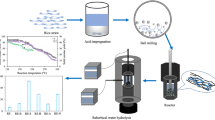
Abstract
The economy of the bioethanol production process can be improved by increasing the initial solid content and reducing the reaction time. The enzymatic approach utilizing intermittent ball milling has been proposed as a viable solution to tackle these challenges effectively. This study demonstrated that intermittent ball milling during high-solids enzymatic hydrolysis for a duration of 12 h showed a significantly higher total sugar yield, with an increase of 25.8% compared to continuous ball milling enzymatic hydrolysis method. The subsequent investigation comprehensively examined the impact of multi-conditional parameter variations on sugar yield during intermittent ball milling. The orthogonal experiment resulted in the determination of optimal process parameters, including a solid–liquid ratio of 25%, ball milling time (per cycle) of 5 min, ball milling frequency of 50 Hz, 6 balls used during the process, and an incubation temperature of 50℃ under optimized conditions. The conditions led to the achievement of a total reducing sugar concentration of 103.8 g/L, a total sugar yield of 415.1 mg/g, and an enzymatic hydrolysis yield of 72.4%. The optimized intermittent ball milling process exhibited superior hydrolysis yield and shorter reaction time compared to other enzymatic methods with untreated biomass. This study holds significant implications for the eventual industrialization of bioethanol refinement.






Similar content being viewed by others
Data availability
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
References
Zhang H, Han L, Dong H (2021) An insight to pretreatment, enzyme adsorption and enzymatic hydrolysis of lignocellulosic biomass: experimental and modeling studies. Renew Sust Energ Rev 140:110758. https://doi.org/10.1016/j.rser.2021.110758
Zhou Z, Lei F, Li P, Jiang J (2018) Lignocellulosic biomass to biofuels and biochemicals: a comprehensive review with a focus on ethanol organosolv pretreatment technology. Biotechnol Bioeng 115(11):2683–2702. https://doi.org/10.1002/bit.26788
Haldar D, Purkait MK (2020) Lignocellulosic conversion into value-added products: a review. Process Biochem 89:110–133. https://doi.org/10.1016/j.procbio.2019.10.001
Nababan MYS, Fatriasari W, Wistara NJ (2020) Response surface methodology for enzymatic hydrolysis optimization of jabon alkaline pulp with Tween 80 surfactant addition. Biomass Convers Bior 12(6):2165–2174. https://doi.org/10.1007/s13399-020-00807-w
Khare SK, Pandey A, Larroche C (2015) Current perspectives in enzymatic saccharification of lignocellulosic biomass. Biochem Eng J 102:38–44. https://doi.org/10.1016/j.bej.2015.02.033
Yang Y, Jalalah M, Alsareii SA, Harraz FA, Almadiy AA, Thakur N, Salama E-S (2023) Enhancement of total reducing sugar content from seaweeds (SWs) biomass via pretreatment for ethanol production: an optimized study. Biomass Convers Bior 1–12. https://doi.org/10.1007/s13399-023-05186-6
Chen H, Qiu W (2010) Key technologies for bioethanol production from lignocellulose. Biotechnol Adv 28(5):556–562. https://doi.org/10.1016/j.biotechadv.2010.05.005
Gatt E, Khatri V, Bley J, Barnabe S, Vandenbossche V, Beauregard M (2019) Enzymatic hydrolysis of corn crop residues with high solid loadings: new insights into the impact of bioextrusion on biomass deconstruction using carbohydrate-binding modules. Bioresour Technol 282:398–406. https://doi.org/10.1016/j.biortech.2019.03.045
Ahmad A, Naqvi SA, Jaskani MJ, Waseem M, Ali E, Khan IA, Faisal Manzoor M, Siddeeg A, Aadil RM (2021) Efficient utilization of date palm waste for the bioethanol production through Saccharomyces cerevisiae strain. Food Sci Nutr 9(4):2066–2074. https://doi.org/10.1002/fsn3.2175
Sarris D, Papanikolaou S (2016) Biotechnological production of ethanol: biochemistry, processes and technologies. Eng Life Sci 16(4):307–329. https://doi.org/10.1002/elsc.201400199
Kumar R, Tabatabaei M, Karimi K, Sárvári Horváth I (2016) Recent updates on lignocellulosic biomass derived ethanol - a review. Biofuel Res J 3(1):347–356. https://doi.org/10.18331/brj2016.3.1.4
Chi X, Liu C, Bi YH, Yu G, Zhang Y, Wang Z, Li B, Cui Q (2019) A clean and effective potassium hydroxide pretreatment of corncob residue for the enhancement of enzymatic hydrolysis at high solids loading. RSC Adv 9(20):11558–11566. https://doi.org/10.1039/c9ra01555h
Yildirim O, Tunay D, Ozkaya B (2021) Optimization of enzymatic hydrolysis conditions of chemical pretreated cotton stalk using response surface methodology for enhanced bioethanol production yield. Biomass Convers Bior 13(8):6623–6634. https://doi.org/10.1007/s13399-021-01692-7
Huang C, Jiang X, Shen X, Hu J, Tang W, Wu X, Ragauskas A, Jameel H, Meng X, Yong Q (2022) Lignin-enzyme interaction: a roadblock for efficient enzymatic hydrolysis of lignocellulosics. Renew Sust Energ Rev 154:111822. https://doi.org/10.1016/j.rser.2021.111822
Zhou H, Tan L, Fu Y, Zhang H, Liu N, Qin M, Wang Z (2019) Rapid nondestructive fractionation of biomass (</=15 min) by using flow-through recyclable formic acid toward whole valorization of carbohydrate and lignin. Chemsuschem 12(6):1213–1221. https://doi.org/10.1002/cssc.201802803
Chu Q, Song K, Bu Q, Hu J, Li F, Wang J, Chen X, Shi A (2018) Two-stage pretreatment with alkaline sulphonation and steam treatment of Eucalyptus woody biomass to enhance its enzymatic digestibility for bioethanol production. Energ Convers Manage 175:236–245. https://doi.org/10.1016/j.enconman.2018.08.100
Hernández-Beltrán JU, Hernández-Escoto H (2018) Enzymatic hydrolysis of biomass at high-solids loadings through fed-batch operation. Biomass Bioenerg 119:191–197. https://doi.org/10.1016/j.biombioe.2018.09.020
Cui M, Li X (2023) Additives Enhancing Enzymatic Hydrolysis of Wheat Straw to Obtain Fermentable Sugar. Appl Biochem Biotechnol 195(2):1059–1071. https://doi.org/10.1007/s12010-022-04200-3
Chu Q, Wang R, Tong W, Jin Y, Hu J, Song K (2020) Improving enzymatic saccharification and ethanol production from hardwood by deacetylation and steam pretreatment: insight into mitigating lignin inhibition. ACS Sustainable Chem Eng 8(49):17967–17978. https://doi.org/10.1021/acssuschemeng.0c05583
Wang W, Wang X, Zhang Y, Yu Q, Tan X, Zhuang X, Yuan Z (2020) Effect of sodium hydroxide pretreatment on physicochemical changes and enzymatic hydrolysis of herbaceous and woody lignocelluloses. Ind Crop Prod 145:112145. https://doi.org/10.1016/j.indcrop.2020.112145
Kadhum HJ, Mahapatra DM, Murthy GS (2019) A comparative account of glucose yields and bioethanol production from separate and simultaneous saccharification and fermentation processes at high solids loading with variable PEG concentration. Bioresour Technol 283:67–75. https://doi.org/10.1016/j.biortech.2019.03.060
Zheng T, Yang L, Ding M, Huang C, Yao J (2022) Metal-organic framework promoting high-solids enzymatic hydrolysis of untreated corncob residues. Bioresour Technol 344(Pt A):126163. https://doi.org/10.1016/j.biortech.2021.126163
Cai X, Hu CH, Wang J, Zeng XH, Luo JX, Li M, Liu ZQ, Zheng YG (2021) Efficient high-solids enzymatic hydrolysis of corncobs by an acidic pretreatment and a fed-batch feeding mode. Bioresour Technol 326:124768. https://doi.org/10.1016/j.biortech.2021.124768
Tai C, Keshwani DR, Voltan DS, Kuhar PS, Engel AJ (2015) Optimal control strategy for fed-batch enzymatic hydrolysis of lignocellulosic biomass based on epidemic modeling. Biotechnol Bioeng 112(7):1376–1382. https://doi.org/10.1002/bit.25552
Perez-Venegas M, Juaristi E (2021) Mechanoenzymology: State of the Art and Challenges towards Highly Sustainable Biocatalysis. Chemsuschem 14(13):2682–2688. https://doi.org/10.1002/cssc.202100624
Battista F, Gomez Almendros M, Rousset R, Bouillon P-A (2019) Enzymatic hydrolysis at high lignocellulosic content: Optimization of the mixing system geometry and of a fed-batch strategy to increase glucose concentration. Renew Energy 131:152–158. https://doi.org/10.1016/j.renene.2018.07.038
Godoy CMd, Machado DL, Costa ACd (2019) Batch and fed-batch enzymatic hydrolysis of pretreated sugarcane bagasse – Assays and modeling. Fuel 253:392–399. https://doi.org/10.1016/j.fuel.2019.05.038
Al Amin Leamon AKM, Venegas MP, Orsat V, Auclair K, Dumont MJ (2022) Semisynthetic transformation of banana peel to enhance the conversion of sugars to 5-hydroxymethylfurfural. Bioresour Technol 362:127782. https://doi.org/10.1016/j.biortech.2022.127782
Arciszewski J, Auclair K (2022) Mechanoenzymatic Reactions Involving Polymeric Substrates or Products. Chemsuschem 15(7):e202102084. https://doi.org/10.1002/cssc.202102084
Hammerer F, Loots L, Do JL, Therien JPD, Nickels CW, Friscic T, Auclair K (2018) Solvent-Free Enzyme Activity: Quick, High-Yielding Mechanoenzymatic Hydrolysis of Cellulose into Glucose. Angew Chem Int Ed 57(10):2621–2624. https://doi.org/10.1002/anie.201711643
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2008) Determination of structural carbohydrates and lignin in biomass laboratory analytical procedure. NREL/TP-510-42618.
Lai C, Tu M, Xia C, Shi Z, Sun S, Yong Q, Yu S (2017) Lignin alkylation enhances enzymatic hydrolysis of lignocellulosic biomass. Energy Fuels 31(11):12317–12326. https://doi.org/10.1021/acs.energyfuels.7b02405
Wu Y, Ge S, Xia C, Mei C, Kim K-H, Cai L, Smith LM, Lee J, Shi SQ (2021) Application of intermittent ball milling to enzymatic hydrolysis for efficient conversion of lignocellulosic biomass into glucose. Renew Sust Energ Rev 136:110442. https://doi.org/10.1016/j.rser.2020.110442
Tao X, Zhang P, Zhang G, Nabi M, Jin S, Wang S, Ye J, Liu X (2018) Thermo-carbide slag pretreatment of turfgrass pruning: Physical-chemical structure changes, reducing sugar production, and enzymatic hydrolysis kinetics. Energ Convers Manage 155:169–174. https://doi.org/10.1016/j.enconman.2017.10.079
Modenbach AA, Nokes SE (2013) Enzymatic hydrolysis of biomass at high-solids loadings – A review. Biomass Bioenerg 56:526–544. https://doi.org/10.1016/j.biombioe.2013.05.031
Chen HZ, Liu ZH (2017) Enzymatic hydrolysis of lignocellulosic biomass from low to high solids loading. Eng Life Sci 17(5):489–499. https://doi.org/10.1002/elsc.201600102
Du J, Liang J, Gao X, Liu G, Qu Y (2020) Optimization of an artificial cellulase cocktail for high-solids enzymatic hydrolysis of cellulosic materials with different pretreatment methods. Bioresour Technol 295:122272. https://doi.org/10.1016/j.biortech.2019.122272
Lin Z, Huang H, Zhang H, Zhang L, Yan L, Chen J (2010) Ball Milling Pretreatment of Corn Stover for Enhancing the Efficiency of Enzymatic Hydrolysis. Appl Biochem Biotech 162(7):1872–1880. https://doi.org/10.1007/s12010-010-8965-5
Sitotaw YW, Habtu NG, Van Gerven T (2022) Intensification of low concentration alkaline pretreatment with planetary ball milling for efficient enzymatic saccharification of enset fiber (Ensete ventricosum). Biomass Convers Bior :1-16. https://doi.org/10.1007/s13399-021-02185-3
Swathy R, Rambabu K, Banat F, Ho S-H, Chu D-T, Show PL (2020) Production and optimization of high grade cellulase from waste date seeds by Cellulomonas uda NCIM 2353 for biohydrogen production. Int J Hydrogen Energ 45(42):22260–22270. https://doi.org/10.1016/j.ijhydene.2019.06.171
Zhang L, Tsuzuki T, Wang X (2015) Preparation of cellulose nanofiber from softwood pulp by ball milling. Cellulose 22(3):1729–1741. https://doi.org/10.1007/s10570-015-0582-6
dos Santos-Rocha MSR, Pratto B, Corrêa LJ, Badino AC, Almeida RMRG, Cruz AJG (2018) Assessment of different biomass feeding strategies for improving the enzymatic hydrolysis of sugarcane straw. Ind Crop Prod 125:293–302. https://doi.org/10.1016/j.indcrop.2018.09.005
Funding
This study was supported by the National Natural Science Foundation of China (No. 32301723), the Postdoctoral Science Foundation of China (No.2020M671367), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (20KJB416009), the Innovation/Entrepreneurship Program of Jiangsu Province and the Priority Academic Program Development of Jiangsu Higher Education Institutions (No. PAPD-2018–87).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Bo Zhang, Yuchen Xing, Guanya Ji and Tianyan You. The first draft of the manuscript was written by Bo Zhang and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Intermittent ball milling effectively mitigated the "high-solids effect".
• The effect of conditional parameters on sugar yield was investigated.
• Optimal process parameters were obtained by orthogonal experiment.
• The hydrolysis efficiency of rice straw reached 72.4% without pretreatment.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, B., Xing, Y., Ji, G. et al. Effect of intermittent ball milling on high-solids enzymatic saccharification of rice straw. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05429-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05429-0