Abstract
Silver and copper nanoparticles were biosynthesized from AgNO3 & CuSO4.5H2O solution by using fruits extract of Solanum xanthocarpum. These nanoparticles (NPs) were characterized by UV, FTIR, and SEM techniques and evaluated for biological activities. The UV absorption peaks of biosynthesized Ag and CuNPs were observed at 452 nm and 549 nm, respectively. The FTIR analyses of the fruits extracts were carried out to identify the possible classes of chemical compounds responsible for NPs stability and reduction. The crystalline structure and size of NPs were characterized by using SEM. The NPs were tested against some selected pathogenic strains. In the current study, the tested bacterial and fungal strains were found sensitive to biosynthesized Ag and CuNPs at concentrations (25μg/mL, 50μg/mL, 75μg/mL, and 100μg/mL). The overall results show that, the antimicrobial activities of CuNPs were comparatively more potent than AgNPs. Antioxidant activities of Ag and CuNPs were evaluated using the DPPH method. The results showed effective free radical scavenging activities, but AgNPs exhibited more significant activities as compared to CuNPs. Anticancer activities were measured by using the MTT assay, Cell viability was used to determine the cytotoxic activity of NPs using HepG2 hepatocellular carcinoma cell line. In the present study, the NPs prevented the growth of tumor cells in a dose-dependent manner (5–500μg/mL). With a gradual increase in NPs concentration, the cell viability of cancer cells decreased. The two highest NPs concentrations (100μg/mL & 250μg/mL) significantly decreased cell viability. The results exhibited that AgNPs are more effective than CuNPs. Additionally, they exhibited effective biological activities, making them to be used in medical sector for cancer treatment, sensors, medications delivery, and several other applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nanotechnology can be defined as “the branch of science and engineering deals with the study of design, synthesis, characterization and application of nanoparticles & devices.” Particles having minimum one dimension and size range 1–100nm are known as nanoparticles (NPs). They cannot be detected by naked eyes and have different physical, chemical and optical properties as compared to their bulk materials. Their applications are based on size, shape, and number of dimensions, which are the fundamental parameters of nanostructures. Mostly NPs are made up of a few hundred atoms. The NPs of different sizes of the same material have different characteristics, as result of surface area to volume ratio. Small size NPs have a large surface area to volume ratio as compared to the bulk materials such as powder and sheet. These characteristics allow the NPs to have unique chemical, physical, and optical characteristics [1]. On the basis of their origin, NPs are classified into three categories natural, incidental, engineered, and their structure may be zero dimension (OD), one dimension (1D), two dimensions (2D), and three dimensions (3D). Recently, it was developed that quantum dots or quantum boxes have zero dimensions [2]. It has been observed that NPs are the determination of Modern Science; however, they have a very prolonged history. For millennia, primitive people have used nanotechnology in different fields. Still, nobody can obviously say about the first initiative to use NPs in different fields. Optical property is the one of basic and vital properties of NPs. For instance, silver nanoparticles (AgNPs) have yellowish gray color, gold nanoparticles (AuNPs) having a size of 20nm possess a special red wine color and, palladium and platinum nanoparticles contain black color [3].
Plant-mediated, metal nanoparticles have increasing importance in the field of nanotechnology, due to its high efficiency, no toxicity, low cost, and ecofriendly manners [4]. Metal nanoparticles have been produced using three different types of methods, including physical, chemical, and biological ones [5]. A lot of work has been done on green synthesis of metal nanoparticles (MNPs) by using reducing agent such as plant extract, algae, and microorganisms, including bacteria and fungi. The plant extract works as the best reducing and capping agent for the production and stability of MNPs [6]. In order to find practical solutions to the environmental problems the world faces and as an alternative to environmentally hazardous methods and goods, green synthesis of MNPs has been developed [7]. Silver nanoparticles (AgNPs) are one of the most significant NPs that have been widely studied. They have distinctive optical, electrical, and biological properties; as a result, they are used in catalysis, biosensors, imaging, the delivery of genes and drugs, the manufacture of nanodevices, and medicine. The most commercialized nanomaterial is AgNPs which are used approximately five hundred tons per year and their use is increased widely in future [8]. Silver nanoparticles are more interesting on account of their antimicrobial activities, nontoxic, safe inorganic antimicrobial agent that is able to kill approximately 650 kinds of microbes, which causes different types of diseases. Biosynthesis of plant mediated NPs, in which plant extract act as capping and reducing agents are more advantageous as compare to the other biological method, because they reduce the drawbacks of maintaining and culturing of cell process and can be used for production of commercial scale nanoparticle [9]. Antioxidant activity depends not only on the substances that are analyzed, but also highly on methodology used for activities. Various methods used for antioxidant activities. However, the most common are ABTS, DPPH, and FC. The antioxidant properties of coffee, tea, beer, apple juice, and dietary supplements were evaluated using the chromogenic radicals 2, 2-azino-bis (3 ethylbenzothiazoline-6-sulfonic acid) (ABTS), 2, 2- diphenyl-1-picrylhydrazyl (DPPH), and Folin-Ciocalteu (FC) techniques. The difference among the antioxidant activity values depends on the reaction medium. In reactions with chromogenic radicals, greater values of antioxidant activity were measure in buffer solution pH 7.4 than in water or methanol. The type of procedure and solvent makes a similar impact on antioxidant reactivity. The pace at which radicals react with antioxidants in samples demonstrates that incubation time has a significant impact on antioxidant activity [10]. Solanum xanthocarpum, commonly known as yellow-berried nightshade, is a prickly plant, which grows wild in different regions of the Indo-Pakistan subcontinent. It belongs to family Solanaceae and plays an important place among medicinal herbs (especially, for the treatment of bronchial asthma, cough, worms etc.) since ancient times. A green synthesis route for the production of silver nanoparticles using methanol extract from Solanum xanthocarpum berry (SXE) is reported. Silver nanoparticles (AgNps), having a surface plasmon resonance (SPR) band centered at 406 nm, were synthesized by reacting SXE (as capping as well as reducing agent) with AgNO3 during a 25 min process at 45 °C. The synthesized AgNps were characterized using UV–Visible spectrophotometry, powdered X-ray diffraction, and transmission electron microscopy (TEM). The results showed that the time of reaction, temperature, and volume ratio of SXE to AgNO3 could accelerate the reduction rate of Ag+ and affect the AgNps size and shape. The nanoparticles were found to be about 10nm in size, mono-dispersed in nature, and spherical in shape [11].
The current study on green synthesis or plant mediated biosynthesis of silver (AgNPs) and copper nanoparticles (CuNPs) from silver nitrate (AgNO3) and copper pentahydrate (CuSO4.5H2O) by using Solanum xanthocarpum fruits aqueous extract at room temperature in dark condition. These NPs are characterized by using the advanced techniques such as UV, FTIR, and SEM and evaluate for biological activities.
2 Materials and methods
2.1 Collection of plant
The Solanum xanthocarpum fruits were collected from different area in Swabi, Khyber Pakhtunkhwa, Pakistan. The fresh whole plant, including fruits, were washed with fresh tap water to clear away the dust particles and other polluted organic material was washed with de-ionized water. Then the fruits from plant separated and air dried at room temperature in shade. The experimental study was done at the Medicinal Botanic Center (MBC) in Pakistan Council of Scientific and Industrial Research (PCSIR) Laboratories Complex Peshawar.
2.2 Chemicals and culture media
Silver nitrate (AgNO3), copper sulfate pentahydrae (CuSO4.5H2O), and NaOH were used as reagents. Culture medium like nutrient agar (NA), nutrient broth (NB), and Muller Hinton agar (MHA) were used for microorganism growth and antibacterial characteristics. Analytical grade chemicals were utilized to conduct the experiment. For the extraction and formation of various stock solutions, de-ionized water was used.
2.3 Preparation of plant crude extract
Dried S. xanthocarpum fruits were first chopped and then grinded on grinding machine. After grinding, 50gm of the plant material was soaked in conical flasks (250mL) in 150mL de-ionized water at 60°C for 30min; afterwards, the aqueous extract was filtered from Whatman No. 41 filter paper to obtain clear aqueous fruits extract from any undeserved materials. The clear filtrate (aqueous extract) was store in a conical flask with glass stopper in a refrigerator for the experimental work.
2.4 Preparation of various solutions
Silver nitrate solution (5mM): 0.4247 g of AgNO3 was dissolved in 500mL de-ionized water to produce 5mM solution of AgNO3. NaOH solution (0.1M): 0.4 g of NaOH was dissolved in 100mL of de-ionized water to prepare 0.1M NaOH solution and further used to adjust the pH values in different experiments. Copper sulfate pentahydrate (CuSO4.5H2O) solution (5mM): 0.6242g of CuSO4.5H2O was dissolved in 500mL de-ionized water to produce 5mM solution of CuSO4.5H2O.
2.5 Green synthesis of silver nanoparticle
The plant-mediated silver nanoparticles (AgNPs) were produced by mixing the dilute aqueous fruit extract of S. xanthocarpum and silver nitrate (AgNO3) solution. A total of 90ml of silver nitrate solution having concentration 5mM were mixed with 10mL of diluted aqueous fruit extract of S. xanthocarpum in the 250-mL conical flask. Put the conical flask on a hot plate having magnetic stirrer for continuous stirring at normal room temperature (27°C) in dark condition. The biosynthesized AgNPs were verified by changing in color of the solution mixture from light red to yellowish gray. By using the naked eye, this shift in color was initially recorded for the creation of AgNPs (visual observation). Because of the reaction mixture’s acidic makeup, it took some time before the color of the mixture changed. It has been said often in the literature that creating NPs in an alkaline medium (8–9 pH) is preferable than in an acidic environment. To create an alkaline medium for the reaction mixture, 0.1 mM of NaOH solution was used. The fact that the aqueous fruit extract was applied gradually using a syringe is more important. A small amount of aqueous fruit extract added to silver salt will cause the proper synthesis of AgNPs. The solution was centrifuged at 5000 rpm for 30 min after the reaction was finished. AgNPs were precipitated and collected, then dried at 60°C in an oven [12].
2.6 Green synthesis of copper nanoparticles
The plant-mediated copper nanoparticles (CuNPs) were synthesized by mixing of copper sulfate pentahydrate (CuSO4.5H2O) solution and aqueous fruits extract of S. xanthocarpum. A total of 20mL of dilute aqueous fruits extract of S. xanthocarpum was mixed with 80mL of the 5mM solution of CuSO4.5H2O in a 250-mL conical flask, and place the conical flask on a magnetic stirrer at room temperature in the dark for overnight. The change in color of reaction mixture from light green to reddish shiny brown indicated the formation of CuNPs. This change in color was an initial indicator for the completion of reaction. After reaction completion, the CuNPs were precipitated and settled at the bottom of the solution. Repetitive centrifugation at 5000 rpm for 30 min was used to separate the reaction mixture, followed by dispersion of the precipitates in de-ionized water. The precipitates of CuNPs were collected and dry in an oven at 60°C. For experimental control, the similar amount of CuSO4.5H2O solution was maintained separately under the similar reaction conditions. The purified and dry CuNPs were characterized by UV, FTIR, and SEM [13].
2.7 Characterization
The biosynthesized NPs were characterized by visual observation (VO) ultraviolet visible spectroscopy (UV), Fourier transform infrared spectroscopy FTIR, and scanning electron Microscope (SEM). These techniques can be used to identify the size, shape, surface, dimension and dispersity of NPs [14].
2.8 Visual observation
Visual observation (VO) served as the initial confirmation. A visible sign of the formation of silver (Ag) and copper nanoparticles (CuNPs) was the color changing of reaction mixture. Plasmonic colors are unique in that the optical properties of metal nanoparticles (MNPs) can be tailored by altering the size, shape, and material composition.
2.9 Ultraviolet visible spectroscopy
The verification of NPs was performed by ultraviolet visible (UV) spectroscopy to determine the involvement of photochemical, which reduced the metal ions into their respective metal nanoparticles (MNPs) by surface plasmon resonance (SPR) technique. UV spectroscopy showed clear absorbance peak at the range of 400–600nm, due to the intercommunication between light and mobile surface electrons of metal MNPs. It provided detail information about size, shape, aggregation, stability, and overall morphological structure of the NPs. The NPs of each metal contain a particular range of absorbance peak depending on size of NPs. Ag and CuNPs were given a particular absorbance peak at range of 400–450nm and 550–600 nm respectively [1, 8].
2.10 Fourier Transform Infrared Spectroscopy
Fourier transform infrared spectroscopy (FTIR) was used to characterize the biosynthesized NPs produced by various techniques. For FTIR analysis, a very small amount of powder sample was required and it explained the surface chemistry of NPs. This instrumentation study helped to determine the functional groups of biosynthesized NPs. Sometimes, small band changes in the FTIR spectrum of plant phytochemicals in free form or attached to NPs are seen. These changes are happening as proof of the metal reduction and NP production. The most important phytochemical components that contribute to the capping and reduction of NPs were identified by FTIR analysis [1, 8].
2.11 Scanning electron microscopy
The most important factors for characterization and verification of NPs were size and shape determination, which were determined using scanning electron microscopy Its operation is based on the electron microscopes basic principles and it has a number of important advantages for morphological structure investigation. This technique is used to determine the morphology of various MNPs, such as gold, silver, and copper. SEM examination was used to determine the morphological structure of NPs, revealing their spherical form and size range of 5–30 nm. The main problem of this method is that it is a slow procedure, expensive, and necessitates thorough data concerning size distribution [8].
2.12 Antimicrobial assay
Media preparation, in de-ionized water, the necessary amounts of Muller Hinton Agar (MHA) (21g / l), nutrient agar (NA) (28g / l), and nutrient broth (NB) (13g / l) were made. The nutritious broth was poured into each test tube in an amount of around 20 ml. The test tubes and media flasks were all sterilized in an autoclave for 15 min at 121°C and 1.5 lbs of pressure. They all had cotton wool plugs. To avoid contamination, sterile nutritional agar was put into sterilized Petri plates in a biosafety cabinet after sterilization (about 25-ml per Petri plate). Petri plates were filled with the media and given about 30 minutes to harden before being inverted for 24 h in a 37°C incubator. The inverted posture was necessary to prevent water from the media inside the plates from evaporating. After 24 h, the infected plates were separated, and the clean plates were utilized to cultivate bacteria and fungi. While nutrient broth in flasks (around 20ml/flask) was used for shaking incubation of microorganisms, nutrient broth in test tubes was employed to standardize microbial colonies.
2.13 Microorganisms
The microbial species, which comprised bacteria and fungi, were acquired from the Microbiology Lab of MBC at PCSIR Laboratories Complex Peshawar. The test organisms used in the current study included five Gram-negative strains, such as Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumonia, Salmonella typhae, and Proteus mirabilis, as well as two Gram-positive strains, Staphylococcus aureus and Bacillus subtilis, and one fungus, Candida albicans.
2.14 Stock solution of nanoparticles
The green syntheses of Ag and CuNPs were evaluated for antimicrobial potential. Stock solutions of Ag and CuNPs were prepared with various concentrations (25μg/mL, 50μg/mL, 75μg/mL, and 100μg/mL) in dimethylsulfoxide (DMSO) have the chemical formula (CH3)2SO. It is a colorless liquid, has strong polarity (7.2), and dissolves both polar and non-polar compounds. This property makes the DMSO miscible in a wide range of organic solvents. It has no inhibitory activities itself due to this property it is used in antimicrobial activities.
2.15 Inoculation of culture
After the preparation and solidification of media, the test microbes were inoculated in the culture media in order to refresh the original culture on nutrient agar (NA) by streaking method in the sterile environment in biosafety cabinet. Refresh cultures of the chosen microorganisms were inoculated in nutrient broth (NB) and placed in a shaking incubator for uniform growth of the culture in nutrient broth at 37°C for 24 h.
2.16 Well diffusion method
Antimicrobial assay of Ag and CuNPs was assessed by well diffusion method follow the [7] with slight modification. The bacterial cultures were inoculated onto nutrient agar (NA) plates after being manually adjusted to 0.5 McFarland turbidity standards. The C. albicans culture was adjusted to a concentration of 108cfu/mL in order to measure the antifungal activity. The C. albicans culture was injected into Muller Hinton Agar (MHA) plates after being suspended in a sterile solution of 0.9% normal saline. Four wells of 6-mm diameter were made by using sterile borer. A total of 50μL of each solution of Ag & CuNPs with different concentrations (25μg/mL, 50μg/mL, 75μg/mL, and 100μg/mL) were applied into the corresponding well. Then the bacterial and C. albicans cultures plates were incubated at 37 °C for 24 h. Generally, fungi are incubated in 25–30°C for 1 week; but here, C. albicans act like bacteria and grow on 37°C [15]. Antibiotics (azithromycin and ciprofloxacin) were used as a positive control for Gram-positive bacteria, Gram-negative bacteria, and C. albicans at the same concentrations well-1 on different plates. The solvent in which the stock solution was prepared (50μL well−1) was poured into the well as a negative control. These plates were then incubated for a further 24 h at 37 °C.
2.17 Antioxidant activity
DPPH radical scavenging activity or antioxidant activity was measure by using the “DPPH Technique” described by Bonomo et al. [16] with slight modification. This method is based on measuring DPPH concentrations (2, 2-diphenyl-1-picryl-hydrazyl). DPPH is a stable free radical that is red in color and turns yellow when scavenged (in powder form). Antioxidant reacted with the stable free radical DPPH, pairing it off in the presence of a hydrogen donor and reducing it to DPPH-H, which lowers the DPPH’s absorbance. The ability of DPPH to scavenge free radicals was demonstrated by this feature. The aliquot of Ag and CuNPs in various concentrations (20–100μg/ml) were mixed with 2ml DPPH (0.1mM in methanol). The samples were put in dark for incubation at 37 °C for 30 min. After incubation, a UV spectrophotometer (Carry 60 UV-Vis Spectrophotometer, Agilent Technologies) was used to measure the absorbance at 520nm. The absorbance decreases as a result of the antioxidants’ interaction with DPPH to create DPPH-H. The degree of discoloration revealed the antioxidant compound or nanoparticle potential to donate hydrogen as well as their scavenging abilities. The following formula was used to measure the percentage of free radical scavenging activity for the analyzed samples.
2.18 Anticancer activity
MTT assay, the tetrazolium dye or 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, was used to measures the metabolic activities of cells. Under specific circumstances, NAD(P)H-dependent cellular oxidoreductases may serve as an indicator of the quantity of alive cells. The MTT can be converted by these enzymes to its insoluble purple formazan. Assays with tetrazolium dye can also be used to quantify cytotoxicity. MTT experiments are often conducted in the dark since the MTT reagent is light sensitive. Anticancer activities were measured by using the MTT assay as described by [17, 18] with slight modifications. Briefly, 1× 106 cells were placed in a 96-well culture dish and left to adhere at 37 °C for 24 h in a moderately humid environment with 5% CO2 and 95% O2. For 48 h, cells were exposed to NPs at different concentrations (5–500μg/ml for AgNPs and (5–300μg/ml for CuNPs). After exposure (10μl/well of 100μl of cell suspension), MTT (5μg/ml stock in PBS) was applied to the plate. The plate was then incubated for 4 h. The reaction mixture was carefully withdrawn once the incubation period was complete, and 200μl of DMSO was added to each well while being gently mixed. After shaking the plates for 10 min at room temperature, the absorbance at 550nm was measured using a microplate reader. Untreated groups were processed under the same circumstances as treated negative controls. The cytotoxicity was calculated with following formula:
3 Results
3.1 Visual observation
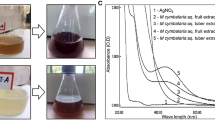
In the present study, the initial confirmation was carried out by visual observation. For the manufacture of AgNPs, silver ions (Ag+) are obtained from silver nitrate solution (AgNO3). Fruit extract from S. xanthocarpum stabilized AgNPs by reducing silver ions (Ag+). It was observed visually that the reaction medium change color from light red to yellowish gray after 24-h incubation dark room is an indication of AgNP formation. While in the case of CuNPs, color of the precursor solution change, the synthesis of CuNPs was confirmed in the form of precipitation on the inner surface of the conical flask, which changes in color from light green to reddish lustrous brown. The CuSO4.5H2O reacts with the fruit extract of S. xanthocarpum to modify the color of the reaction mixture as shown in Fig. 1–F.
A Silver nitrate solution and plant extract before reaction. B Reaction mixture of synthesized silver nanoparticles. C Dry silver nanoparticles (AgNPs). D Copper sulfate pentahydrate solution and plant extract before reaction. EReaction mixture of synthesized copper nanoparticles. F Dry copper nanoparticles (CuNPs)
3.2 UV–visible spectroscopic analysis
Ultraviolet visible spectroscopy (Carry 60 UV-Vis Spectrophotometer) is a fast identification and characterization of nanoparticles (NPs). The UV-visible absorption spectra of plant extract and biosynthesized Ag and CuNPs from S. xanthocarpum fruits extract are shown in Fig. 2. Silver nanoparticles with mobile surface electrons interacted with light to form a large absorbance peak, or surface plasmon resonance (SPR), in the 400-–500-nm range. The absorption peak of synthesized AgNPs was observed at 452nm which is within expected range. The absorption peak of copper nanoparticles (CuNPs) occurs at 549nm. The CuNPs have been shown to have absorption bands in the range of 500–600 nm.
3.3 FTIR analysis of synthesized nanopaarticles
Fourier transform infrared (FTIR) spectroscopic (FTIR Prestige 21, Schimadzu Japan) analyses were carried out to identify the possible categories of chemical compounds in the fruits extracts of S. xanthocarpum responsible for reduction and stabilization of the metal ions into their NPs. These compounds are identified by their functional groups in FTIR spectra having different range of absorption peaks shown in Figs. 3, 4, and 5. The range of 4000−500 cm−1 was employed. The presence of polyphenols, polysaccharides, or proteins is indicated by the plant extracts prominent and broad peak at 3315 cm−1, which is caused by the presence of the OH group. Alkanes are identified by the C-H stretching and bending vibration, which assigns the miner peak at 2900 cm−1. N-H bend of primary amines is given the peak at 1614 cm−1. The peaks at 1380 and 1200 cm−1 indicating C−O stretching vibration strong peak presence of alcohols, carboxylic acid, ester, and ether, 1050 cm−1 identifying the C–N presence of aliphatic amines. After synthesis of AgNPs, the above peaks shift into 2920, 2380, 2110, 1506, and 1001.1 cm−1, which assigned for C-H stretching of aromatic, C−N of aliphatic amines, C=C, and C=O groups which are due to alkenes, aldehydes, ketones, and carboxylic acid respectively. While in the case of CuNP formation, the peaks shift into 3273, 2922, 2328, 1606, 1539, 1456, 1373, 1275, and 1038 cm−1 were assigned to OH stretching for alcohol, CH, COO, C=C, and C=O groups which indicated alkyl alcohol and alkyl amine.
3.4 SEM analysis of nanoparticles
Morphological, crystalline structure, and size of biosynthesized Ag and CuNPs were measured by using scanning electron microscopy (SEM). The SEM images showed that the clump nature of AgNPs was conspicuously cubic and some were spherical in shape (Fig. 6). The amplified SEM image indicates that the silver nanocubes are in a well-cubical structure with soft surface and sharp edges. The normal SEM image of the CuNPs exhibited in Fig. 7 described that the morphology of prepared NPs was noticed as an approximately spherical shape with a rough surface. It was exhibited that the biosynthesized CuNPs have separated particles from each other as well as clusters. However, it was observed that the biosynthesized NPs were stabilized by secondary metabolites present in the fruit extract of S. xanthocarpum. The monodispersity of NPs is a useful parameter for biomedical application.
3.5 SEM analysis of nanoparticles
Morphological, crystalline structure, and size of biosynthesized Ag and CuNPs were measured by using scanning electron microscopy (SEM) [SEM, JEOL JSM-5910]. The SEM images showed that the clump nature of AgNPs was conspicuously cubic and some were spherical in shape (Fig. 6). The amplified SEM image indicates that the silver nanocubes are in a well-cubical structure with soft surface and sharp edges. The normal SEM image of the CuNPs exhibited in Fig. 7 described that the morphology of prepared NPs was noticed as an approximately spherical shape with a rough surface. It was exhibited that the biosynthesized CuNPs have separated particles from each other as well as clusters. However, it was observed that the biosynthesized NPs were stabilized by secondary metabolites present in the fruit extract of S. xanthocarpum. The monodispersity of NPs is a useful parameter for biomedical application.
3.6 Antimicrobial activities
The biosynthesized NPs were tested against some pathogenic bacteria and fungus. In which, five Gram-negative including Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumonia, Salmonella typhae, and Proteus mirabilis, two are Gram-positive Bacillus subtilis and Staphylococcus aureus, and one fungal Candida albicans strain. All concentration (25μg/mL, 50μg/mL, 75μg/mL and 100μg/mL) of both AgNPs and CuNPs were very sensitive to all of the bacterial and fungal species tested in the study. Overall results show that the antimicrobial activities of CuNPs are comparatively more significant than AgNPs. Azithromycin and ciprofloxacin, which were sensitive to the investigated microorganisms, were used as positive control antibiotics at the same doses in well−1 on separate plates, as indicated in Fig. 8. As depicted in Fig. 8, the stock solution’s solvent (DMSO) of 50μL well was used as a negative control produced no zone of inhibition.
3.7 Antioxidant activities
Antioxidant or free radical scavenging activities of Ag and CuNPs were assessed utilizing the DPPH free radical scavenging technique. The stable molecule DPPH can take on electrons or hydrogen from NPs. The results showed effective free radical scavenging activities of Ag and CuNPs, but AgNPs show more significant activities as compared to CuNPs. The antioxidant potential of Ag and CuNPs is credited by functional groups attached to NPs from S. xanthocarpum fruits extract. The IC50 value is inversely proportional to the antioxidant potential of a compound. The compounds having strong antioxidant activities have low value of IC50. On the bases of IC50 values, antioxidant potential of AgNPs is significantly higher than the CuNPs as shown (Fig. 9).
3.8 Anticancer activities
Cell viability assay was used to determine the cytotoxic activity of AgNPs using HepG2 hepatocellular carcinoma cell line. MTT solution was utilized primarily to determine the viability of cells with mitochondrial dysfunction. In the present study, the NPs prevented the growth of tumor cells in a dose-dependent manner (5–500μg/ml). Figure 10 depicts the proliferation of HepG2 cancer cells and their interactions with various doses of AgNPs. With a gradual increase in NPs concentration, the cell viability of cancer cells decreased. At the two highest NP concentrations (100μg/ml and 250μg/ml), cell viability was significantly reduced. Furthermore, subsequent increases in the NPs concentration did not have a significant cytotoxic effect on cancer cells. In the present study, CuNPs were used against hepatocellular carcinoma (HepG2) cell line at concentrations (5–300ug/ml) and the viability of cells decreased with an increase in concentrations, as shown in Fig. 10. HepG2 cells were subjected to CuNPs at doses of 5–300μg/ml. It is also evident that synchronization occurs with cellular uptake because, as shown Fig. 10, as absorbance increases, cell viability declines progressively. At a concentration of 200μg/ml, the cytotoxic activity was at its peak; however, subsequent increases in concentration did not result in any significant anti-proliferative activity.
4 Discussion
Nanoparticles (NPs) of each metal contain a particular range of absorbance peak depends on size of NPs. For instance, AuNPs give a specific absorbance peak at range of 500–550nm, AgNPs give a particular absorbance peak at range of 400–450nm, and CuNPs give absorbance peak at ranges of 550–600 nm [1]. However, some researchers have manufactured NPs with Surface plasmon resonance (SPR) peaks for sizes smaller than 400nm. Silver ions, complexes, contaminants, and plant phytochemicals are likely to absorb the absorbance bands below 400 nm. The size and form of the NPs as well as the dielectric constant of the surrounding medium have a significant impact on the properties of SPR [8]. In the current studies, the UV- visible absorption spectra of biologically produced Ag and CuNPs from S. xanthocarpum fruits extract were examined in the present studies. The absorption peak of synthesized Ag and CuNPs was observed at 452 and 549 nm respectively which is within expected range (Fig. 2).
FTIR spectroscopy was utilized for a qualitative investigation of biosynthesized NPs. The FTIR spectra with various absorption peak ranges are depicted in Figs. 3, 4, and 5. The presence of polyphenols, polysaccharides, or proteins is indicated by the plant extracts prominent and broad peak at 3315 cm−1, which is caused by the presence of the OH group. Alkanes are identified by the C-H stretching and bending vibration, which assigns the miner peak at 2900 cm−1. N-H bend of primary amines is given the peak at 1614 cm−1. The peaks at 1380 and 1200 cm−1 indicate C−O stretching vibration strong peak presence of alcohols, carboxylic acid, ester, and ether, and 1050 cm−1 identifies C–N presence of aliphatic amines. After the synthesis of AgNPs, the above peaks shift into 2920, 2380, 2110, 1506, and 1001.1 cm-1, which are assigned for C-H stretching of aromatic, C−N of aliphatic amines, C=C, and C=O groups which are due to alkenes, aldehydes, ketone, and carboxylic acid respectively. While in the case of CuNP formation, the peaks shift into 3273, 2922, 2328, 1606, 1539, 1456, 1373,1275, and 1038 cm−1 were assigned to OH stretching for alcohol, CH, COO, C=C, and C=O groups which indicated alkyl alcohol and alkyl amine. In the previous investigation, it was determined that the strong absorption bands at 1603 cm−1 and 1616 cm−1 in the FTIR spectra of Ag and CuNPs, respectively, were caused by the binding of NHC=O to metal ions. In addition, there are peaks for secondary amine at 2922 cm−1, C-N stretching vibration of aromatic amine at 1383 cm−1, AgNPs at 1138 cm−1, 821 cm−1, 764 cm−1, and 595 cm−1, and CuNPs at 1383 cm−1, 1074 cm−1, and 601 cm−1.
The presence of the O-H group in proteins, polysaccharides, or polyphenols may explain the peaks at 3186 cm−1 and 3341 cm−1. The free amine groups or carboxylate ions of the amino acid residues in proteins, according to some research, can bind to metal nanoparticles (MNPs). The phytochemical analysis of Cassia occidentalis revealed the presence of phenolic compounds such as apigenin, emodin, aloe-emodin, rhein, and vitexin, which are responsible for the development of MNPs [4].
In the present study, the SEM images showed that clump nature of AgNPs were conspicuously cubic and some spherical in shape (Fig. 6). The amplified SEM image indicates that the silver nanotubes are in well cubical structure with soft surface and sharp edges. The normal SEM image of the CuNPs exhibited in Fig. 7, which described that the morphology of prepared NPs, was noticed as approximately spherical shape with rough surface. It was exhibited that the biosynthesized CuNPs have separated particles from each other as well as clusters. However, it was observed that, the synthesized nanoparticles were stabilized by secondary metabolites present in the fruits extract of S. xanthocarpum. The monodispersity of NPs is a useful parameter for the biomedical application. In the previous study, Fig. 5 displays the surface morphological and nanostructural SEM findings. The SEM micrographs clearly show aggregates of AgNPs from C. occidentalis with typical particle sizes ranging from 20 to 65 nm and copper nanoparticles with a size range of 30–65 nm [4]. In another study, the form and crystalline structures of bio-prepared Ag NPs were examined using FE-SEM and TEM techniques. The spherical NPs were easily distinguished and ranged in size from 20 to 80 nm. It is fascinating to see how, as the amount of root extract grows, the Ag NPs’ SEM and TEM images change. It is crucial to understand that the shapes and sizes of Ag NPs have an impact on the NPs’ optical and electrical characteristics [19].
In the present study various concentration (25μg/mL, 50μg/μL, 75μg/mL, and 100μg/mL) of both Ag and CuNPs were used for bacterial and fungal species. It shows high sensitivity against K. pneumonia (20, 22, 28, and 30mm), S. typhae (16, 20, 27, and 29mm), S. aureus (15, 23, 25, and 28mm), P. aeruginosa (14, 17, 20, and 24mm), B. subtilis (15, 18, 19, and 22mm), C. albicans (12, 17, 20, and 23mm), and followed by E. coli (12, 15, 18, and 21mm). The lowest sensitivity was against P. mirabilis (10, 12, 15 and 17mm). The CuNPs give high sensitivity against P. aeruginosa (20, 24, 26 and 29mm), S. typhae (20, 23, 25 and 28mm), S. aureus (15, 24, 26, and 30mm), C. albicans (18, 22, 25, and 28mm), E. coli (17, 20, 25, and 28mm), P. mirabilis (16, 22, 24, and 27mm) and followed by B. subtilis (12, 16, 20, and 23mm). The lowest sensitivity was against K. pneumonia (12, 15, 17, and 20mm). Overall results show that the antimicrobial activities of CuNPs are comparatively more significant than AgNPs. Elumalai et al. [20] described that, the evaluation of antimicrobial activities of the plant mediated (Ocimum basilicum) biosynthesis of AgNPs. Disc diffusion technique was used for determination of antimicrobial efficacy, against some selected human pathogenic bacteria, Staphylococcus aureus, Escherichia coli, K. pneumonia, Proteus mirabilis, and Proteus valgaris, showed results 8.9, 9.2, 7.8, 8.1, and 7.4mm zone of inhibition respectively. They also exhibiting antifungal activity against Aspergillus flavus, Aspergillus terreus, Aspergillus nigar, and Aspergillus fumigates at the range of 7.8-, 7.5-, 6.9-, and 7.1-mm zone of inhibition respectively. In the previous study, it was found that the CuNPs of roots and leaves extract of Asparagus addscendes have greater zone of inhibition as compared to crude extract of their roots and leave. The zone of inhibition for E. coli (19mm), B subtilis (17mm), S. typhi (19mm), K. pneumonia (18mm), and S. aureus (16mm) in case leave nanoparticles while in case of roots extracts E. coli (20mm), B. subtilis (18mm), S. typhi (21mm), K. pneumonia (18mm), and S. aureus (17mm) [21].
Antioxidants are chemicals, either natural or synthetic, that prevent oxidation reactions. The process of oxidation produces free radicals (oxidants), which can cause a chain reaction and harm to an organism’s cells. These are also known as “free radical scavengers.” These are present naturally in vegetable and fruits, which are good sources of antioxidants and may reduce risk of several diseases. However, in high concentration antioxidants may be harmful. At high concentration antioxidants, may act as pro-oxidants, increasing oxidation, which protect and accelerate the formation of dangerous cells (such as tumor and cancer cells). The common commercially available antioxidants are DPPH, hydrogen peroxide (H2O2), nitric oxide (NO). In the current studies, the results showed effective free radical scavenging activities of Ag and CuNPs, but copper nanoparticles show more significant activities as compared to AgNPs. The antioxidant potential of AgNPs and CuNPs is credited by functional groups attached to nanoparticles from S. xanthocarpum fruit extract. Antioxidant activities of AgNPs and CuNPs were evaluated using the DPPH method. On the bases of IC50 values 98, 285, and 39 of AgNPs, CuNPs and positive control ascorbic acid respectively showed effective free radical scavenging activities, but AgNPs exhibited more significant activities as compared to CuNPs. The compounds having strong antioxidant activities have low value of IC50. Antioxidant potential of AgNPs is significantly higher than the copper nanoparticles as shown in Fig. 9. In the previous study, Khan et al. [15] reported that DPPH technique was used to test the free radical scavenging abilities of AuNPs and AgNPs. These NPs had greater antioxidant activity as indicated by the proportion of DPPH they were able to scavenge (75.85% + 0.67% and 78.87% + 0.19%, respectively). The results showed that these NPs revealed excellent antioxidant activities, so their biomedical performance enhanced from their herbal sources.
To reduce the prevalence of liver cancer, more individualized therapeutic approaches must be developed. Due to the promising medicinal benefits of trace metals on malignancies, metal and metal nanoparticles are the preferred candidates for cancer diagnosis and treatment. Trace elements including silver (Ag), zinc (Zn), copper (Cu), iron (Fe), and chromium are essential for the activity of metalloproteases, the immune system, DNA synthesis, enzymes, and antioxidants. In the current study, the green synthesis of AgNPs exhibited profound cytotoxicity against HepG-2 liver cancer cell line (Fig. 10). The viability of hepatocellular carcinoma cells decreases significantly with increase as the concentration of AgNPs. When examining the cytotoxic effect of nanoparticles (NPs), numerous criteria can be evaluated, including their size, surface charge and dissolution rate. The viability of multiple cancer cell lines was shown to be decreased by AgNPs in a dose-dependent way by earlier investigations. Another study contrasted the standard method and the eco-friendly method for determining the toxicity of AgNPs. Green synthesis was noticeably more harmful than industrial synthesis against the MCF-7 cell line. Krishnan et al. [11] also looked into the cytotoxicity of AgNPs against MCF-7 at a range of concentrations (10–100μg/mL). In comparison to Piper nigrum extract, plant-mediated AgNPs exhibit substantial cytotoxic activity against both MCF-7 and Hep-2 cells. Since the results show that Piper nigrum is an excellent plant for employing in the production of silver nanoparticles. Numerous human cell lines, including HepG2, A549, and certain major rat cell lines, have been used to examine the toxicological implications of chemically derived AgNPs. A number of studies have shown that AgNPs are only cytotoxic to malignant cells and not to normal cells. The results of the current MTT assay justify the cytotoxic potential of AgNPs. The MTT experiment demonstrated that cell viability decreased proportionally to concentration of nanoparticles. These results are in accordance with previous studies. At a concentration of 250μg/ml, AgNPs had the most anticancer effects against the HeLa and Hep-2 cell lines. The growth of the cell lines CRL-1451, 4T1, CT-26, and WEHI-3B was suppressed by green-synthesized AgNPs in a dose-dependent manner. Additionally, they demonstrated that normal murine fibroblasts are not cytotoxic by AgNPs. These results are in line with earlier research that showed exposure to chemically derived AgNPs significantly reduced the number of SH- SY5Y cells that were viable at all-time points.
Globally, hepatocellular carcinoma (HepG2) is the leading cause of death from gynecological tumors. Conventional treatments such as chemoradiotherapy and surgery have limited applications due to the side effects and pharmacotherapy resistance associated with them. Due to their tumor-specific regime, low toxicity, and increased biocompatibility, nanomaterials such as liposomes, metal oxides, and polymers have recently been identified as promising delivery vehicles. The FDA has authorized the use of numerous nanoformulation-based chemotherapy dosage forms in clinical trials for the treatment of breast, cervical, and ovarian cancer. Doxil® and Abraxane® are the first anticancer nanoformulations available on the market. According to multiple studies, the cytotoxicity of CuNPs against Hep-G2 and HT-29 cancer cells increased as their concentration increased. Particle size, specific surface area, and Cu+2 ion release rate all affect how harmful CuNPs are. They demonstrated that biosynthesized CuNPs are very lethal to the A549 cancer cell line due to the generation of reactive oxygen species and oxidative stress. In a study, the cytotoxicity of biosynthesized CuNPs was evaluated against human airway epithelial (HEp2) and breast cancer (MCF-7) cells. It has been suggested that ROS production and oxidative stress mediate the cytotoxicity of nanoparticles. The current study demonstrated that produced CuNPs exhibited an optimal level of cytotoxicity against Hep-G2 cancer cell lines; consequently, they may be a viable option for the treatment of multiple types of cancer. Prior research has demonstrated that CuNPs satisfy two fundamental requirements for an effective chemotherapeutic agent: tumor selectivity and low toxicity to normal cells. Figure 10 clearly demonstrates that the HeLa cancer cell line took up a substantial amount of NPs. This investigation may contribute significantly to the development of research into prolonged drug exposure, which will be advantageous for biomedical application such as cancer treatment and drug delivery systems. Our study indicates that biosynthesized AgNPs possess favorable physiochemical properties and are appropriate for use in medical applications.
5 Conclusions
Green method for producing silver and copper nanoparticles has been created utilizing the aqueous fruit extract of S. xanthocarpum. AgNPs and CuNPs can be produced by S. xanthocarpum fruit extract. It is observed visually that the reaction medium change color from light red to yellowish gray, which indicates of AgNPs formation. Confirmation of the production of CuNPs is provided by the precursor solution’s color changing from light green to reddish glossy brown during precipitation. Utilizing UV-visible spectroscopy, FTIR analysis, and SEM examination, the biosynthesized NPs are described. AgNPs and CuNPs produced through plant-mediated biosynthesis displayed efficient biological activity (such as antimicrobial, antioxidant, and anticancer activities). These NPs show a potent antimicrobial activity against some selected pathogenic microbial strains, such as E. coli, P. aeruginosa, K. pneumonia, S. typhae, P. mirabilis, S. aureus, B. subtilis, and C. albicans. The antioxidant activities of AgNPs and CuNPs, on the bases of IC50 values 98 and 285 respectively exhibited effective activities; however, the former exhibited more potent activities as compared to later one. The compounds having strong antioxidant activities have low value of IC50. The cytotoxic activity of NPs is determined by using HepG2 hepatocellular carcinoma cell line. In the present study, the NPs prevented the growth of tumor cells in a dose-dependent manner (5–500μg/ml). With a gradual increase in NPs concentration, the cell viability of cancer cells decreased. At the two highest NP concentrations (100μg/ml and 250μg/ml), cell viability is significantly reduced. So, it is concluded that AgNPs and CuNPs exhibited effective biological activities, making them to be used in medical sector for cancer treatment, sensors, medications delivery and several other applications. On the bases of present study, the conventional physical and chemical methods used to make MNPs may be replaced by green synthesis techniques.
Data availability
Data will be made available on request.
Code availability
Not applicable.
References
Ahmed RH, Mustafa DE (2020) Green synthesis of silver nanoparticles mediated by traditionally used medicinal plants in Sudan. Int Nano Lett 10(1):1–14
Bagherzade G, Tavakoli MM, Namaei MH (2017) Green synthesis of silver nanoparticles using aqueous extract of saffron (Crocus sativus L.) wastages and its antibacterial activity against six bacteria. Asian Pac J Trop Biomed 7(3):227–233
Ijaz I, Gilani E, Nazir A, Bukhari A (2020) Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chem Lett Rev 13(3):223–245
Gondwal M, Joshi Nee Pant G (2018) Synthesis and catalytic and biological activities of silver and copper nanoparticles using Cassia occidentalis. Int J Biomater 2018:6735426. https://doi.org/10.1155/2018/6735426
Lee SH, Jun B-H (2019) Silver nanoparticles: synthesis and application for nanomedicine. Int J Mol Sci 20(4):865
Ahmed S, Ahmad M, Swami BL, Ikram S (2016) Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J Radiat Res Appl Sci 9(1):1–7
Ramzan M, Obodo RM, Mukhtar S, Ilyas S, Aziz F, Thovhogi N (2021) Green synthesis of copper oxide nanoparticles using Cedrus deodara aqueous extract for antibacterial activity. Mater Today: Proc 36:576–581
Rafique M, Sadaf I, Rafique MS, Tahir MB (2017) A review on green synthesis of silver nanoparticles and their applications. Artif Cells Nanomed Biotechnol 45(7):1272–1291
Ibrahim HM (2015) Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J Radiat Res Appl Sci 8(3):265–275
Abramovič H, Grobin B, Ulrih NP, Cigić B (2017) The methodology applied in DPPH, ABTS and Folin-Ciocalteau assays has a large influence on the determined antioxidant potential. Acta Chim Slov 64(2):491–499
Amin M, Anwar F, Janjua MRSA, Iqbal MA, Rashid U (2012) Green synthesis of silver nanoparticles through reduction with Solanum xanthocarpum L. berry extract: characterization, antimicrobial and urease inhibitory activities against Helicobacter pylori. Int J Mol Sci 13(8):9923–9941
Lade BD, Shanware AS (2020) Phytonanofabrication: methodology and factors affecting biosynthesis of nanoparticles. In: Smart nanosystems for biomedicine, optoelectronics and catalysis. IntechOpen. https://doi.org/10.5772/intechopen.83226
Olajire A, Ifediora N, Bello M, Benson N (2018) Green synthesis of copper nanoparticles using Alchornea laxiflora leaf extract and their catalytic application for oxidative desulphurization of model oil. Iran J Sci Technol Trans A Sci 42:1935–1946
Awwad A, Amer M (2020) Biosynthesis of copper oxide nanoparticles using Ailanthus altissima leaf extract and antibacterial activity. Chem Int 6:210–217
Khan SA, Shahid S, Lee C-S (2020) Green synthesis of gold and silver nanoparticles using leaf extract of Clerodendrum inerme; characterization, antimicrobial, and antioxidant activities. Biomolecules 10(6):835
Bonomo MG, Cafaro C, Russo D, Calabrone L, Milella L, Saturnino C et al (2020) Antimicrobial activity, antioxidant properties and phytochemical screening of Aesculus hippocastanum mother tincture against food-borne bacteria. Lett Drug Des Discov 17(1):48–56
Majid A, Faraj HR (2022) Green synthesis of copper nanoparticles using aqueous extract of yerba mate (llex Paraguarients St. Hill) and its anticancer activity. Int J Nanosci Nanotechnol 18(2):99–108
Sarkar S, Kotteeswaran V (2018) Green synthesis of silver nanoparticles from aqueous leaf extract of pomegranate (Punica granatum) and their anticancer activity on human cervical cancer cells. Adv Nat Sci: Nanosci Nanotechnol 9(2):025014
Benakashani F, Allafchian A, Jalali SAH (2017) Green synthesis, characterization and antibacterial activity of silver nanoparticles from root extract of Lepidium draba weed. Green Chem Lett Rev 10(4):324–330
Elumalai D, Sathiyaraj M, Vimalkumar E, Kaleena PK, Hemavathi M, Venkatesh P (2019) Bio fabricated of silver nanoparticles using Ocimum basilicum and its efficacy of antimicrobial and antioxidant activity. Asian J Green Chem 3(1):1–124
Thakur S, Sharma S, Thakur S, Rai R (2018) Green synthesis of copper nano-particles using Asparagus adscendens roxb. Root and leaf extract and their antimicrobial activities. Int J Curr Microbiol Appl Sci 7(4):683–694
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
All authors have contributed equally and read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable
Conflict of interest
The authors declare no competing interests.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rahman, G., Fazal, H., Ullah, A. et al. Empowering silver and copper nanoparticles through aqueous fruit extract of Solanum xanthocarpum for sustainable advancements. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05270-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05270-5