Abstract
Green biomass from leguminous and gramineous forage crops, such as alfalfa and grass-clover, has been proposed as a potential new source of feed protein concentrates for non-ruminant livestock. However, the efficient separation of the protein fraction from the non-digestible cell components, primarily comprising cell walls (fiber) and starch, presents a significant technological challenge. Moreover, it is crucial to optimize the process to preserve the optimal nutritional value of the final product. This study comprehensively analyzed the non-digestible fiber content and composition across all biorefinery fractions using two different feedstocks: green biomass from alfalfa and grass-clover. The pilot scale refining process involved a combination of screw pressing, lactic acid fermentation, and protein separation via centrifugation. We observed variations in carbohydrate composition and abundance between alfalfa and grass-clover. The lactic acid fermentation led to a reduction in cellulose and total glucose content. Our findings indicate that the final protein concentrate still contains residual cell wall components, including lignin, indicating potential inefficiencies in the filtration, fermentation, and isolation steps. The presented analytical approach provides a valuable framework for optimizing processing conditions and tailoring enzyme cocktails for enhanced valorization of the by-products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Constant pressure on global agriculture to sustainably feed the growing world population while facing the socioeconomic dynamics, consequences of climate change, and shifting eating habits have spurred the exploration of alternative food sources. One of the strategies involves reducing the reliance on animal-derived proteins in feed and food production while utilizing a wider range of raw materials such as plants, insects, or microorganisms [1, 2]. While plants are already a staple food providing nutrients, fiber, and a range of essential amino acids, the availability of suitable sources for large-scale protein production still needs to be improved.
Soybeans, a legume species, are a primary source of plant protein in the global food and feed supply predominantly due to favorable processing characteristics and nutritional value [3]. However, large-scale soybean cultivation is confined to relatively limited geographical regions, thus leading to over-intensification of land use and further negative environmental impacts, including deforestation, to accommodate the escalating food demand [4]. The production of concentrated protein feed from green biomass has emerged as a promising alternative to conventional protein sources like soybeans [1]. Some of the plants considered as the alternative include forage crops such as alfalfa (Lucerne; Medicago sativa) or mixtures consisting of several species (grass-clover), for example, white/red clover (Trifolium sp.), and different species of ryegrass (Lolium sp.) or Festuca, which traditionally can be grown on less fertile arable lands. These multipurpose crops are already used for cattle grazing, green feed/supplement, silage production, and improving soil quality through atmospheric nitrogen fixation. Leguminous plants like alfalfa and clover are enriched in high-quality proteins within their green biomass, displaying digestibility characteristics in their protein concentrates comparable to proteins found in legume seeds such as soybean, pea, or lupine [5, 6].
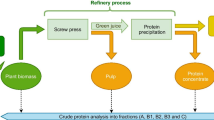
The utilization of this green biomass feedstock to produce concentrated feed protein for non-ruminant livestock, mainly poultry and pigs, has generated significant interest in recent years. Industrial production of protein from green biomass requires the establishment of an efficient biorefining stream. Among others, a refining process utilizing screw pressing and lactic acid-based fermentation has been developed on a lab scale [7] and was tested at a demo scale [8]. Briefly, this multistep refining process (Fig. 1) commences with a screw pressing of freshly harvested green biomass (fresh matter, FM), yielding a solid press cake fraction (PC) and a liquid green juice (GJ). The green juice is filtered to retain larger, insoluble fragments of cell debris (the fiber filtrate (FF)). Subsequently, the liquid fraction undergoes lactic acid fermentation for isoelectric precipitation of soluble proteins. The proteins are then separated by centrifugation, producing brown juice (BJ) and protein concentrate (LPC), which is further dried to obtain the final feed supplement. The main bottleneck of the current experimental technology for refining green biomass into feed protein concentrate is separating protein from nutritionally poor fiber, a mixture of plant cell wall components, namely complex carbohydrates, lignin, and glycoproteins. The overarching goal of the biorefinery process is to maximize the yield and quality of the extracted protein concentrate while also exploring the potential applications for by-products (fiber), such as bioenergy production in biogas plants [9, 10]. For instance, in the case of bioenergy production, minimizing the residual protein content in the press cake is desirable, whereas, for cattle feed, it is preferred to retain a certain amount of protein in the press cake [6]. Fiber in feed was previously believed to have a negative effect on animals [11] as the presence and composition of fiber in the protein concentrate may impact its digestibility by influencing intestinal viscosity, nutrient uptake, and hydrolytic enzyme activity [12]. However, recent studies suggest that the content and composition of fiber play a critical role and can offer multiple beneficial effects to animals [13, 14]. Therefore, monitoring fiber content and composition throughout the refining process is crucial for enhancing protein recovery and ensuring the purity and nutritional value of the final feed product. Furthermore, further valorization of the non-digestible by-products (for biomaterials, fermentable sugars, source of green chemicals) or their re-entry to the refining stream requires knowledge of their specific composition and content. This knowledge will facilitate the application of specialized hydrolytic enzyme cocktails and microbial strains tailored to the specific fiber fraction.
Simplified green biomass processing stream scheme used in this study. Plant material harvested from a field (green biomass, FM) was passed through a screw press to release proteins into the liquid fraction, while a large portion of insoluble fiber was retained as press cake (PC). The liquid fraction was further centrifuged to collect smaller fragments of cell wall debris (fiber filtrate, FF), while the liquid fraction of the filtered green juice (GJ) was fermented with lactic acid bacteria. Fermented juice was centrifuged to separate the final product of protein concentrate (LPC) from brown juice (BJ). The green color indicates the main input and output fractions obtained during the biomass processing; the yellow color indicates processes used during the protein concentrate production; purple indicates the process’s by-products. Fractions collected and used in this study are written in bold. *Shredding was only applied to the alfalfa biomass. The dashed line indicates the recirculation of fibers back to the screw press; this step was only included for grass-clover processing
In this study, we systematically characterized fractions generated during the production of green biomass protein concentrates derived from alfalfa and grass-clover mixture. Our approach involved a combination of in situ and in vitro analytical techniques, including high-pressure liquid chromatography (HPLC) and comprehensive microarray polymer profiling (CoMPP or MAPP [15, 16]), to assess the content and composition of fiber components (cell wall polysaccharides and lignin) as well as starch. By employing these methods, we elucidated variations in the cell wall composition of alfalfa and grass-clover across different fractions during biomass processing. Furthermore, our findings revealed the impact of lactic acid fermentation on the fiber content and the purity of the final protein product, highlighting the intricate interplay between processing techniques and fiber characteristics.
2 Materials and methods
2.1 Cultivation and harvest of the feedstocks
Organically grown alfalfa (Medicago sativa) was harvested in September 2019 from an experimental field at Aarhus University Foulum, Denmark, using a Grass Tech Grazer GT120 (Future Grass Technology Ltd, Carlow, Ireland) without chopping. Organically grown grass-clover was harvested from a commercial farm in Søttrup near Skive, Denmark, in August 2019 using commercial grass harvester machinery, which chopped it into smaller pieces (1–3 cm). The differences in biomass preprocessing at harvest result from technical differences in harvesters available at each field station. The grass-clover refers to ForageMax45 (DLF, Roskilde, Denmark), consisting of a mixture of perennial ryegrass (Lolium perenne), hybrid ryegrass (Italian ryegrass x meadow fescue (Festulolium)), white clover (Trifolium repens) and red clover (Trifolium pratense) (mixed in percentage ratio 40:42:9:9).
2.2 Biomass processing and production of LPCs
Alfalfa biomass was processed shortly after harvesting at a pilot-scale biorefining plant at Aarhus University Foulum. Briefly, the green biomass was cut in a shredder to an average length of 5 cm and then loaded into a Vincent CP4 single screw press (Vincent Corporation, Tampa, Florida, USA). The resulting juice was filtrated through a 150-μm mesh gravitation bow sieve (WestCoast, Esbjerg, Denmark), removing the bulk of larger fiber particles. After filtration, the juice was inoculated with a pre-culture of the lactic acid strain Lactobacillus salivarius BC1001 [17] and fermented overnight at 38°C in a stainless-steel tank as earlier described by Santamaria-Fernandez et al. [8]. The formation of lactic acid lowered the pH to around 4.2, coagulating the proteins in the juice. The resulting protein curd was dewatered in a horizontal decanter centrifuge (Alfa Laval, Søborg, Denmark).
The grass-clover processing took place at a pilot plant in Skive and followed a similar concept as described for the alfalfa, with few modifications. Grass-clover was cut into smaller pieces at harvest; thus, no shredder was employed at the pilot plant. For fractionation, twin-screw presses were used. In the case of the presented data, a Cir-tech Twin-Press (model EFB 25, Cir-tech, Skærbæk, Denmark) was employed, and a 2-layer vibrating sieve with 250 and 100 μm mesh (Cir-tech, Skærbæk, Denmark) separated fiber particles from the juice. Contrary to the alfalfa processing, grass-clover fibers were circulated back into the screw press (dashed line, Fig. 1). The fermented juice was dewatered in a decanter centrifuge (GEA, Düsseldorf, Germany) at a flow of 12 L min−1.
Samples of all refining fractions (FM, FF, PC, GJ, LPC, and BJ, Fig. 1) were collected and freeze-dried in a Telstar LyoQuest -55 freeze dryer (Azbil Co., Japan) for approx. 24 h (0.500 mBar, room temperature). The dried samples were then milled to a fine powder in a Pulverisette 6 planetary mill (Fritsch, Germany) using rounds of 30-s milling at 600 rpm. Powder samples were stored within airtight containers in the dark at room temperature.
2.3 Histochemistry and imaging
Small pieces of plant material were fixed in 4% formaldehyde solution in 1x phosphate-buffered saline (PBS) under vacuum, washed twice with PBS, and dehydrated through ethanol series (30%, 50%, 70%, 96%, and absolute ethanol). The embedding in LR White resin (Medium grade; Agar Scientific, Stansted, UK) was performed by submerging the samples first in the 1:1 mixture of LR resin: absolute ethanol (Sigma-Aldrich) for 24 h and then in pure LR resin. The samples were orientated in gelatin capsules (size 1, Electron Microscopy Sciences, Hatfield, Pennsylvania, USA) filled with LR White resin and polymerized at 60°C overnight. The gelatin capsule was removed, and the blocks were trimmed and sectioned using Leica EM-UC7 ultramicrotome (Leica, Germany) into 1-μm-thin sections collected and adhered on charged SuperFrost slides (Fisher Scientific).
Eosin Y/Calcofluor White (CW) staining: The slides were stained with a mixture of β-(1,4)-glucan specific dye Calcofluor White M2R (Fluorescent Brightener 28 at 1:100 dilution in PBS from 10 mg mL−1 stock) and 1% of basophilic dye Eosin Y for 10 min, washed twice with PBS and observed with the Leica SP5 (Leica, Germany) confocal microscope. CW: excitation 350 nm/emission 450 nm. Eosin Y: 496 nm excitation/emission 550 nm.
Toluidine blue staining: The sections were stained for 5 min with 1% of Toluidine Blue in water. After two washes with water, the stained sections were directly observed with a light microscope Olympus BX41 (Olympus, Japan).
2.4 Comprehensive microarray polymer profiling (CoMPP)
Homogenized freeze-dried samples collected at each of the processing steps were washed twice with 70% (v/v) ethanol, followed by CHCl3:CH3OH (1:1, v/v) and a final wash with 100% acetone; the remaining cell wall-enriched insoluble residue (AIR) was left to air-dry.
The CoMPP analysis was performed according to the method reported by Moller et al. [15] with modifications. An additional water extraction step was added compared to the original protocol. Each of the samples was weighed out in triplicate. Briefly, 10 mg of dry material was directly extracted with 300 μL Milli-Q water. In addition, 10 mg AIR was sequentially treated with 300 μL 50 mM trans-1,2-diaminocyclohexane-N,N,N′,N′-tetraacetic acid (CDTA) pH 7.5, followed by extraction with 300 μL 4 M NaOH containing 0.1% (v/v) NaBH4. Each extraction was carried out for 2 h in a TissueLyser II (Qiagen AB, Sollentuna, Sweden) at 6 s−1 at room temperature. Samples were centrifuged after each extraction, and the supernatant was collected and printed on nitrocellulose using an ArrayJet Marathon printer (ArrayJet, Roslin, UK). Four dilution points were prepared for each sample and were printed in two technical replicates.
The arrays were first blocked for 1 h with 5% (w/v) low-fat milk powder solution in phosphate-buffered saline (MP/PBS) and then probed with a set of specific primary monoclonal antibodies (Table 1) for 2 h. After several washes with PBS, arrays were incubated with 1:5000 solutions of either anti-mouse or anti-rat secondary antibodies conjugated with alkaline phosphatase for another 2 h. After three washes with PBS, followed by a final wash in Milli-Q water, the arrays were developed using a 5-bromo-4-chloro-3-indolyl-phosphate (BCIP)/nitro-blue tetrazolium chloride (NBT) substrate and scanned using a flatbed scanner (CanoScan 9000 Mark II; Canon, Søborg, Denmark) at 2400 dpi. The images were then converted to grayscale, and the calculated intensity of the signal was quantified using the microarray analysis software ProScanArray Express (PerkinElmer, Waltham, MA, USA). The relative intensity values were normalized to a scale from 0 to 100 and transformed into a heatmap.
2.5 Determination of non-cellulosic polysaccharide and crystalline cellulose content
The AIR material (10 mg) was hydrolyzed with 2 M trifluoroacetic acid (TFA) for 90 min at 121°C to release the non-cellulosic polysaccharides. Samples were cooled down and centrifuged for 10 min at 10000 rpm; the supernatant was collected and dried overnight in a centrifuge concentrator (Eppendorf, Hamburg, Germany). The pellet was collected and used for crystalline cellulose determination, as described below. The composition of monosaccharides and uronic acids in the supernatant was determined and quantified using high-pressure liquid chromatography using a Dionex Ultimate 3000 LC system (Thermo Scientific, Waltham, MA, USA) coupled with a refractive index detector (Dionex, Waltham, MA, USA). Before HPLC-RI, samples were pH-adjusted (depending on the used column), centrifuged (10 000 g, 10 min), and filtered (0.45 μM). For analysis of fucose, glucuronic acid, galacturonic acid, and rhamnose, an Aminex HPX-87H column (Bio-Rad Laboratories, Hercules, CA, USA) was used with 0.005 M H2SO4 as mobile phase, 0.6 mL min−1 flow rate, and 65°C column temperature. An Aminex HPX-87P column was used to analyze the remaining neutral sugars with Milli-Q water as mobile phase, 0.6 mL min−1 flow rate, and 85°C column temperature.
The crystalline cellulose content in the pellet was quantified using the Updegraff method following the protocol by Dampanaboina et al. [18]. The released glucose was quantified using the colorimetric anthrone reagent. The absorbance was measured at 625 nm in a microplate reader (Eon™ High-Performance Microplate Spectrophotometer, BioTek, Winooski, VT, USA) against a standard curve prepared with glucose. The anthrone assay was performed in three technical replicates for each sample.
2.6 Acetyl bromide soluble lignin
The acetyl bromide soluble lignin (ABSL) was quantified following the method by Moreira-Vilar et al. [19]. The analysis was carried out in a protein-free AIR prepared according to dos Santos et al. [20] to assess the effect of protein and other UV-absorbing components on the measurement. The concentration of ABSL was calculated from the UV absorbance measured at 280 nm (Eon™ High-Performance Microplate Spectrophotometer, BioTek, Winooski, VT, USA) using a molar extinction coefficient of 18.126 g−1 l cm−1 [21] and a path length of 0.6345 cm. Quantification of ABSL was performed in triplicate for each sample.
3 Results
3.1 Microscopic evaluation of the efficacy of screw pressing and filtration
We used microscopy to assess the protein extraction process’s efficiency and the tissue disruption level. Here, we present only data obtained from alfalfa as it represents a more homogenous single species material in contrast to grass-clover. Tissues were stained using the polychromatic dye, toluidine blue, which colored cell walls and nuclei purple, while the lignified tissues and the cytoplasmic proteins appeared blue (Fig. 2a, c). Laser scanning confocal microscopy and two fluorescent dyes were used to examine tissue morphology. Calcofluor White, a β-glucans specific dye, served as a general cell wall counterstain, while a nucleophilic dye, Eosin Y, allowed simultaneous visualization of cytoplasmic content [22]. The latter dye produced a distinct red granular signal within the cells, particularly those of mesophyll tissue (Fig. 2b, d). Microscopic imaging revealed that the screw pressing primarily disrupted mesophyll cells causing loss of cytosolic content (Fig. 2c, d). However, a substantial portion of cells in the pressed cake remained unbroken, as indicated by prominent Eosin Y labeling (Fig. 2d). Most of the cell wall material remained in the pressed cake fraction with discernible tissue types (e.g., vascular bundles and epidermis (Fig. 2c, d).
The effect of screw pressing on the cellular structure integrity of alfalfa. Images of resin-embedded sections of leaf material (a, b) and pressed cake (c, d). The sections were stained with Toluidine blue (a, c) or co-labeled with Calcofluor White (CW), a dye specific for cell wall β-glucans (blue) and Eosin Y, a nucleophilic dye with a preference towards proteins (red) (b, d). The efficiency of screw pressing was monitored by loss of intracellular protein content as indicated by arrowheads. Scale bars: 50μm
3.2 Immuno-microarray profiling of carbohydrate content in the different fractions along the refining stream
We prepared an alcohol-insoluble residue (AIR) to analyze the content and detailed composition of the cell wall material in the fractions obtained during green biomass protein concentrate production. AIR is a cell wall-enriched material free of highly soluble proteins, lipids, and primary/secondary metabolites [16]. The AIR content was comparable between alfalfa and grass-clover fractions, except for GJ, where the value was two-fold higher for the grass-clover (Fig. 3).
Plant cell walls are highly complex structures consisting of various polysaccharides, polyphenols, glycoproteins, and polyesters. The microscopy observation and AIR content determination revealed the presence of cell wall debris in all the fractions. Thus, we sought to analyze further the relative presence of cell wall polysaccharides, proteoglycans, and starch in the samples using immune-microarray-based profiling (comprehensive microarray polymer profiling, CoMPP, alternatively called MAPP [16, 23]. This method involved sequential extraction of carbohydrates from AIR using CDTA followed by NaOH, with the former mainly releasing pectin and loosely bound proteoglycans and the latter targeting mostly hemicelluloses. Additionally, water extraction was directly performed on the dry matter to assess the presence of easily extractable compounds. All resulting extracts were printed as nitrocellulose microarrays and probed with a panel of specific anti-glycan monoclonal antibodies (mAbs; see Table 1 for designations and specificity). The resulting glycan profiles exhibited variation between feedstock types and, notably, among the fractions.
Water extraction was particularly efficient in releasing glycosylated proteins (Fig. 4). Epitopes of extensins and arabinogalactan proteins (AGPs) were abundantly observed in almost all alfalfa fractions. At the same time, only AGPs were detected in grass-clover (Fig. 4). Several pectin epitopes of homogalacturonan (HG) and rhamnogalacturonan I (RG-I) were also found to be released in water, primarily at the early steps of processing (Fig. 4). Moreover, heteroxylan epitopes, recognized with LM27 antibody, were detected in all grass-clover fractions except LPC.
Comprehensive microarray polymer profiling (CoMPP) of extractable polysaccharides and glycosylated proteins in different fractions obtained from alfalfa and grass-clover. a The dry material of alfalfa and grass-clover was incubated with water to release soluble and weakly-bound cell wall components. Additionally, b AIR material was subjected to extraction with CDTA followed by NaOH. The heatmaps present average signal intensities, and the color intensity is correlated with the signal strength; white color indicates low and red high intensity. The names of antibodies and the epitopes they recognize are indicated along the x-axis. Antibodies that did not show a signal for all the samples are not included in the heatmap but are listed in Table 1. AGP, arabinogalactan protein; BJ, brown juice; DE, degree of methylesterification; FF, fiber filtrate; FM, fresh matter; GAX, glucuronoarabinoxylan; GJ, green juice; HG, homogalacturonan; LPC; green biomass protein concentrate; PC, press cake; RG-I, rhamnogalacturonan I
Extractions performed on the AIR material resulted in a higher release of cell wall-associated epitopes. As expected, the CDTA fraction exhibited a notable abundance of HG (JIM5, JIM7) and RG-I (LM5, LM6, LM13, INRA-RU1) (Fig. 4). FM and PC fractions from both feedstocks displayed the highest signal. However, while all the anti-pectin antibodies exhibited a strong signal in the FF and LPC fractions of alfalfa, in grass-clover, the signal was predominantly observed in the GJ fraction (Fig. 4). The LM25 mAb, recognizing xyloglucan and unsubstituted β-glucans, was the only hemicellulose-targeting antibody displaying substantial signal in the CDTA fractions of both feedstocks, exhibiting a labeling pattern similar to that of pectin antibodies. Additionally, heteromannans (LM21) and grass-specific heteroxylans (LM27) were detected in grass-clover at the early and late processing stages, respectively. All fractions contained substantial amounts of AGPs, and in alfalfa, extensin epitopes also gave a particularly high signal in the BJ fraction.
Some pectin epitopes were still detectable in NaOH fraction, mainly those of RG-I. However, the majority of the signals in this fraction originated from antibodies targeting various hemicelluloses (Fig. 4). The FM and PC fractions of alfalfa had similar profiles with abundant signals for mAbs recognizing xyloglucan (LM25 and LM15), unsubstituted xylan (LM10), and heteromannan (LM21). These epitopes were also detected in the FF and GJ fractions but with much lower signal intensity. Some signals for heteroxylan (LM11, LM27) and (1,3;1,4)-β-glucan (BS-400-3) were also detected in alfalfa but almost exclusively in the FM (Fig. 4). Conversely, all hemicellulose epitopes were very abundant in the FM and PC fraction of grass-clover, and to a lesser extent in the GJ, while the FF fraction displayed minimal signal (Fig. 4). Similar to the previous extractions, NaOH successfully released glycosylated proteins, with epitopes of AGPs and extensins predominantly present in all fractions except BJ. Signals for starch (INCh1) and (1,3)-β-glucan (BS-400-2) targeting antibodies were also detected in the NaOH extract. While signals for (1,3)-β-glucan were observed across all the fractions except BJ, starch epitopes were only detected in the FM and BJ fractions of alfalfa and GJ of grass-clover.
3.3 Monosaccharide composition analysis supports CoMPP results
In addition to semi-quantitative CoMPP profiling, we analyzed monosaccharide composition using HPLC-RI on a trifluoroacetic acid (TFA)-hydrolyzed AIR material. The monosaccharide content of non-cellulosic polysaccharides in alfalfa fractions showed minor variations, constituting approximately 10–11% of AIR, except from LPC, which had only 5% of non-cellulosic polysaccharides (Table 2). Conversely, grass-clover fractions exhibited more substantial variation, with higher amounts of non-cellulosic polysaccharides detected in all fractions than in alfalfa (Table 2). GJ and BJ fractions contained the highest amounts of non-cellulosic polysaccharides, 27 and 19% of AIR, respectively (Table 2). Similar to alfalfa, the LPC fraction in grass-clover exhibited the lowest non-cellulose polysaccharide content, about 7% of AIR. The composition of the released monosaccharides also varied between the fractions and the biomass types. FM and PC fractions of alfalfa had comparable monosaccharide profiles, with glucose and xylose comprising about half of the detected sugars. In GJ, the contribution of these sugars increased to 60%, while the relative amount of neutral sugars decreased compared to FM and PC. As expected, the glucose content decreased below 10% in BJ, while xylose content doubled compared to GJ, with neutral sugar content remaining primarily unaffected. All monosaccharides in the initial FM fraction were also detected in the LPC.
Contrary to alfalfa, the relative content of glucose and xylose showed little changes at the early processing steps of grass-clover, while the other neutral monosaccharides showed alterations instead (Table 2). Glucose content dropped to 10% in the BJ of grass-clover, accounting for 28% of all detected monosaccharides. All the monosaccharides were also detected in the LPC fraction of grass-clover, with glucose being the most abundant, as in alfalfa.
3.4 Biochemical determination of crystalline cellulose and lignin content
While cellulose and lignin are part of the non-digestible fiber fraction, their insolubility prevents their inclusion in the nitrocellulose-based analysis of non-cellulosic polysaccharides. Hence, we quantified the content of these two cell wall components using established biochemical assays.
Crystalline cellulose accounted for about 10% of AIR material in the FM of both feedstocks, with its contribution increasing to 12% in alfalfa PC and 14% in grass-clover PC (Fig. 5a). FF of grass-clover exhibited a high cellulose content of 9%, which was about three times higher than the value measured in alfalfa FF. Only trace amounts of crystalline cellulose were detected in GJ and BJ fractions of both feedstocks, specifically 1.86 and 0.507 μg cellulose per mg AIR in GJ of alfalfa and grass-clover, respectively, with further reductions in BJ. The two feedstocks displayed very different cellulose content in the LPC fraction, as grass-clover accounted for only 1.2% of the fraction, while alfalfa exhibited a value approximately 16 times higher.
Concentration of crystalline cellulose and acetyl bromide soluble lignin (ABSL) in the fractions of alfalfa and grass-clover. a Crystalline cellulose was released from the cell wall material using the Updegraff method, followed by quantification of glucose with the anthrone reagent (n=4, each sample was analyzed in three technical replicates). b Concentrations of acetyl bromide soluble lignin were calorimetrically measured in protein-free AIR material (n=4; each sample was analyzed in 3 technical replicates). BJ, brown juice; FF, fiber filtrate; FM, fresh matter; GJ, green juice; LPC; protein concentrate; PC, press cake
Distribution of ABSL varied across different fractions, exhibiting distinct patterns compared to crystalline cellulose (Fig. 5). The content in FM and PC of alfalfa was identical, about 12% AIR. In comparison, only 8.7% was observed in FM of grass-clover and 11% in PC (Fig. 5b). The ABSL in GJ, FF, and LPC of alfalfa was comparable and more than twofold lower than that measured in FM. Conversely, the corresponding fractions from grass-clover had much higher ABSL levels, the same (as observed in the FF fraction) or 20% lower than in the FM. Notably, in contrast to cellulose, lignin content in BJ was among the highest for all fractions, with similar values for both feedstock types.
4 Discussion
4.1 Differences in fiber composition in green biomass during industrial processing
Various types of green biomass are considered an alternative for protein production; they are often mixes of several species, harvested at different growth stages, which can result in variation in cell wall composition (fiber) [24], consequently affecting the processing. We were particularly interested in how this diversity would manifest in the composition of different fractions and whether the process needed to be tailored separately for individual feedstocks. Thus, in this study, we compared the processing of two types of feedstocks with distinct cell wall compositions: grass-clover, a mixture of grasses and legumes, representing a combination of type I and type II cell walls [25], and alfalfa possessing exclusively type I cell wall. It is important to note that our analysis was conducted on the AIR preparations in which most alcohol-soluble proteins were released during the process [16]. Thus, the data reflects the relative abundance of the fiber types rather than the fiber-to-protein content ratio. The grass-specific glucuronoarabinoxylan (LM27 mAb) and mixed-linkage glucan (BS-400-3 mAb) epitopes were more abundant in grass-clover than in alfalfa-derived samples (Fig. 4) [26]. Surprisingly, the pectin content was comparable between the two feedstocks despite expectations based on type I/type II cell wall characteristics [26]. Only a minor starch signal was observed in a few samples; however, it must be clarified if the low signal results from a suboptimal detection system or due to circadian regulation of starch content [27].
The most significant compositional differences appeared in the GJ fraction, where grass-clover possessed almost twice as much fiber per gram dry weight as alfalfa (Fig. 3). Those differences were mainly identified as pectin, hemicelluloses (Fig. 4 and Table 2) and lignin (Fig. 5b). We speculate that the differences observed in GJ samples may indicate anatomical variations in tissue types and extractability resulting from inherent variation in cell wall structure. Another explanation could be the effect of the degree of sample cutting at harvest. Increased contribution of soluble cell wall components and improved fiber digestion was demonstrated previously in ruminants due to mechanical processing that led to higher cell disruption [28].
4.2 Effect of lactic acid fermentation on fiber content of BJ and purity of LPC
We observed an expected effect of lactic acid fermentation in the BJ samples, such as a sharp decrease in glucose content, an increase in xylose content, and a reduction in crystalline cellulose content. We detected monosaccharides in BJ using HPLC but not CoMPP, which indicates polysaccharide breakdown into smaller fragments during the fermentation. Detection of low-molecular-weight oligosaccharides (typically with a DP < 6) is impossible with CoMPP as they do not adhere to the nitrocellulose microarray matrix. Although the BJ was relatively poor in polysaccharides, this was not the case for LPC, as epitopes of all classes of polysaccharides were still detected there, although in lower quantities compared to other fractions (Fig. 4 and Table 2). Our in vitro analysis revealed the presence of non-fermentable fibers, such as xylan and lignin, in the final protein concentrate. However, the absolute amounts in fresh weight have to be determined.
This indicates that the fermentation is not 100% effective; a post-fermentation refining process should be considered that concentrates both polysaccharides and proteins. As the content of non-digestible fibers may lower the quality of the LPC [29], cell wall degrading enzymes may be added during or after the fermentation to reduce the degree of such fibers in the final product. There are numerous examples of the positive effect of LPC inclusion in animal feed as a full or partial substitution of soy proteins, but the inclusion level may depend on the protein and especially the non-digestible fiber content of the LPC [13, 30].
4.3 Optimization of the protein extraction
Green biomass is an exciting source for food protein production, as the primary photosynthesis-related protein, ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCo), possesses desirable nutritional qualities and functional properties for food applications, such as solubility, foaming, and gelling [31,32,33]. However, the efficiency of the extraction process is a focal point for advancing the sustainability of plant-based protein production. Our methods, particularly microscopy observation, demonstrated that screw pressing only partially disrupted the tissues. This aligns with the general observation that only a modest portion of the starting dry matter and protein is typically recovered in the green juice fraction when processing this biomass. A previous study has reported that 65–75% of dry weight and crude protein remain in the press cake fraction after pressing grass-clover, alfalfa, and oilseed radish using a lab-scale twin screw presser [7]. Similar extraction efficiency has been observed for other leaf biomass types, such as cassava leaves, where approximately 30% of the protein was recovered in the juice during a lab-scale production of cassava leaf protein concentrate [34]. Higher extraction efficiency could be achieved through multiple pressings and the implementation of pretreatment steps, such as chopping or pulping. The pressing step itself could also be optimized, as demonstrated by a study that achieved 58% protein recovery in green juice from alfalfa by employing an optimized screw profile and adding water [35]. However, it is crucial to consider the associated costs of additional processing and material input, especially in large-scale production and potentially higher content of fiber released into the solution during the pretreatment steps.
4.4 Potential use of structural cell wall proteoglycan
Interestingly, our approach identified cell wall structural proteoglycans, specifically AGPs and extensins, in all the fractions throughout the technological process and was detectable in the BJ and protein concentrate, suggesting that those forms of the glycan chains are less fermentable. As some proteoglycan-rich extracts, such as gum Arabic, serve as functional food additives with emulsifying and plasticizing properties [36], it would be interesting to precisely analyze the structure and amount of these cell wall components and assess their impact on the nutritional value and other characteristics, such as viscosity, of the final protein product.
4.5 Strategies for elimination of unwanted fiber
The negative impact of fiber in animal diets has been mostly associated with the anti-nutritional value of the fiber; however, recent studies consider other beneficial effects of fiber, such as anti-inflammatory or growth stimulating [13, 37]. For example, lignin, which has consistently been found in all the tested fractions (Fig. 5b), may have a beneficial role as an antioxidant. Still, they can also obstruct the accessibility of microorganisms or hydrolytic enzymes to carbohydrates. The knowledge about the fiber composition in the fractions allows better utilization of by-products for a more diverse range of applications. It enables the development of targeted strategies to remove unwanted components.
Enzymatic hydrolysis is critical in numerous biomass processing lines, including bioethanol production from lignocellulosic biomass [38]. Incorporating enzymatic hydrolysis into green biomass protein extraction can serve multiple purposes; previous experimental work has demonstrated the effective integration of enzymatic hydrolysis in both the processing and precipitation steps and downstream fiber removal in the LPC (data not published). Enzymatic treatments using commercial products could be costly; however, alternative sources of enzymatic activity are available. One option is the on-site production of lignocellulolytic enzymes by cultivating filamentous fungi on the press cake fraction, an excellent growth substrate (data not published). This could be achieved using a simple solid-state fermentation setup, as demonstrated by a study that utilized a wheat bran/sphagnum substrate combined with different combinations of Trichoderma and Aspergillus strains to produce cost-effective enzyme broth with high activity towards pretreated wheat straw [39]. Again, the results presented in our study can aid in selecting potential fungal candidates based on their secretomes and guide the choice of microorganisms for bioconversion in the biorefinery system.
5 Conclusions
This study used various analytical techniques to evaluate the distribution of different cell wall components during green biomass protein concentrate production from alfalfa and grass-clover. The presence of fiber was detected along the entire industrial stream, and the use of CoMPP provided detailed information about fiber profiles in tested feedstocks. Although the grass-clover and alfalfa share strong compositional similarities (pectin content), they possess unique polysaccharide epitopes (glucuronoarabinoxylans) and striking differences in content and composition of polysaccharides in the GJ fraction. Lactic acid fermentation successfully removes part of the fiber, which can be collected as low-molecular-weight oligosaccharide fragments in the BJ fraction. The presence of non-fermentable fibers and cell wall structural proteoglycans in the protein concentrate warrants further investigation regarding their quantities and potential impact on the nutritional and functional properties of the final product.
In conclusion, efficient extraction of pure protein concentrates from plant biomass might require optimization due to variations in the fiber composition of the used feedstocks. However, the combination of microscopic observation, immuno-microarray profiling, and monosaccharide composition analysis is an effective evaluation approach to identify polysaccharide targets for targeted enzymatic hydrolysis and the utilization of alternative enzymatic sources and microbial strains for improving the efficiency of green biomass protein extraction and refining processes.
Data availability
The data and material of the current study are available from the corresponding author at reasonable request.
References
Kim SW, Less JF, Wang L, Yan T, Kiron V, Kaushik SJ, Lei XG (2019) Meeting global feed protein demand: challenge, opportunity, and strategy. Annu Rev Anim Biosci 7(1):221–243. https://doi.org/10.1146/annurev-animal-030117-014838
Colgrave ML, Dominik S, Tobin AB, Stockmann R, Simon C, Howitt CA, Belobrajdic DP, Paull C, Vanhercke T (2021) Perspectives on future protein production. J Agric Food Chem 69(50):15076–15083. https://doi.org/10.1021/acs.jafc.1c05989
Qin P, Wang T, Luo Y (2022) A review on plant-based proteins from soybean: health benefits and soy product development. J Agric Food Res 7:100265. https://doi.org/10.1016/j.jafr.2021.100265
da Silva RFB, Viña A, Moran EF, Dou Y, Batistella M, Liu J (2021) Socioeconomic and environmental effects of soybean production in metacoupled systems. Sci Rep 11(1):18662. https://doi.org/10.1038/s41598-021-98256-6
Olukosi OA, Walker RL, Houdijk JGM (2019) Evaluation of the nutritive value of legume alternatives to soybean meal for broiler chickens. Poult Sci 98(11):5778–5788. https://doi.org/10.3382/ps/pez374
Stødkilde L, Damborg VK, Jørgensen H, Lærke HN, Jensen SK (2019) Digestibility of fractionated green biomass as protein source for monogastric animals. Animal 13(9):1817–1825. https://doi.org/10.1017/S1751731119000156
Santamaría-Fernández M, Molinuevo-Salces B, Kiel P, Steenfeldt S, Uellendahl H, Lübeck M (2017) Lactic acid fermentation for refining proteins from green crops and obtaining a high quality feed product for monogastric animals. J Clean Prod 162:875–881. https://doi.org/10.1016/j.jclepro.2017.06.115
Santamaria-Fernandez M, Ambye-Jensen M, Damborg VK, Lübeck M (2019) Demonstration-scale protein recovery by lactic acid fermentation from grass clover – a single case of the production of protein concentrate and press cake silage for animal feeding trials. Biofuels Bioprod Biorefin 13(3):502–513. https://doi.org/10.1002/bbb.1957
Solarte-Toro JC, Cardona Alzate CA (2023) Sustainability of biorefineries: challenges and perspectives. Energies 16(9). https://doi.org/10.3390/en16093786
Calvo-Flores FG, Martin-Martinez FJ (2022) Biorefineries: achievements and challenges for a bio-based economy. Front Chem 10. https://doi.org/10.3389/fchem.2022.973417
Bach Knudsen KE (2001) The nutritional significance of “dietary fibre” analysis. Anim Feed Sci Technol 90(1):3–20. https://doi.org/10.1016/S0377-8401(01)00193-6
Plouhinec L, Neugnot V, Lafond M, Berrin J-G (2023) Carbohydrate-active enzymes in animal feed. Biotechnol Adv 65:108145. https://doi.org/10.1016/j.biotechadv.2023.108145
Jha R, Mishra P (2021) Dietary fiber in poultry nutrition and their effects on nutrient utilization, performance, gut health, and on the environment: a review. J Anim Sci Biotechnol 12(1):51. https://doi.org/10.1186/s40104-021-00576-0
Tiwari UP, Fleming SA, Abdul Rasheed MS, Jha R, Dilger RN (2020) The role of oligosaccharides and polysaccharides of xylan and mannan in gut health of monogastric animals. J Nutr Sci 9:e21. https://doi.org/10.1017/jns.2020.14
Moller I, Sørensen I, Bernal AJ, Blaukopf C, Lee K, Øbro J, Pettolino F, Roberts A, Mikkelsen JD, Knox JP, Bacic A, Willats WG (2007) High-throughput mapping of cell-wall polymers within and between plants using novel microarrays. Plant J 50(6):1118–1128. https://doi.org/10.1111/j.1365-313X.2007.03114.x
Fangel JU, Jones CY, Ulvskov P, Harholt J, Willats WGT (2021) Analytical implications of different methods for preparing plant cell wall material. Carbohydr Polym 261:117866. https://doi.org/10.1016/j.carbpol.2021.117866
Thomsen MH, Kiel P (2008) Selection of lactic acid bacteria for acidification of brown juice (grass juice), with the aim of making a durable substrate for L-lysine fermentation. J Sci Food Agric 88(6):976–983. https://doi.org/10.1002/jsfa.3176
Dampanaboina L, Yuan N, Mendu V (2021) Estimation of crystalline cellulose content of plant biomass using the updegraff method. J Vis Exp 171. https://doi.org/10.3791/62031
Moreira-Vilar FC, Siqueira-Soares RDC, Finger-Teixeira A, Oliveira DMD, Ferro AP, da Rocha GJ, Ferrarese MDLL, dos Santos WD, Ferrarese-Filho O (2014) The acetyl bromide method is faster, simpler and presents best recovery of lignin in different herbaceous tissues than klason and thioglycolic acid methods. PLoS One 9(10):e110000. https://doi.org/10.1371/journal.pone.0110000
dos Santos WD, Ferrarese MLL, Nakamura CV, Mourão KSM, Mangolin CA, Ferrarese-Filho O (2008) Soybean (Glycine max) root lignification induced by ferulic acid. The Possible Mode of Action. J Chem Ecol 34(9):1230–1241. https://doi.org/10.1007/s10886-008-9522-3
Fukushima RS, Hatfield RD (2004) Comparison of the acetyl bromide spectrophotometric method with other analytical lignin methods for determining lignin concentration in forage samples. J Agric Food Chem 52(12):3713–3720
Chau HW, Goh YK, Si BC, Vujanovic V (2011) An innovative brilliant blue FCF method for fluorescent staining of fungi and bacteria. Biotech Histochem 86(4):280–287. https://doi.org/10.3109/10520295.2010.492733
Moller I, Marcus SE, Haeger A, Verhertbruggen Y, Verhoef R, Schols H, Ulvskov P, Mikkelsen JD, Knox JP, Willats W (2008) High-throughput screening of monoclonal antibodies against plant cell wall glycans by hierarchical clustering of their carbohydrate microarray binding profiles. Glycoconj J 25(1):37–48. https://doi.org/10.1007/s10719-007-9059-7
Sarkar P, Bosneaga E, Auer M (2009) Plant cell walls throughout evolution: towards a molecular understanding of their design principles. J Exp Bot 60(13):3615–3635. https://doi.org/10.1093/jxb/erp245
Zhang B, Gao Y, Zhang L, Zhou Y (2021) The plant cell wall: biosynthesis, construction, and functions. J Integr Plant Biol 63(1):251–272. https://doi.org/10.1111/jipb.13055
Vogel J (2008) Unique aspects of the grass cell wall. Curr Opin Plant Biol 11(3):301–307. https://doi.org/10.1016/j.pbi.2008.03.002
Izumi M (2019) Roles of the clock in controlling starch metabolism. Plant Physiol 179(4):1441–1443. https://doi.org/10.1104/pp.19.00166
Pintens DA, Shinners KJ, Friede JC, Kalscheur KF, Digman MF, Combs DK (2022) Intensive mechanical processing of forage crops to improve fibre digestion. Grass Forage Sci 77(1):55–65. https://doi.org/10.1111/gfs.12559
Stødkilde L, Ambye-Jensen M, Krogh Jensen S (2020) Biorefined grass-clover protein composition and effect on organic broiler performance and meat fatty acid profile. J Anim Physiol Anim Nutr (Berl) 104(6):1757–1767. https://doi.org/10.1111/jpn.13406
Domokos-Szabolcsy É, Yavuz SR, Picoli E, Fári MG, Kovács Z, Tóth C, Kaszás L, Alshaal T, Elhawat N (2023) Green biomass-based protein for sustainable feed and food supply: an overview of current and future prospective. Life 13(2). https://doi.org/10.3390/life13020307
Nissen SH, Lübeck M, Møller AH, Dalsgaard TK (2022) Protein recovery and quality of alfalfa extracts obtained by acid precipitation and fermentation. Bioresour Technol Rep 19:101190. https://doi.org/10.1016/j.biteb.2022.101190
Hadidi M, Hossienpour Y, Nooshkam M, Mahfouzi M, Gharagozlou M, Aliakbari FS, Aghababaei F, McClement DJ Green leaf proteins: a sustainable source of edible plant-based proteins. Crit Rev Food Sci Nutr:1–18. https://doi.org/10.1080/10408398.2023.2229436
Santamaría-Fernández M, Lübeck M (2020) Production of leaf protein concentrates in green biorefineries as alternative feed for monogastric animals. Anim Feed Sci Technol 268:114605. https://doi.org/10.1016/j.anifeedsci.2020.114605
Ayele HH, Latif S, Bruins ME, Müller J (2021) Partitioning of proteins and anti-nutrients in cassava (Manihot esculenta Crantz) leaf processing fractions after mechanical extraction and ultrafiltration. Foods 10(8). https://doi.org/10.3390/foods10081714
Colas D, Doumeng C, Pontalier PY, Rigal L (2013) Green crop fractionation by twin-screw extrusion: influence of the screw profile on alfalfa (Medicago sativa) dehydration and protein extraction. Chem Eng Process 72:1–9. https://doi.org/10.1016/j.cep.2013.05.017
Barak S, Mudgil D, Taneja S (2020) Exudate gums: chemistry, properties and food applications – a review. J Sci Food Agric 100(7):2828–2835. https://doi.org/10.1002/jsfa.10302
Hussein SM, Frankel TL (2019) Effect of varying proportions of lignin and cellulose supplements on immune function and lymphoid organs of layer poultry (Gallus gallus). Poult Sci 56(1):71–77. https://doi.org/10.2141/jpsa.0180032
Vasić K, Knez Ž, Leitgeb M (2021) Bioethanol production by enzymatic hydrolysis from different lignocellulosic sources. Molecules 26(3). https://doi.org/10.3390/molecules26030753
Kolasa M, Ahring BK, Lübeck PS, Lübeck M (2014) Co-cultivation of Trichoderma reesei RutC30 with three black Aspergillus strains facilitates efficient hydrolysis of pretreated wheat straw and shows promises for on-site enzyme production. Bioresour Technol 169:143–148. https://doi.org/10.1016/j.biortech.2014.06.082
Acknowledgements
We thank Associate Professor Morten Ambye-Jensen for giving us access to their pilot-scale biorefining plant at Aarhus University Foulum to produce alfalfa fractions and the company BiomassProtein Aps for harvesting, processing, and delivering grass-clover fractions from their pilot plant in Skive.
Funding
Open access funding provided by Royal Library, Copenhagen University Library. This study was funded by the Danish Green Development and Demonstration Program (GUDP) project GræsProteinFoder grant no. 35009-19-1508. SG was additionally supported by the Independent Research Fund Denmark (grant number 0170-00009B), and JM is supported by the grant IMPULZ (IM-2021-23) from the Slovak Academy of Sciences.
Author information
Authors and Affiliations
Contributions
Mette Lübeck obtained the resources and provided the first concept and design of the study, with the contribution of all authors at the later stages. Bodil Jørgensen provided the immuno-microarray profiling platform. Sylwia Głazowska, Emil Gundersen, Stefan Heiske, and Jozef Mravec performed material preparation, data collection, and analysis. Sylwia Głazowska and Jozef Mravec wrote the first draft of the manuscript, and all authors commented on previous versions. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
This declaration is not applicable.
Competing interests
The authors declare no competing interests, except for Mette Lübeck, who co-founded BiomassProtein Aps, a company aiming at commercializing green biorefining. The biorefined samples from grass-clover were obtained from BiomassProtein’s pilot plant.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Głazowska, S., Gundersen, E., Heiske, S. et al. Tracking digestible and non-digestible cell wall components during protein concentrate production from grass-clover and alfalfa. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-05125-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-05125-5