. Neuzillet, A. Tijeras-Raballand, C. Ragulan et al., Inter- and intra-tumoural heterogeneity in cancer-associated fibroblasts of human pancreatic ductal adenocarcinoma. J. Pathol. 248, 51–65 (2019)
C. Neuzillet, R. Nicolle, J. Raffenne et al., Periostin- and podoplanin-positive cancer-associated fibroblast subtypes cooperate to shape the inflamed tumor microenvironment in aggressive pancreatic adenocarcinoma. J. Pathol. 258, 408–425 (2022)
D.S. Foster, M. Januszyk, D. Delitto et al., Multiomic analysis reveals conservation of cancer-associated fibroblast phenotypes across species and tissue of origin. Cancer Cell. 40, 1392–1406 (2022)
C.X. Dominguez, S. Muller, S. Keerthivasan et al., Single-cell RNA sequencing reveals stromal evolution into LRRC15(+) Myofibroblasts as a determinant of patient response to Cancer Immunotherapy. Cancer Discov. 10, 232–253 (2020)
A.T. Krishnamurty, J.A. Shyer, M. Thai et al., LRRC15(+) myofibroblasts dictate the stromal setpoint to suppress tumour immunity. Nature 611, 148–154 (2022)
R. Francescone, D. Barbosa Vendramini-Costa, J. Franco-Barraza et al., Netrin G1 promotes pancreatic tumorigenesis through Cancer-Associated Fibroblast-Driven Nutritional Support and Immunosuppression. Cancer Discov. 11, 446–479 (2021)
C. Hutton, F. Heider, A. Blanco-Gomez et al., Single-cell analysis defines a pancreatic fibroblast lineage that supports anti-tumor immunity. Cancer Cell. 39, 1227–1244e20 (2021)
Y. Chen, J. Kim, S. Yang et al., Type I collagen deletion in alphaSMA(+) myofibroblasts augments immune suppression and accelerates progression of pancreatic cancer. Cancer Cell. 39, 548–565 (2021)
M.V. Apte, P.S. Haber, T.L. Applegate et al., Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut. 43, 128–133 (1998)
P.S. Haber, G.W. Keogh, M.V. Apte et al., Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis. Am. J. Pathol. 155, 1087–1095 (1999)
M.V. Apte, P.A. Phillips, R.G. Fahmy et al., Does alcohol directly stimulate pancreatic fibrogenesis? Studies with rat pancreatic stellate cells. Gastroenterology. 118, 780–794 (2000)
A.T. Lee, Z. Xu, S.P. Pothula et al., Alcohol and cigarette smoke components activate human pancreatic stellate cells: implications for the progression of chronic pancreatitis. Alcohol Clin. Exp. Res. 39, 2123–2133 (2015)
X. Yang, J. Chen, J. Wang et al., Very-low-density lipoprotein receptor-enhanced lipid metabolism in pancreatic stellate cells promotes pancreatic fibrosis. Immunity. 55, 1185–1199 (2022). e8
H. Zhao, L. Yang, J. Baddour et al., Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. Elife. 5, e10250 (2016)
T. Shan, S. Chen, X. Chen, W. Lin, Run, W. Li, Cancer-associated fibroblasts enhance pancreatic cancer cell invasion by remodeling the metabolic conversion mechanism. Oncol. Rep. 37(4), 1971–1979 (2017)
F.R. Auciello, V. Bulusu, C. Oon et al., A stromal Lysolipid-Autotaxin Signaling Axis promotes pancreatic tumor progression. Cancer Discov. 9, 617–627 (2019)
C.M. Sousa, D.E. Biancur, X. Wang et al., Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature. 536, 479–483 (2016)
O. Olivares, J.R. Mayers, V. Gouirand et al., Collagen-derived proline promotes pancreatic ductal adenocarcinoma cell survival under nutrient limited conditions. Nat. Commun. 8, 16031 (2017)
M. Yuan, B. Tu, H. Li et al., Cancer-associated fibroblasts employ NUFIP1-dependent autophagy to secrete nucleosides and support pancreatic tumor growth. Nat. Cancer. 3, 945–960 (2022)
H. Liu, H. Zhang, X. Liu et al., Pancreatic stellate cells exploit Wnt/beta-catenin/TCF7-mediated glutamine metabolism to promote pancreatic cancer cells growth. Cancer Lett. 555, 216040 (2023)
J.M. Bailey, B.J. Swanson, T. Hamada et al., Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin. Cancer Res. 14, 5995–6004 (2008)
R.L. Yauch, S.E. Gould, S.J. Scales et al., A paracrine requirement for hedgehog signalling in cancer. Nature. 455, 406–410 (2008)
D.E. Rosow, A.S. Liss, O. Strobel et al., Sonic hedgehog in pancreatic cancer: from bench to bedside, then back to the bench. Surgery. 152, S19–32 (2012)
J.J. Lee, R.M. Perera, H. Wang et al., Stromal response to hedgehog signaling restrains pancreatic cancer progression. Proc. Natl. Acad. Sci. U S A 111, E3091–E3100 (2014)
N.G. Steele, G. Biffi, S.B. Kemp et al., Inhibition of hedgehog signaling alters fibroblast composition in pancreatic Cancer. Clin. Cancer Res. 27, 2023–2037 (2021)
P. Provenzano Paolo, C. Cuevas, E. Chang Amy et al., Enzymatic targeting of the Stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 21, 418–429 (2012)
C.B. Thompson, H.M. Shepard, P.M. O’Connor et al., Enzymatic depletion of tumor hyaluronan induces antitumor responses in preclinical animal models. Mol. Cancer Ther. 9, 3052–3064 (2010)
A.N. Hosein, R.A. Brekken, A. Maitra, Pancreatic cancer stroma: an update on therapeutic targeting strategies. Nat. Rev. Gastroenterol. Hepatol. 17, 487–505 (2020)
K.M. McAndrews, Y. Chen, J.K. Darpolor et al., Identification of functional heterogeneity of Carcinoma-Associated fibroblasts with distinct IL6-Mediated Therapy Resistance in Pancreatic Cancer. Cancer Discov. 12, 1580–1597 (2022)
K.E. Richards, A.E. Zeleniak, M.L. Fishel et al., Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene. 36, 1770–1778 (2017)
Y. Shi, W. Gao, N.K. Lytle et al., Targeting LIF-mediated paracrine interaction for pancreatic cancer therapy and monitoring. Nature. 569, 131–135 (2019)
S. Dalin, M.R. Sullivan, A.N. Lau et al., Deoxycytidine Release from pancreatic stellate cells promotes Gemcitabine Resistance. Cancer Res. 79, 5723–5733 (2019)
J. Cao, J. Li, L. Sun et al., Hypoxia-driven paracrine osteopontin/integrin alphavbeta3 signaling promotes pancreatic cancer cell epithelial-mesenchymal transition and cancer stem cell-like properties by modulating forkhead box protein M1. Mol. Oncol. 13, 228–245 (2019)
D. Zhang, L. Li, H. Jiang et al., Tumor-stroma IL1beta-IRAK4 Feedforward Circuitry drives Tumor Fibrosis, Chemoresistance, and poor prognosis in pancreatic Cancer. Cancer Res. 78, 1700–1712 (2018)
P. Nan, X. Dong, X. Bai et al., Tumor-stroma TGF-β1-THBS2 feedback circuit drives pancreatic ductal adenocarcinoma progression via integrin αvβ3/CD36-mediated activation of the MAPK pathway. Cancer Lett. 528, 59–75 (2022)
S. Schuth, Le S. Blanc, T.G. Krieger et al., Patient-specific modeling of stroma-mediated chemoresistance of pancreatic cancer using a three-dimensional organoid-fibroblast co-culture system. J. Exp. Clin. Cancer Res. 41, 312 (2022)
D. Cui Zhou, R.G. Jayasinghe, S. Chen et al., Spatially restricted drivers and transitional cell populations cooperate with the microenvironment in untreated and chemo-resistant pancreatic cancer. Nat. Genet. 54, 1390–1405 (2022)
M. Blauer, M. Laaninen, J. Sand et al., Reciprocal stimulation of pancreatic acinar and stellate cells in a novel long-term in vitro co-culture model. Pancreatology. 16, 570–577 (2016)
P.A. Phillips, L. Yang, A. Shulkes et al., Pancreatic stellate cells produce acetylcholine and may play a role in pancreatic exocrine secretion. Proc. Natl. Acad. Sci. U S A 107, 17397–17402 (2010)
J.S. Liu, Z.J. Cui, Pancreatic stellate cells serve as a Brake mechanism on pancreatic Acinar Cell Calcium Signaling modulated by Methionine Sulfoxide reductase expression. Cells 8, 109 (2019).
W. Yao, D. Luo, Z. Lv et al., The Rabep1-Mediated endocytosis and activation of trypsinogen to promote pancreatic stellate cell activation. Biomolecules 12, 1063 (2022).
M.A. Jakubowska, P.E. Ferdek, O.V. Gerasimenko et al., Nitric oxide signals are interlinked with calcium signals in normal pancreatic stellate cells upon oxidative stress and inflammation. Open. Biol. 6, 160149 (2016)
O. Gryshchenko, J.V. Gerasimenko, S. Peng et al., Calcium signalling in the acinar environment of the exocrine pancreas: physiology and pathophysiology. J. Physiol. 596, 2663–2678 (2018)
S. Hausmann, I. Regel, K. Steiger et al., Loss of Periostin results in impaired regeneration and pancreatic atrophy after Cerulein-Induced Pancreatitis. Am. J. Pathol. 186, 24–31 (2016)
X. Liu, J.R. Pitarresi, M.C. Cuitino et al., Genetic ablation of smoothened in pancreatic fibroblasts increases acinar-ductal metaplasia. Genes Dev. 30, 1943–1955 (2016)
V.K. Singh, D. Yadav, P.K. Garg, Diagnosis and management of chronic pancreatitis: a review. JAMA 322, 2422–2434 (2019)
F.W. Shek, R.C. Benyon, F.M. Walker et al., Expression of transforming growth factor-beta 1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis. Am. J. Pathol. 160, 1787–1798 (2002)
J. Tahara, K. Shimizu, K. Shiratori, Engulfment of necrotic acinar cells by pancreatic stellate cells inhibits pancreatic fibrogenesis. Pancreas. 37, 69–74 (2008)
K. Kikuta, A. Masamune, S. Hamada et al., Pancreatic stellate cells reduce insulin expression and induce apoptosis in pancreatic beta-cells. Biochem. Biophys. Res. Commun. 433, 292–297 (2013)
X. Zhu, D. Liu, G. Li et al., Exosomal mir-140-3p and mir-143-3p from TGF-beta1-treated pancreatic stellate cells target BCL2 mRNA to increase beta-cell apoptosis. Mol. Cell. Endocrinol. 551, 111653 (2022)
Q. Li, Y. Zhu, L. Liu et al., Pancreatic stellate cells and TGFβ in the Pathogenesis of Pain in Chronic Pancreatitis. Gastroenterology 2017;152
X. Mao, J. Xu, W. Wang et al., Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol. Cancer. 20, 131 (2021)
Z. Zhang, H. Zhang, L. Shi et al., Heterogeneous cancer-associated fibroblasts: a new perspective for understanding immunosuppression in pancreatic cancer. Immunology. 167, 1–14 (2022)
De L. Monte, M. Reni, E. Tassi et al., Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J. Exp. Med. 208, 469–478 (2011)
C. Feig, J.O. Jones, M. Kraman et al., Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl. Acad. Sci. U S A 110, 20212–20217 (2013)
A. Ene-Obong, A.J. Clear, J. Watt et al., Activated pancreatic stellate cells sequester CD8 + T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma. Gastroenterology. 145, 1121–1132 (2013)
D. Goehrig, J. Nigri, R. Samain et al., Stromal protein betaig-h3 reprogrammes tumour microenvironment in pancreatic cancer. Gut. 68, 693–707 (2019)
H. Huang, Z. Wang, Y. Zhang et al., Mesothelial cell-derived antigen-presenting cancer-associated fibroblasts induce expansion of regulatory T cells in pancreatic cancer. Cancer Cell. 40, 656–673 (2022). e7
F.S.R. Picard, V. Lutz, A. Brichkina et al., IL-17A-producing CD8(+) T Cells Promote PDAC via Induction of Inflammatory cancer-associated Fibroblasts. Gut 72, 1510–1522 (2023)
L. Gorchs, C. Fernandez Moro, P. Bankhead et al., Human Pancreatic Carcinoma-Associated fibroblasts promote expression of co-inhibitory markers on CD4(+) and CD8(+) T-Cells. Front. Immunol. 10, 847 (2019)
S.K. Daniel, K.M. Sullivan, K.P. Labadie et al., Hypoxia as a barrier to immunotherapy in pancreatic adenocarcinoma. Clin. Transl Med. 8, 10 (2019)
T.A. Mace, Z. Ameen, A. Collins et al., Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res. 73, 3007–3018 (2013)
K. Kesh, V.T. Garrido, A. Dosch et al., Stroma secreted IL6 selects for stem-like population and alters pancreatic tumor microenvironment by reprogramming metabolic pathways. Cell. Death Dis. 11, 967 (2020)
A. Zhang, Y. Qian, Z. Ye et al., Cancer-associated fibroblasts promote M2 polarization of macrophages in pancreatic ductal adenocarcinoma. Cancer Med. 6, 463–470 (2017)
C.J. Garcia Garcia, Y. Huang, N.R. Fuentes et al., Stromal HIF2 Regulates Immune Suppression in the Pancreatic Cancer Microenvironment. Gastroenterology 2022;162:2018–2031
J.A. Myers, J.S. Miller, Exploring the NK cell platform for cancer immunotherapy. Nat. Rev. Clin. Oncol. 18, 85–100 (2021)
Q. Huang, M. Huang, F. Meng et al., Activated pancreatic stellate cells inhibit NK cell function in the human pancreatic cancer microenvironment. Cell. Mol. Immunol. 16, 87–89 (2019)
J. Xue, V. Sharma, M.H. Hsieh et al., Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis. Nat. Commun. 6, 7158 (2015)
N. Wu, X.F. Xu, J.Q. Xin et al., The effects of nuclear factor-kappa B in pancreatic stellate cells on inflammation and fibrosis of chronic pancreatitis. J. Cell. Mol. Med. 25, 2213–2227 (2020)
K. Shimizu, M. Kobayashi, J. Tahara et al., Cytokines and peroxisome proliferator-activated receptor gamma ligand regulate phagocytosis by pancreatic stellate cells. Gastroenterology. 128, 2105–2118 (2005)
E.F. Carapuca, E. Gemenetzidis, C. Feig et al., Anti-stromal treatment together with chemotherapy targets multiple signalling pathways in pancreatic adenocarcinoma. J. Pathol. 239, 286–296 (2016)
M.H. Sherman, R.T. Yu, D.D. Engle et al., Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 159, 80–93 (2014)
M. Rickmann, E.C. Vaquero, J.R. Malagelada et al., Tocotrienols induce apoptosis and autophagy in rat pancreatic stellate cells through the mitochondrial death pathway. Gastroenterology. 132, 2518–2532 (2007)
A.R. Dosch, S. Singh, X. Dai et al., Targeting tumor–stromal IL6/STAT3 signaling through IL1 receptor inhibition in pancreatic Cancer. Mol. Cancer Ther. 20, 2280–2290 (2021)
T.A. Mace, R. Shakya, J.R. Pitarresi et al., IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut. 67, 320–332 (2018)
D. Melisi, R. Garcia-Carbonero, T. Macarulla et al., Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer. Br. J. Cancer. 119, 1208–1214 (2018)
E. O’Reilly, T. Golan, M. Ikeda et al., P-22 phase III study (daNIS-2) of the anti–TGF-β monoclonal antibody NIS793 with nab-paclitaxel/gemcitabine vs nab-paclitaxel/gemcitabine alone in patients with first-line metastatic pancreatic ductal adenocarcinoma. Ann. Oncol. 33, S254 (2022)
E. Borazanci, A.M. Schram, E. Garralda et al., Phase I, first-in-human study of MSC-1 (AZD0171), a humanized anti-leukemia inhibitory factor monoclonal antibody, for advanced solid tumors. ESMO Open. 7, 100530 (2022)
O. Gryshchenko, J.V. Gerasimenko, O.V. Gerasimenko et al., Ca(2+) signals mediated by bradykinin type 2 receptors in normal pancreatic stellate cells can be inhibited by specific ca(2+) channel blockade. J. Physiol. 594, 281–293 (2016)
V. Szabo, N. Csakany-Papp, M. Gorog et al., Orai1 Calcium Channel Inhibition Prevents Progression of Chronic Pancreatitis. JCI Insight 8, e167645 (2023)
X.P. Zeng, L.J. Wang, H.L. Guo et al., Dasatinib ameliorates chronic pancreatitis induced by caerulein via anti-fibrotic and anti-inflammatory mechanism. Pharmacol. Res. 147, 104357 (2019)
H.M. Kocher, B. Basu, F.E.M. Froeling et al., Phase I clinical trial repurposing all-trans retinoic acid as a stromal targeting agent for pancreatic cancer. Nat. Commun. 11, 4841 (2020)
Funding
This work is supported by National Natural Science Foundation of China (82122010 and 82070659 to Li Wen), Shanghai Natural Science Foundation (22ZR1480500 to Li Wen), National High Level Hospital Clinical Research Funding (2022-PUMCH-E-003 to Li Wen) and CAMS Innovation Fund for Medical Sciences (2023-I2M-3-001 to Li Wen and 2022-I2M-1-004).
Author information
Authors and Affiliations
Contributions
LW designed and supervised the study, drafted and revised the manuscript and obtained the funding. HH performed the literature search and critical assessment of the published papers, drafted the manuscript. HH, WL and XZ drew the diagrams. XZ, WL, JP and FC revised the manuscript and participated in the intellectual discussions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, H., Lu, W., Zhang, X. et al. Fibroblast subtypes in pancreatic cancer and pancreatitis: from mechanisms to therapeutic strategies. Cell Oncol. (2023). https://doi.org/10.1007/s13402-023-00874-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s13402-023-00874-x