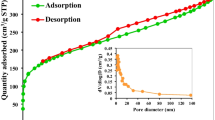
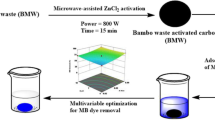
Abstract
Herein, blended mandarin (Citrus reticulata) peel (MP) and algae (AG) biomass were thermochemically treated (TCTMPAG) to yield a cost-effective and renewable adsorbent for removal of methylene blue (MB), a known toxic cationic dye. The preparation included microwave irradiation, in conjunction with H3PO4 activation at 800 W for 15 min in a nitrogen atmosphere. The adsorption characteristics of TCTMPAG were studied by assessing its capacity to remove methylene blue (MB) dye from aqueous media. The Box-Behnken design (BBD) was used to optimize key adsorption factors, namely A: TCTMPAG dosage (0.02–0.12 g/0.1 L), B: pH (4–10), and C: contact period (30–420) min. The BBD model determined that the highest elimination of MB (98.4%) occurred for a TCTMPAG dosage of 0.12 g/0.1L, pH 10, and a contact time of 225 min. The MB dye adsorption rate profile conformed to a pseudo-second-order (PSO) model, while the Langmuir and Temkin model adequately represented the equilibrium adsorption profile (R2 = 0.97). The highest adsorption capacity (qmax) of TCTMPAG for MB dye was determined to be 48.5 mg/g. Various contributions to the adsorption mechanism include various contributions such as electrostatic forces, H-bonding, pore filling, and π-π stacking onto the TCTMPAG adsorbent surface.









Similar content being viewed by others
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Cigeroglu Z, El Messaoudi N, Şenol ZM, Baskan G, Georgin J, Gubernat S (2024) Clay-based nanomaterials and their adsorptive removal efficiency for dyes and antibiotics: a review. Mater 26:100735. https://doi.org/10.1016/j.mtsust.2024.100735
Wang X, Zhang P, Xu F, Sun B, Hong G, Bao L (2022) Adsorption of methylene blue on azo dye wastewater by molybdenum disulfide nanomaterials. Sustainability 14(13):7585. https://doi.org/10.3390/su14137585
Yu YQ, Liu SH, Pei Y, Luo XG (2021) Growing Pd NPs on cellulose microspheres via in-situ reduction for catalytic decolorization of methylene blue. Int J Biol Macromol 166:1419–1428
Alvarenga G, Lima JP, Goszczynski A, Rosa CH (2020) Methylene blue adsorption by timbaúva (Enterolobium contortisiliquum)- derived materials. Environ Sci Pollut Res 27:27893–27903
Azam K, Shezad N, Shafiq I, Akhter P, Akhtar F, Jamil F, Shafique S (2022) A review on activated carbon modifications for the treatment of wastewater containing anionic dyes. Chemosphere 306:135566. https://doi.org/10.1016/j.chemosphere.2022.135566
Sultana M, Rownok MH, Sabrin M, Rahaman MH, Alam SN (2022) A review on experimental chemically modified activated carbon to enhance dye and heavy metals adsorption. Clean Eng Technol 6:100382. https://doi.org/10.1016/j.clet.2021.100382
Ramutshatsha-Makhwedzha D, Mavhungu A, Moropeng ML, Mbaya R (2022) Activated carbon derived from waste orange and lemon peels for the adsorption of methyl orange and methylene blue dyes from wastewater. Heliyon 8:09930. https://doi.org/10.1016/j.heliyon.2022.e09930
Zaharia MM, Vasiliu AL, Trofin MA, Pamfil D, Bucatariu F, Racovita S, Mihai M (2021) Design of multifunctional composite materials based on acrylic ion exchangers and CaCO3 as sorbents for small organic molecules. React Funct Polym 166:104997–105012
Raninga M, Mudgal A, Patel VK, Patel J, Sinha MK (2023) Modification of activated carbon-based adsorbent for removal of industrial dyes and heavy metals: a review. Materials Today: Proceedings 77:286–294. https://doi.org/10.1016/j.matpr.2022.11.358
Arslan DŞ, Ertap H, Şenol ZM, El Messaoudi N, Mehmeti V (2023) Preparation of polyacrylamide titanium dioxide hybrid nanocomposite by direct polymerization and its applicability in removing crystal violet from aqueous solution. J Environ Polym Degrad 2:1–5. https://doi.org/10.1007/s10924-023-03004-8
de Toledo WDMC, Pinheiro RA, Trava-Airoldi VJ, Corat EJ (2022) Development of boron-doped diamond (BDD) deposited on carbon nanotubes (CNT) to form BDD/CNT structures relevant for electrochemical degradation. Diamond Relat Mater 127:109159–1109170
Ihaddaden S, Aberkane D, Boukerroui A, Robert D (2022) Removal of methylene blue (basic dye) by coagulation-flocculation with biomaterials (bentonite and Opuntia ficus indica). J Water Process Eng 49:102952–102964
Ruthiraan M, Mubarak NM, Abdullah EC, Khalid M, Nizamuddin S, Walvekar R, Karri RR (2019) An overview of magnetic material: preparation and adsorption removal of heavy metals from wastewater. Magnetic Nanostruct. Nanotechnology in the Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-16439-3_8
Khan FSA, Mubarak NM, Khalid M, Walvekar R, Abdullah EC, Mazari SA, Karri RR (2020) Magnetic nanoadsorbents’ potential route for heavy metals removal—a review. Environ Sci Pollut Res 27(19):24342–24356
Saghi MH, Qasemi M, Alidadi H, Alahabadi A, Rastegar A, Kowsari MH, Shams M (2020) Vanadium oxide nanoparticles for methylene blue water remediation: exploring the effect of physicochemical parameters by process modeling. J Mol Liq 318:114046. https://doi.org/10.1016/j.molliq.2020.114046
Jawad AH, Sahu UK, Mastuli MS, ALOthman ZA, Wilson LD, (2022) Multivariable optimization with desirability function for carbon porosity and methylene blue adsorption by watermelon rind activated carbon prepared by microwave assisted H3PO4. Biomass Convers Biomass Convers Biorefinery 1:15. https://doi.org/10.1007/s13399-022-02423-2
Mustafa I (2019) Methylene blue removal from water using H2SO4 crosslinked magnetic chitosan nanocomposite beads. Microchem J 144:397–402. https://doi.org/10.1016/j.microc.2018.09.032
He K, Zeng G, Chen A, Huang Z, Peng M, Huang T, Chen G (2019) Graphene hybridized polydopamine-kaolin composite as effective adsorbent for methylene blue removal. Compos B Eng 161:141–149. https://doi.org/10.1016/j.compositesb.2018.10.063
Hoang AT, Kumar S, Lichtfouse E, Cheng CK, Varma RS, Senthilkumar N, Nguyen XP (2022) Remediation of heavy metal polluted waters using activated carbon from lignocellulosic biomass: an update of recent trends. Chemosphere 302:134825–134848
Arumugasamy SK, Chellasamy G, Sekar S, Lee S, Govindaraju S, Yun K (2022) TriMOF synergized on the surface of activated carbon produced from pineapple leaves for the environmental pollutant reduction and oxygen evolution process. Chemosphere 286:131893. https://doi.org/10.1016/j.chemosphere.2021.131893
El Khomri M, El Messaoudi N, Dbik A, Bentahar S, Lacherai A, Chegini ZG, Bouich A (2021) Removal of Congo red from aqueous solution in single and binary mixture systems using Argan nutshell wood. PRT 51(5):477–488. https://doi.org/10.1108/PRT-04-2021-0045
Jimoh OS, Ibrahim AO, Bello OS (2022) Metformin adsorption onto activated carbon prepared by acid activation and carbonization of orange peel. Int J Phyto 25(2):125–136. https://doi.org/10.1080/15226514.2022.2064815
Karri RR, Sahu JN, Meikap BC (2020) Improving efficacy of Cr (VI) adsorption process on sustainable adsorbent derived from waste biomass (sugarcane bagasse) with help of ant colony optimization. Ind Crops Prod 143:111927
Tripathy A, Mohanty S, Nayak SK, Ramadoss A (2021) Renewable banana-peel-derived activated carbon as an inexpensive and efficient electrode material showing fascinating supercapacitive performance. J Environ Chem Eng 9(6):106398. https://doi.org/10.1016/j.jece.2021.106398
Saadi W, Rodríguez-Sánchez S, Ruiz B, Najar-Souissi S, Ouederni A, Fuente E (2022) From pomegranate peels waste to one-step alkaline carbonate activated carbons. Prospect as sustainable adsorbent for the renewable energy production. J Environ Chem Eng 10(1):107010. https://doi.org/10.1016/j.jece.2021.107010
Herrera-Barros A, Bitar-Castro N, Villabona-Ortíz A, Tejada-Tovar C, Gonzalez-Delgado AD (2020) Nickel adsorption from aqueous solution using lemon peel biomass chemically modified with TiO2 nanoparticles. Sustain Chem Pharm 17:100299
Alamze M, Madiha Tullah M, Ihsanullah SA, Khan B, Setzer WN, Al-Zaqri N, Ibrahim MNM (2022) Kinetic, thermodynamic and adsorption isotherm studies of detoxification of Eriochrome Black T dye from wastewater by native and washed garlic peel. Water 14:3713
Eleryan A, Yılmaz M, El-Nemr MA, Ragab S, Helal M, Hassaan MA, Nemr AE (2022) Mandarin Biochar-TETA (MBT) prepared from Citrus reticulata peels for adsorption of Acid Yellow 11 dye from water. Sci Rep 12:17797
Anticona M, Blesa J, Lopez-Malo D, Frigola A, Esteve MJ (2021) Effects of ultrasound-assisted extraction on physicochemical properties, bioactive compounds, and antioxidant capacity for the valorization of hybrid Mandarin peels. Food Biosci 42:101185
Mahmoud ME, Mohamed AK (2020) Novel derived pectin hydrogel from mandarin peel based metal-organic frameworks composite for enhanced Cr(VI) and Pb(II) ions removal. Int J Biol Macromol 164:920
Pavan FA, Lima IS, Lima EC, Airoldi C, Gushikem Y (2006) Use of Ponkan mandarin peels as biosorbent for toxic metals uptake from aqueous solutions. J Hazard Chem Mater 137:527–533
Koyuncu F, Güzelb F, Saygılı H (2018) Role of optimization parameters in the production of nanoporous carbon from mandarin shells by microwave-assisted chemical activation and utilization as dye adsorbent. Adv Powder Technol 29:2108
Besegatto SV, Martins ML, Lopes TJ, da Silva A (2021) Multivariated calibration as a tool for resolution of color from mandarin peel and dyes in aqueous solution for bio-adsorption studies. J Environ Chem Eng 9:104605
Park H, Kim J, Lee YG, Chon K (2021) Enhanced adsorptive removal of dyes using mandarin peel biochars via chemical activation with NH4Cl and ZnCl2. Water 13:1495
Eldeeb TM, Aigbe UO, Ukhurebor KE, Onyancha RB, El-Nemr MA, Hassaan MA, Osibote OA, Ragab S, Okundaye B, Balogun VA (2024) Biosorption of acid brown 14 dye to mandarin-CO-TETA derived from mandarin peels. Bioref 14:5053–5073. https://doi.org/10.1007/s13399-022-02664-1
Sun W, Sun W, Wang Y (2019) Biosorption of Direct Fast Scarlet 4BS from aqueous solution using the green-tide-causing marine algae Enteromorpha prolifera. Spectrochim Acta Part A: Molecul Biomol Spect 223:117347
El Messaoudi N, Ciğeroğlu Z, Şenol ZM, Kazan-Kaya ES, Fernine Y, Gubernat S, Lopicic Z (2024) Green synthesis of CuFe2O4 nanoparticles from bioresource extracts and their applications in different areas: a review. Biomass Conversion and Biorefinery 6:1–22. https://doi.org/10.1007/s13399-023-05264-9
Almomani F, Bohsale RR (2021) Bio-sorption of toxic metals from industrial wastewater by algae strains Spirulina platensis and Chlorella vulgaris: application of isotherm, kinetic models and process optimization. Sci Total Environ 755:142654
Sarojini G, Babu SV, Rajamohan N, Rajasimman M, Pugazhendhi A (2022) Application of a polymer-magnetic-algae based nano-composite for the removal of methylene blue–characterization, parametric and kinetic studies. Environ Pollut 292:118376
Hoang AT, Kumar S, Lichtfouse E, Cheng CK, Varma RS, Senthilkumar N, Nguyen XP (2022) Remediation of heavy metal polluted waters using activated carbon from lignocellulosic biomass: an update of recent trends. Chemosphere 302:134825. https://doi.org/10.1016/j.chemosphere.2022.134825
Foong SY, Liew RK, Yang Y, Cheng YW, Yek PNY, Mahari WAW, Lam SS (2020) Valorization of biomass waste to engineered activated biochar by microwave pyrolysis: progress, challenges, and future directions. Chem Eng J 389:124401
Sirajo L, MaZ Z (2022) Adsorption of water pollutants using H3PO4-activated lignocellulosic agricultural waste: a mini review. Toxin Reviews 42(1):349–361. https://doi.org/10.1080/15569543.2022.2062775
Genli N, Kutluay S, Baytar O, . Şahi̇N Ö, (2021) Preparation and characterization of activated carbon from hydrochar by hydrothermal carbonization of chickpea stem: an application in methylene blue removal by RSM optimization. Int J Phyto 24(1):88–100. https://doi.org/10.1080/15226514.2021.1926911
Uçar S, Erdem M, Tay T, Karagöz S (2014) Removal of lead (II) and nickel (II) ions from aqueous solution using activated carbon prepared from rapeseed oil cake by Na2CO3 activation. Clean Tech and Env Pol 17(3):747–756. https://doi.org/10.1007/s10098-014-0830-8
Al-Qaessi F (2010) Production of activated carbon from date stones by using zinc chloride. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects 32(10):917–930. https://doi.org/10.1080/15567030903493062
Wong S, Yac’cob NaN, Hassan O, Inuwa IM (2018) From pollutant to solution of wastewater pollution: synthesis of activated carbon from textile sludge for dye adsorption. Chinese J Chem Eng 26(4):870–878. https://doi.org/10.1016/j.cjche.2017.07.015
Chiang CH, Chen J, Lin JH (2020) Preparation of pore-size tunable activated carbon derived from waste coffee grounds for high adsorption capacities of organic dyes. J Environ Chem Eng 8(4):103929. https://doi.org/10.1016/j.jece.2020.103929
Jawad AH, Saber SEM, Abdulhameed AS, Reghioua A, ALOthman ZA, Wilson LD (2022) Mesoporous-activated carbon from mangosteen (Garcinia mangostana) peels by H3PO4 assisted microwave: optimization, characterization, and adsorption mechanism for methylene blue dye removal. Diamond Relat Mater 129:109389. https://doi.org/10.1016/j.diamond.2022.109389
Farki NNANLT, Abdulhameed AS, Surip SN, Alothman ZA, Jawad AH (2023) Tropical fruit wastes including durian seeds and rambutan peels as a precursor for producing activated carbon using H3PO4 -assisted microwave method: RSM-BBD optimization and mechanism for methylene blue dye adsorption. Int J Phyto 25(12):1567–1578. https://doi.org/10.1080/15226514.2023.2175780
Dalvand A, Nabizadeh R, Ganjali MR, Khoobi M, Nazmara S, Mahvi AH (2016) Modeling of Reactive Blue 19 azo dye removal from colored textile wastewater using L-arginine-functionalized Fe3O4 nanoparticles: optimization, reusability, kinetic and equilibrium studies. J Magnet Magnet Mater 404:179–189
Hu L, Peng Y, Wu F, Peng S, Li J, Liu Z (2017) Tubular activated carbons made from cotton stalk for dynamic adsorption of airborne toluene. J Taiwan Institute Chem Eng 80:399–405. https://doi.org/10.1016/j.jtice.2017.07.029
Sing KS (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl Chem 57(4):603–619
Abdul Khalil HPS, Jawaid M, Firoozian P, Rashid U, Islam A, Akil HM (2013) Activated carbon from various agricultural wastes by chemical activation with KOH: preparation and characterization. J Biobased Mater Bioenergy 7(6):708–714
Bisht D, Sinha S, Nigam S, Bisaria K, Mehrotra T, Singh R (2021) Adsorptive decontamination of paper mill effluent by nano fly ash: response surface methodology, adsorption isotherm and reusability studies. Water Sci Technol 83(7):1662–1676
Xu J, Chen L, Qu H, Jiao Y, Xie J, Xing G (2014) Preparation and characterization of activated carbon from reedy grass leaves by chemical activation with H3PO4. Appl Surface Sci 320:674–680. https://doi.org/10.1016/j.apsusc.2014.08.178
Köseoğlu E, Akmil-Başar C (2015) Preparation, structural evaluation and adsorptive properties of activated carbon from agricultural waste biomass. Adv Powder Technol 26(3):811–818
Masoudian N, Rajabi M, Ghaedi M (2019) Titanium oxide nanoparticles loaded onto activated carbon prepared from bio-waste watermelon rind for the efficient ultrasonic-assisted adsorption of Congo red and phenol red dyes from wastewaters. Polyhedron 173:114105–114114
Kutluay S, Temel F (2021) Silica gel based new adsorbent having enhanced VOC dynamic adsorption/desorption performance. Colloids Surf A: Physicochem Eng Asp 609:125848
Foroutan R, Peighambardoust SJ, Mohammadi R, Peighambardoust SH, Ramavandi B (2022) Development of new magnetic adsorbent of walnut shell ash/starch/Fe3O4 for effective copper ions removal: treatment of groundwater samples. Chemosphere 296:133978
Ashrafi SD, Safari GH, Sharafi K, Kamani H, Jaafari J (2021) Adsorption of 4-Nitrophenol on calcium alginate-multiwall carbon nanotube beads: modeling, kinetics, equilibriums and reusability studies. Int J Biol Macromol 185:66–76
Shukla SK, Pandey S, Saha S, Singh HR, Mishra PK, Kumar S, Jha SK (2021) Removal of crystal violet by Cu-chitosan nano-biocomposite particles using Box-Behnken design. J Environ Chem Eng 9(5):105847
Jayan N, Laxmi DBM, Akbar ST (2021) Central composite design for adsorption of Pb(II) and Zn(II) metals on PKM-2 Moringa oleifera leaves. ACS Omega 6(39):25277–25298. https://doi.org/10.1021/acsomega.1c03069
Rose PK, Poonia V, Kumar R, Kataria N, Sharma P, Lamba J, Bhattacharya P (2023) Congo red dye removal using modified banana leaves: adsorption equilibrium, kinetics, and reusability analysis. Groundw Sustain Dev 23:101005. https://doi.org/10.1016/j.gsd.2023.101005
Amin MT, Alazba AA, Shafiq M (2021) Successful application of eucalyptus camdulensis biochar in the batch adsorption of crystal violet and methylene blue dyes from aqueous solution. Sustain 13(7):3600
Lagergren S (1898) Zur theorie der sogenannten adsorption geloster stoffe. Vet Akad Handl 24:1–39
Ho YS, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70:115–124
Theamwong N, Intarabumrung W, Sangon S, Aintharabunya S, Ngernyen Y, Hunt AJ, Supanchaiyamat N (2021) Activated carbons from waste Cassia bakeriana seed pods as high-performance adsorbents for toxic anionic dye and ciprofloxacin antibiotic remediation. Bioresour Technol 341:125832–125842
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Frenudlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–471
Temkin MI (1940) Kinetics of ammonia synthesis on promoted iron catalysts, Acta physiochim. URSS 12:327–356
Jin Y, Liu F, Li Y, Du Q, Song F, Chen B, Chen K, Zhang Y, Wang M, Sun Y, Zhao S, Jing Z, Pi X, Wang YQ, Wang D (2023) Efficient adsorption of azo anionic dye Congo Red by micro-nano metal-organic framework MIL-68(Fe) and MIL-68(Fe)/chitosan composite sponge: preparation, characterization and adsorption performance. Int J Biol Macromol 252:126198. https://doi.org/10.1016/j.ijbiomac.2023.126198
Francis AO, Kevin OS, Zaini MAA (2023) Vitex doniana seed activated carbon for methylene blue adsorption: equilibrium and kinetics. Int J Phytoremediation 25(12):1625–1635. https://doi.org/10.1080/15226514.2023.2179013
Durrani W, Nasrullah A, Khan A, Fagieh TM, Bakhsh EM, Akhtar K, Khan SB, Din IU, Khan MS, Bokhari A (2022) Adsorption efficiency of date palm based activated carbon-alginate membrane for methylene blue. Chemosphere 302:134793. https://doi.org/10.1016/j.chemosphere.2022.134793
Yadav S, Dhakate SR, Singh BP (2022) Carbon nanotube incorporated eucalyptus-derived activated carbon-based novel adsorbent for efficient removal of methylene blue and eosin yellow dyes. Bioresour Technol 344(Pt B):126231. https://doi.org/10.1016/j.biortech.2021.126231
Tuli F, Hossain A, Kibria AF, Tareq ARM, Mamun S, Ullah AKMA (2020) Removal of methylene blue from water by low-cost activated carbon prepared from tea waste: a study of adsorption isotherm and kinetics. Environ Nanotechnol Monit Manag 14:100354. https://doi.org/10.1016/j.enmm.2020.100354
Gupta S, Vishesh Y, Sarvshrestha N, Bhardwaj AS, Kumar A, Topare NS, Raut-Jadhav S, Bokil SA, Asiri AM (2022) Adsorption isotherm studies of methylene blue using activated carbon of waste fruit peel as an adsorbent. Mater Today Proc 57:1500–1508. https://doi.org/10.1016/j.matpr.2021.12.044
Gao X, Xu D, Gao Y, Chen G, Zhai R, Huang X, Liu G (2021) Mussel-inspired triple bionic adsorbent: facile preparation of layered double hydroxide@ polydopamine@ metal-polyphenol networks and their selective adsorption of dyes in single and binary systems. J Hazard Mater 420:126609
Al-musawi HA, Al-Mammar DE (2021) Adsorption of naphthol green B dye on to Ca-montmorillonite and nano-composite Ca-montmorillonite clay. Annals Romanian Soc Cell Biol 25(5):2797–2810
Ahmad R, Ansari K (2022) Fabrication of alginate@ silver nanoparticles (Alg@AgNPs) bionanocomposite for the sequestration of crystal violet dye from aqueous solution. Int J Biol Macromol 218:157–167
Ali NM, Mohammed AJ (2023) Removal of malachite green by poly acrylic beads. Wasit Journal of Pure Sciences 2(1):55–66. https://doi.org/10.31185/wjps.117
Mahapatra U, Chatterjee A, Das C, Manna AK (2021) Adsorptive removal of hexavalent chromium and methylene blue from simulated solution by activated carbon synthesized from natural rubber industry biosludge. Environ Technol Innova 22:101427
Dao MU, Le HS, Hoang HY, Tran VA, Doan VD, Le TTN, Sirotkin A (2021) Natural core-shell structure activated carbon beads derived from Litsea glutinosa seeds for removal of methylene blue: facile preparation, characterization, and adsorption properties. Environ Res 198:110481
Acknowledgements
The authors are thankful to the Faculty of Applied Sciences, Universiti Teknologi MARA (UiTM) Shah Alam, Malaysia for the research facilities. The author (Zeid A. ALOthman) is grateful to the Researchers Supporting Project No. (RSP2024R1), King Saud University, Riyadh, Saudi Arabia.
Funding
The author (Zeid A. ALOthman) is grateful to the Researchers Supporting Project No. (RSP2024R1), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Ali H. Jawad: conceptualization, resources, visualization, supervision, project administration, writing—review and editing. Siti Nabihah Jumadi: formal analysis, validation, data curation, methodology, software, writing—original. Zeid A. ALOthman: formal analysis, validation, visualization. Lee D. Wilson: writing—review and editing, visualization.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jawad, A.H., Jumadi, S.N., ALOthman, Z.A. et al. Thermochemical treatment of mixed mandarin peel and algae via microwave and H3PO4 activation: process optimization and adsorption mechanism for methylene blue dye. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05598-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05598-y