Abstract
Date plum (Diospyros lotus L.) fruits are a good source of bioactive compounds and antioxidants. Drying can increase the shelf life of the fruit and its applications in the food development industry. Optimizing the drying conditions can help to produce prime-quality dried date plum fruits and conserve nutrients including phytochemicals. This study used a two-factor graphics-optimal design to optimize convective drying considering the air velocity and drying temperature of date plum fruits. The independent factors considered included drying temperature (43.78–86.21 °C) and air velocities (0.54–1.96 m/s), and the responses included total phenolic content (TPC), total flavonoid content (TFC), ferric reducing antioxidant power (FRAP), and 2,2-diphenyl-1-picrylhydrazy (DPPH) radical scavenging activity of date plum fruit. The optimized drying conditions (68 °C and 1.75 m/s) resulted in desirable TPC, TFC, FRAP, and DPPH values. The findings indicated that long drying time at low temperatures significantly decreased the phenolics and antioxidants. Date plum tea with different decoction times (5, 10, and 15 min) was prepared from fruits dried at optimum conditions. A decoction time of 5 min resulted in the highest catechin, vanillic, epicatechin, syringic acid, and quercetin-3-glucoside content, which were 2.45 ± 0.04, 11.06 ± 0.11, 22.03 ± 0.11, 12.95 ± 0.08, and 9.37 ± 0.10 mg/L; respectively. In vitro gastrointestinal digestion revealed that the tea product can be a source of highly bioaccessible (> 80%) gallic acid, catechin, vanillic acid, and quercetin-3-glucoside. Applying optimized drying conditions to dehydrate date plum fruit can be useful in preparing a highly bioaccessible polyphenol-rich tea.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Date plum (Diospyros lotus L.) is a plant from the Ebenaceae family. It has been widely cultivated in subtropical southwest Asia and southeast Europe [1] and naturally grows in northeast Turkey in the Black Sea region [2]. Data from several studies suggest that date plum fruits are a valuable source of (poly)phenols and antioxidants [2,3,4]. According to the literature, date plum fruits contain gallic acid, ellagic acid, vanillic acid, resveratrol, caffeic acid, trans-ferulic acid, p-coumaric acid, sinapic acid, naringin, rutin trihydrate, trans-cinnamic acid, and quercetin [5]. It contains a high content of gallic acid and chrysin [2], as well as fatty acids and sugars. Similarly, recent evidence suggests that date plum seeds potentially have unsaturated fatty acids (e.g., oleic acid and linoleic acid) as edible oil [6].
The rich phytochemical compositions, especially polyphenols, present in date plums have given it a wide range of bioactivities. Previous research established that date plum has potential antimicrobial, antioxidant [2, 4, 7], muscle relaxation [8], antidiarrhea, antiseptic, antitumor, antidiabetic, and laxative activities [9, 10], and the ability to reduce cardiac damage [7]. Polyphenols are capable of reducing the risk of cardiovascular disease, cancer, and rheumatoid arthritis in the body [11, 12].
Date plum fruits are consumed fresh or dried. Drying is a traditional food preservation method since it is applied to control microbial growth, reduce deterioration, and stabilize food products by lowering water activity [13]. Dehydration of fruits is a conventional strategy that is applied to preservation by reducing water content, consequently decreasing the risk of microbial growth and enzymatic modifications. Date plum pulp is quickly deteriorated by microbial and enzymatic activity due to high moisture content of 70.5% [4]. According to a previous study, the date plum fruits dried at 70 °C have the highest flavonoid content, antioxidant potential, and phenolic content [14]. The high moisture of fruits is associated with a raised risk of perishable, nutrient loss, and microorganism attacks.
Considering that date plum fruits have reasonable amounts of nutrients and bioactive compounds, drying could establish to extend the shelf-life and preserve the phytochemicals and antioxidant compounds of date plum fruits. The convective drying or hot air–drying method is an alternative technique to improve the shelf life of food products by exposure to hot air [15, 16], preferred in industrial, commercial, and agricultural products due to its lesser operating costs and uniform applications [17, 18]. The previous findings highlighted the potential of convective drying as an efficient and safe drying method for fruits and vegetables with the ability to maintain nutritional and antioxidant properties [19]. Nevertheless, drying can affect agricultural products’ total phenol contents, antioxidant capacity, flavonoids, carotenoids, fatty acids, and polyphenols [20]. The drying temperature causes degradation of polyphenols and antioxidant potential of fruits [21, 22].
To date, two studies have investigated drying date plum. A study evaluated the effect of hot air drying, vacuum drying, and freeze drying on the phytochemical content, bioactivity, and sensory properties [23]. However, the drying conditions and variables in each method were not investigated. Another study, by our group, investigated the effect of convective drying temperature and air velocity on phenolics and antioxidant properties, yet the conditions were not optimized [14]. Therefore, one of the aims of this research was to evaluate the convective drying of date plum fruits and determine its optimum conditions to conserve its phenolic content and antioxidant activity.
Once fruits are dried, they can be utilized for different purposes in food production. Dried fruits can be used to produce fruit tea. Fruit tea has recently attracted many consumers, especially in Europe, due to its flavor variety, health-promoting benefits, being a caffeine-free option, providing a flavorful alternative to plain water (contributing to daily fluid intake), and being refreshing and relaxing during different seasons (can be served hot or cold) [24]. Nevertheless, there has been limit research on developing fruit teas and evaluated their bioactive constituents. Most of the fruit teas that have been evaluated were based on berries such as blackcurrant, bilberry, chokeberry [25], and elderberries [26]. Thus, the second aim of the current study was to produce a date plum tea, using dried fruit at optimum condition, to have the highest total phenolic content, total flavonoid content, and antioxidant capacity. Finally, the polyphenol-rich date plum tea was subjected to in vitro gastrointestinal digestion to evaluate the bioaccessibility of polyphenols.
2 Materials and methods
2.1 Sample preparation and extraction
Mature date plum fruits were obtained from Samsun city in Northern Turkey during Turkey’s spring harvest season in 2022. The healthy fruit samples were sorted, cleaned, and kept in PTE bags (about 300 g) before being brought to the lab. In the lab, the fruit pulp was blended with 20 mL of 80% methanol and allowed to macerate for 12 h at 25 °C. The solutions were suitably filtered and diluted for analysis.
2.2 Physico-chemical characteristics of date plum fruit
2.2.1 Determination of total soluble solids
Total soluble solids were measured at 20 °C after the date plum fruit pulps (10 g) were combined with a volume of 20 mL of distilled water (dw H2O), filtered through filter paper, and poured into the Abbe refractometer (Atago, Japan).
2.2.2 Determination of fruit flesh-to-seed ratio
The flesh-to-seeds fruit ratio (F/S) was calculated using Eq. 1:
where \({\text{Sm}}\) is the mass (g) of the seeds and \({\text{Fm}}\) is the weight (g) of the fruit flesh.
2.2.3 pH analysis
The sample was diluted with dw H2O at a ratio of 1:10, and the pH of the mixture was measured using a pH meter.
2.2.4 Color determination
The fresh samples’ color characteristics, L*, a*, and b*, were measured using a colorimeter (Model DP 400, Minolta, Japan).
2.3 Drying experiment and design
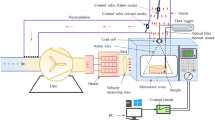
A cabinet dryer with a convection regime (EKSIS, Turkey), as detailed in our previous study [27], was used for drying. The fruit samples were placed into perforated trays in triplicate, evenly divided into three different sections for each drying batch of 480 g and then dried in the convective cabinet dryer until reaching 10% moisture content. The weight reduction of the samples was monitored during the drying process for moisture content determination. As independent variables, the drying process was optimized using the two-factor design: air velocity (m/s) and temperature (°C). Thirteen experimental points were selected in the temperature range of 43.78 and 86.21 °C and air velocity range of 0.54 and 1.95 m/s. Once samples were dried, they were kept in sealed polyethylene LDPE bags and stored at room temperature (25 °C). The samples were analyzed for total phenolic content (TPC), total flavonoid content (TFC), ferric reducing antioxidant power (FRAP), and 2,2-diphenyl-1-picrylhydrazy (DPPH) radical scavenging activity. All experiments were conducted within a week.
2.4 Total phenolic content
The TPC was determined according to the Folin-Ciocalteu method [28]. Experimental measurements were obtained using the SPECTRONIC™ 200 Spectrophotometer (Thermo Scientific™, United Kingdom) at 769 nm, and results were reported in terms of milligrams of gallic acid equivalent (GAE) per gram (mg GAE/g).
2.5 Total flavonoid content
The TFC was determined according to the aluminum chloride colorimetric method [28]. Experimental measurements were obtained using the SPECTRONIC™ 200 Spectrophotometer (Thermo Scientific™, UK) at 510 nm, and results were reported in terms of milligrams of epicatechin equivalent (ECE) per gram (mg ECE/g).
2.6 Ferric reducing antioxidant power (FRAP)
The FRAP assay was carried out using FeSO4 as a standard [28]. Experimental measurements were obtained using the SPECTRONIC™ 200 Spectrophotometer (Thermo Scientific™, UK) at 593 nm, and results were reported in terms of mmol FeSO4 equivalents (mmol ISE/g).
2.7 DPPH radical scavenging activity
The DPPH radical scavenging activity assay was another assay conducted to assess antioxidant activity [28]. Experimental measurements were obtained using the SPECTRONIC™ 200 Spectrophotometer (Thermo Scientific™, United Kingdom) at 517 nm, and the percentage of reduction was calculated according to Eq. 2:
where \({A}_{c}\) is the absorbance of the control and \({A}_{s}\) is the absorbance of the extract. The DPPH radical scavenging activity in each extract was then calculated using a Trolox calibration curve (reduction ratio was considered as Y and concentration of Trolox was considered as X). The results were reported as Trolox equivalents (mmol TE/g).
2.8 Scanning electron microscope (SEM)
The microstructures of samples have been obtained using a field emission scanning electron microscope (JEOL JSM-7001F). A sample was carefully attached to a stainless stub with double sticky tape, and using a sputter coater, it was sputtered with a gold/palladium target (60/40) at 10 nm, functioning with argon and plasma current for 2 min. The images were captured at the two different voltages (5 and 10 kV).
2.9 Preparation of date plum tea
Date plum fruits dried at optimum conditions of 68 °C and 1.75 m/s were selected for tea preparation. The dried fruits were deseeded and sieved. An amount of 4 g of the dried D. lotus fruit was mixed with 100 mL of boiling dw H2O (≈ 100 °C) and heated for 5, 10, or 15 min to produce three decoctions. Finally, the teas were filtered and transferred to volumetric flasks to adjust their volume.
2.10 LC–MS/MS analysis of polyphenols
The amount of phenolic acids (gallic acid, vanillic acid, syringic acid, and salicylic acid) and flavonoids (catechin, epicatechin, and quercetin-3-glucoside) in the fruit sample dried at optimum conditions (1 g of sample extracted with 20 mL dw H2O) and the three decoctions of D. lotus fruit were determined using liquid chromatography with tandem mass spectrometry (LC–MS/MS). An LC–MS/MS (Shimadzu LC–MS 8040) equipped with electrospray ionization (ESI) and two pumps (LC-30 AD), a column oven (CTO-10AS VP), an autosampler (SIL- 30AC), and a degassing unit (DGU-20A 3R) was used. The mobile phase consisted of two solvents: A: water and 0.1% formic acid, and B: methanol and 0.1% formic acid, and separation was conducted according to our previous study [27]. The phenolic acids and flavonoids of were quantified using the peak area of each compound. The compounds of interest were quantified by mixing external standards of each phenolic acid and flavonoid of interest in methanol with 0, 50, 75, 100, 150, and 200 mg/L concentrations.
2.11 Assessment of in vitro bioaccessibility
In vitro gastrointestinal digestion was conducted for the three tea samples prepared at different decoctions times to determine the bioaccessibility of phenolic acids and flavonoids. A volume of 5 mL of each sample was combined with 20 mL of simulated gastric fluid having 1.5 mL of 3.2 g/L pepsin. Using 1 M of HCl, the pH of the mixture was brought to 1.7. Next, the mixture was incubated at 37 °C for a period of 2 h with constant shaking. Following the first 2 h, the pH of the mixture was brought to 7 using 1 M of NaOH. A volume of 4 mL of 5 g/L bile salt and 1 mL of 0.75 M CaCl2 were added to the mixture, as well as 2.5 mL of 4.8 g/L of lipase. Using 1 M of NaOH, the pH of the mixture was adjusted to 7 incubated at 37 °C for 2 h with constant shaking. After 2 h of incubation, an aliquot of the sample was collected and centrifuged at 4 °C for 15 min at 5000 × g. The supernatant was straight away analyzed for phenolic acids (gallic acid, vanillic acid, syringic acid, and salicylic acid) and flavonoids (catechin, epicatechin, and quercetin-3-glucoside) using LC–MS/MS as described earlier (Section 2.10). The bioaccessibility of each polyphenol was then calculated using Eq. 3:
where Cd and Cud are the concentrations of the individual polyphenols in the digested tea sample and the undigested tea sample, respectively.
2.12 Data analysis
The generated optimization models and two-factor graphics were developed using the Design-Expert software 9.0 (Trial version, Stat-Ease Inc., Minneapolis, USA). One-way ANOVA with post-hoc Duncan’s test (SPSS, version 21) was used to assess the statistical significance of independent variables and their correlation. The significance of the results was given at p ≤ 0.05. The optimum parameters were estimated considering the desirability function. The adequacy of the optimization model was determined based on the coefficient of determination (R2), adjusted coefficient of determination (adj. R2), coefficient of variation (CV), and Fisher’s test value (F-value). The regression coefficients were considered significant at p < 0.05.
3 Results and discussion
3.1 Physical, chemical, and antioxidant characteristics of date plum fruit
The current study found that fresh fruits are almost spherical (21.7 ± 4.03 mm in width and 19.2 ± 1.34 mm in length) with a flesh/seed ratio of 3.1 ± 0.65 for each fruit at the fully ripe stage. The color attribute of fresh fruits was presented as L*, a*, and b* values were 37.2 ± 3.9, 6.4 ± 2.5, and 14.5 ± 3.9, respectively. Similar findings were also reported previously [14]. The dry matter, pH, and total soluble solids were 44.6 ± 2.0%, 7.1 ± 0.7, and 11.3 ± 1.0%, respectively. It has previously been observed that the total soluble solids of D. lotus fresh pulp were about 13% [4].
Data from numerous research indicate that date plum has a wide range of bioactivities as a good source of minerals, polyphenols, and antioxidants [2, 3, 9, 14, 29, 30]. The results of the current study show that the total phenolic and flavonoid contents of date plum fruits were 0.99 ± 0.09 mg GAE/g and 0.26 ± 0.22 mg ECE /g, respectively. These findings are slightly higher than those in our previous study [14] in which we reported TPC = 0.81 ± 0.01 mg GAE/g, and TFC = 0.23 ± 0.10 mg ECE/g. The TPC is also higher than the value reported by a previous study 12.7 mg GAE/100 g (= 0.13 mg GAE/g) [5]. The FRAP and DPPH values determined in the current study were 8.01 ± 0.80 mmol ISE/g and 12.38 ± 1.02 mmol TE/g, respectively. The FRAP value is slightly higher than our previously reported results (7.15 ± 1.09 mmol ISE/g), while the DPPH value is slightly lower than our previously reported results (14.92 ± 0.88 mmol TE/g).
3.2 Optimization of convective drying of date plum fruit
The drying conditions were optimized with two factors of response surface methodology. Different air velocities of 0.54, 0.75, 1.25, 1.75, and 1.96 m/s, and temperatures of 43.78, 50, 65, 80, and 86.21 °C were applied. The two-factor design was used to examine the effect of drying temperature and air velocities on the TPC, TFC, FRAP, and DPPH of date plum (Table 1). The investigated responses of each run are given in Table 1. The responses included TPC, TFC, FRAP, and DPPH values.
As presented in Table 1, TPC, TFC, FRAP, and DPPH radical scavenging values ranged from 0.61 to 4.92 mg GAE/g, 0.71 to 1.41 mg ECE/g, 3.68 to 16.47 mmol ISE/g, and 4.25 to 18.21 mmol TE/g, respectively. The current study studied 13 experimental runs. The highest FRAP, DPPH, TPC, and TFC values (16.4723 mmol ISE/g, 18.208 mmol TE/g, 1.41 mg ECE/g, and 14.0889 mg GAE/g) were detected at Run 8 (65 °C and 1.9571 m/s), while the lowest TPC, TFC, and DPPH values (0.61143 mg GAE/g, 0.62 mg ECE/g, and 4.24764 mmol TE/g, respectively) were detected at run 11 (43.7868 °C and 1.25 m/s). The minimum value of FRAP (3.67897 mmol TE/g) was identified at run 2 (86.2132 °C and 1.25 m/s).
The coded factors’ reduced second-order models were described as follows:
The outcomes of the ANOVA of the models are presented in Table A – Supplementary File. The models of TPC and TFC showed F values of 29.41 and 25.39 (p < 0.0001 and 0.0002), R2 of 0.9546 and 0.9477, adjusted R2 of 0.9221 and 0.9104, predicted R2 of 0.8418 and 0.8612, CV of 11.63 and 7.78%, and adequate precision of 18.9445 and 13.3960 with an insignificant lack of fit F value 0.3047 and 0.3272 (p < 0.5972 and 0.8072), respectively. These results indicate that the applied models can be suitable for predicting the TPC and TFC of dried D. lotus fruits. The models of FRAP and DPPH radical scavenging are significant (F values: 22.31 and 22.94) and had good R2 of 0.9410 and 0.9425, adjusted R2 of 0.8988 and 0.9014, predicted R2 of 0.7055 and 0.6899, CV of 12.90 and 14.16%, and adequate precision of 15.3454 and 15.5639 with non-significant lack of fits F value 2.15 and 2.97, respectively.
The evidence in Table 1 shows that the high TPC, TFC, and FRAP values ranged from 65 °C, 1.25 m/s, and 65 °C, 1.96 m/s, while the DPPH radical scavenging activity ranged between 65 °C, 1.96 m/s, and 80 °C, 1.75 m/s. The results found that the optimum drying conditions (temperature and air velocity) were at 68 °C and 1.75 m/s with a functional sample. Under these conditions, the predicted values were 4.61 mg GAE/g, 1.39 mg ECE/g, 12.9 mmol TE/g, and 14.2 mmol ISE/g for TPC, TFC, FRAP, and DPPH radical scavenging, respectively.
The long drying time at low temperatures has a significant effect in decreasing the bioactive compounds of fruits. This effect was also seen in previous studies in which the amount of nutrients in fruits, such as apricots [31] and cranberries [32], decreased with longer drying periods at lower temperatures. The two-factor response surface plots of the different responses are shown in Fig. 1. The response surface methodology was carried out to determine the optimum conditions of dried date plum with the maximum amount of TPC, TFC, and antioxidant properties. According to statistical analysis outlined in Table A – Supplementary File, the drying conditions had significant effects on TPC (p < 0.0001), TFC (p < 0.0002), FRAP (p < 0.0004), and DPPH (p < 0.0003). As seen in Fig. 1, the two-factor response surface plots of the responses increased with an elevation in temperature up to 68 °C, and then, these values were lower at 80 and 86 °C. Thus, based on the results above, the optimizated drying condition was identified as 68 °C (drying temperature) and 1.75 m/s (air velocity).
3.3 Effect of optimum drying conditions on the date plum fruit microstructure
The evidence presented thus far suggests that a considerable variation in the plant material structure and properties can occur during the drying process [33]. The impact of optimum drying on the microstructure and distribution of cells in fresh and dried date plum are shown in Fig. 2. The fresh date plum fruit tissue showed an organized structure consisting of small and precise spherical to oval cells and intercellular spaces. This finding was also reported previously [14] indicating that the fresh date plum fruit tissue showed a well-arranged structure comprising of small and clear spherical to oval cells and intercellular spaces. The SEM micrographs of fruit dried at 68 °C and 1.75 m/s have large, distinguished cell spaces, structured large cells, and intercell contact. As seen in Fig. 2, the cells of the fresh fruit had fewer holes and empty spaces compared with the cell structures of dried fruits, which became deep and large. Thus, the results show that the difference for fresh date plum in terms of structural characteristics is significantly different from the dried sample.
3.4 Preparation of date plum tea at optimum drying conditions
Prior to the preparation of fruit tea, the amount of phenolic acids and flavonoids in the dried fruit (at optimum conditions) was determined using LC–MS/MS. The initial results under optimum conditions showed that date plum tea contained the highest amount of gallic acid, epicatechin, vanillic acid, quercetin-3-glucoside, catechin, salicylic acid, and syringic acid were 219, 40.1, 32.6, 11.0, 7.36, 4.86, and 4.00 mg/L, respectively. This sample was then used to prepare fruit tea samples of different decoction times (5, 10, and 15 min) to identify the best decoction time for the highest polyphenol content. The values of gallic acid, catechin, syringic acid, vanillic acid, epicatechin, quercetin-3-glucoside, and salicylic acid were found in a range of 63.6–103.3, 0.40–2.45, 11.4–13.4, 11.0–11.8, 19.8–23.5, 6.52–9.37, and 9.56–10.95 mg/L, respectively (Table 2).
The decoction process decreased the gallic acid, catechin, vanillic acid, epicatechin, and quercetin-3-glucoside values, except for the syringic acid and salicylic acid values which were increased (Table 2). There were no significant differences between all decoction times on the vanillic acid content. The date plum tea prepared for a decoction time of 5 min contained the highest amount of catechin, vanillic, epicatechin, syringic, and quercetin-3-glucoside. The greatest values of gallic acid and salicylic acid were determined in the 10-min decoction time sample. Based on these results, it is not recommended to extend the decoction time, especially over 10 min. This could potentially result in the oxidation of the phenolic acids and flavonoids thus decreasing their concentration in the tea. Overall, the result in Table 2 shows that the decoction time of tea after 5 min has the highest values of polyphenols. Thus, 5 min of decoction time seems suitable to consume a good amount of polyphenols. Previous studies considering the effect of tea brewing time on polyphenols and antioxidants have also reported similar findings. For example, white tea brewed at 98 °C for 7 min was ideal to extract the highest amount of phenolic compounds and antioxidants [34]. Nevertheless, more studies are required to assess this effect with fruit matrix.
3.5 Bioaccessibility of date plum tea polyphenols
The bioaccessibility of the tea sample of the highest polyphenol content (5 min decoction time) was assessed by in vitro gastrointestinal digestion. Most of the phenolic acids and flavonoids had a very high bioaccessibility, with the highest being salicylic acid, followed by gallic acid (97.2 ± 2.5%), catechin (90.0 ± 0.6%), vanillic acid (87.8 ± 1.7%), quercetin-3-glucoside (84.8 ± 0.4%), and epicatechin (68.3 ± 0.0%). Syringic acid was the only polyphenol with low bioaccessibility (25.0 ± 0.1%). Based on these results, the fruit tea prepared from dried date plum fruit (at optimum conditions) at 5 min decoction time could potentially be a source of highly bioaccessible phenolic acids and flavonoids that are known antioxidants. A very limited number of studies to date have investigated the bioaccessibility of specific polyphenols from tea or herbal drinks. Nevertheless, the tea product in the current study has a higher bioaccessibility of catechin (90.0 ± 0.6%) in comparison to catechin from green tea (23.8%), white tea (19.3%), and black tea (13.0%) after upper gut in vitro digestion [35]. In addition, epicatechin in this study also had a higher bioaccessibility (68.3 ± 0.02%) compared to epicatechin from green tea (23.6%), white tea (19.0%), and black tea (12.7%) [35].
Based on the current findings, date plum could a potential candidate to produce a highly bioaccessible polyphenol-rich tea. Furthermore, the bioactive composition of this tea may be enhanced by combining date plum with ginger (source of gingerols, shogaols and zingerone) [36].
If the fruit tea will be marketed, then it is important to evaluate the stability of polyphenols and antioxidant activity under different conditions. In fact, other bioactivities of the tea could be explored as well as sensory properties for enhanced marketing.
4 Conclusions
The current study looked at the physical and chemical properties, antioxidant capacity, and ideal drying conditions of date plum to obtain polyphenol-rich tea with high bioaccessibility. The optimum drying conditions date plum fruit were at 68 °C and 1.75 m/s. The most prominent finding to emerge from this study is that date plum dried at optimum point has considerable polyphenols. These findings suggest that the date plum tea extracted at a decoction time of 5 min contained the highest values of polyphenols. In addition, the tea product was also a good source of highly bioaccessible (> 80%) gallic acid, catechin, vanillic acid, and quercetin-3-glucoside. The research advances our knowledge of the best conditions for drying date plum fruits and for producing a polyphenol-rich tea with high bioaccessibility.
Data availability
All data supporting this study are included in this manuscript.
References
Hu D, Zhang Q , Luo Z (2008) Phylogenetic analysis in some Diospyros spp.(Ebenaceae) and Japanese persimmon using chloroplast DNA PCR-RFLP markers. Sci Hortic 117(1):32–38
Zeynep AKAR, Karakurt A, Okumuş F, Cinemre S, Düzgün AÖ, Bülent AKAR, Zehra CAN (2020) RP-HPLC-UV Analysis of the phenolic compounds, antimicrobial activity against multi-drug resistant bacteria and antioxidant activity of fruit and seed of diospyros lotus L. ctivity of fruit and seed of diospyros lotus L. Int J Second Metab 7(4):237–246
Gao H, Cheng N, Zhou J, Wang B, Deng J, Cao W (2014) Antioxidant activities and phenolic compounds of date plum persimmon (Diospyros lotus L.) fruits. J Food Sci Technol 51:950-956
Ayoub A, et al (2020) Evaluation of secondary metabolites (antibacterial and antioxidant activity) of Amlok (Diospyros lotus L) fruit extracts of Jammu Region. Studies 7:8
Murathan ZT (2020) Phytochemical screening and antioxidant activity of Diospyros Lotus L. fruits grown in Turkey. Acta Sci Pol Hortorum Cultus 19(2):49–55
Sodeifian G, Saadati Ardestani N, Sajadian SA (2019) Extraction of seed oil from Diospyros lotus optimized using response surface methodology. J For Res 30(2):709–719
Türkmen NB, Özek DA, Taşlidere A, Çiftçi O, Saral Ö, Gül CC (2022) Protective role of Diospyros lotus L. in cisplatin-induced cardiotoxicity: cardiac damage and oxidative stress in rats. Turkish J Pharm Sci 19(2):132
Rauf A et al (2015) In vivo sedative and muscle relaxants activity of Diospyros lotus L. Asian Pac J Trop Biomed 5(4):277–280
Uddin G et al (2014) Anti-nociceptive, anti-inflammatory and sedative activities of the extracts and chemical constituents of Diospyros lotus L. Phytomedicine 21(7):954–959
Rashed K et al (2012) Anti-HIV-1 activity of phenolic compounds isolated from Diospyros lotus fruits. Phytopharmacology 3(2):199–207
Akbari B, Baghaei‐Yazdi N, Bahmaie M, Mahdavi Abhari F (2022) The role of plant‐derived natural antioxidants in reduction of oxidative stress. BioFactors 48(3):611–633
Sahiner M et al (2022) Therapeutic and nutraceutical effects of polyphenolics from natural sources. Molecules 27(19):6225
Raghavi L, Moses J, Anandharamakrishnan C (2018) Refractance window drying of foods: a review. J Food Eng 222:267–275
Hassan AM, Zannou O, Pashazadeh H, Ali Redha A, Koca I (2022) Drying date plum (Diospyros lotus L.) fruit: Assessing rehydration properties, antioxidant activity, and phenolic compounds. J Food Sci 87(10):4394–4415
Khraisheh M, Cooper T, Magee T (1997) Shrinkage characteristics of potatos dehydrated under combined microwave and convective air conditions. Drying Technol 15(3–4):1003–1022
Chen Q et al (2017) Effect of hybrid drying methods on physicochemical, nutritional and antioxidant properties of dried black mulberry. Lwt 80:178–184
Zhang Y et al (2020) Combined medium-and short-wave infrared and hot air impingement drying of sponge gourd (Luffa cylindrical) slices. J Food Eng 284:110043
Srikanth K et al (2019) Convective drying and quality attributes of elephant foot yam (Amorphophallus paeoniifolius). Lwt 99:8–16
Oliveira SM, Brandão TRS, Silva CLM (2015) Influence of drying processes and pretreatments on nutritional and bioactive characteristics of dried vegetables: a review. Food Eng Rev 8(2):134–163
Özcan MM et al (2021) Influence of drying techniques on bioactive properties, phenolic compounds and fatty acid compositions of dried lemon and orange peel powders. J Food Sci Technol 58(1):147–158
Lohachoompol V, Srzednicki G, Craske J (2004) The change of total anthocyanins in blueberries and their antioxidant effect after drying and freezing. J Biomed Biotechnol 2004(5):248
Martin-Gomez J, Varo M, Merida J, Serratosa MP (2017) Bioactive compounds of chamber-dried blueberries at controlled temperature and wines obtained from them. J Chem 2017
Goktas H (2023) Effect of different drying techniques on the bioactive, color, antibacterial and sensory features of date plum fruits (Diospyros Lotus L.). Gıda 48(6):1254–1263
Sahin S (2013) Evaluation of antioxidant properties and phenolic composition of fruit tea infusions. Antioxidants (Basel) 2(4):206–215
Šavikin K et al (2014) Berry fruit teas: phenolic composition and cytotoxic activity. Food Res Int 62:677–683
Kiselova-Kaneva Y et al (2022) High resolution LC-MS/MS characterization of polyphenolic composition and evaluation of antioxidant activity of Sambucus ebulus fruit tea traditionally used in Bulgaria as a functional food. Food Chem 367:130759
Pashazadeh H, Redha AA, Koca I (2024) Effect of convective drying on phenolic acid, flavonoid and anthocyanin content, texture and microstructure of black rosehip fruit. J Food Compos Anal 125:105738
Kaba B, Yıkılkan Y, Pashazadeh H, Ali Redha A, Koca I (2023) Production of cornelian cherry (Cornus mas L.) pulp powder by foam-mat drying: analysis of physicochemical and antioxidant properties. Biomass Convers Biorefinery 1–16
Ahmad MS, Gul H, Ahmad M, Sarwar N, Abbasi SK, Gulfraz M (2014) Phytochemical analysis and biological activities of Diospyros lotus L. fruit extracts. Int J Pharm Chem 4(4):155–159
Ayaz FA, Kadıoǧlu A, Reunanen M (1997) Changes in phenolic acid contents of Diospyros lotus L. during fruit development. J Agric Food Chem 45(7):2539–2541
Vega-Gálvez A, Quispe-Fuentes I, Uribe E, Martinez-Monzo J, Pasten A, Lemus-Mondaca R (2019) Bioactive compounds and physicochemical characterization of dried apricot (Prunus armeniaca L.) as affected by different drying temperatures. CyTA - J Food 17(1):297-306
Zielinska M, Zielinska D (2019) Effects of freezing, convective and microwave-vacuum drying on the content of bioactive compounds and color of cranberries. Lwt 104:202–209
Witrowa-Rajchert D, Rząca M (2009) Effect of drying method on the microstructure and physical properties of dried apples. Drying Technol 27(7–8):903–909
Perez-Burillo S et al (2018) Effect of brewing time and temperature on antioxidant capacity and phenols of white tea: relationship with sensory properties. Food Chem 248:111–118
Annunziata G, Maisto M, Schisano C, Ciampaglia R, Daliu P, Narciso V, Tenore GC, Novellino E (2018) Colon bioaccessibility and antioxidant activity of white, green and black tea polyphenols extract after in vitro simulated gastrointestinal digestion. Nutrients 10(11):1711
Garza-Cadena C et al (2023) A comprehensive review on Ginger (Zingiber officinale) as a potential source of nutraceuticals for food formulations: towards the polishing of gingerol and other present biomolecules. Food Chem 413:135629
Author information
Authors and Affiliations
Contributions
HP and AMAH: conceptualization, methodology, software, data curation, investigation, formal analysis, and writing — original draft. AAR: formal analysis, writing — original draft, and writing — review and editing. IK: conceptualization and supervision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests. For the purpose of open access, the author, Ali Ali Redha, has applied a “Creative Commons Attribution (CC BY) license to any Author Accepted Manuscript version arising.”
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pashazadeh, H., Ali Redha, A., Hassan, A.M.A. et al. Optimizing the drying conditions of date plum (Diospyros lotus L.) to conserve its phenolic content and antioxidants for preparing a highly bioaccessible polyphenol-rich tea. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05683-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05683-2