Abstract
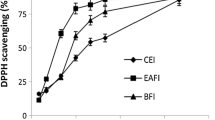
This work is a component of the development of the aerial parts (leaves and stems) of Oudneya africana R., of the Brassicaceae family. For that purpose, the total phenolic content (TPC), total flavonoid content (TFC), antioxidant activities (DPPH, ABTS, β-carotene, and phenanthroline assays), and antimicrobial activity of the ethanolic extracts of this plant were evaluated. Ethanolic leaf extract had a higher quantity of phenolics (69.75 ± 1.87 μg GAE/mg) and flavonoids (91.88 ± 1.94 μg QE/mg) than stem extract. Despite their significant antioxidant activities, leaf and stem ethanolic extracts had a weaker capacity to block the DPPH radical, as well as the ABTS radical, the β-carotene-linoleate bleaching assay, and the phenanthroline test, than industrial antioxidants, namely butylhydroxytoluene (BHT) and butylhydroxyanisole (BHA). The remarkable antioxidant activity was observed in ethanolic stem extract on the ABTS assay with an IC50 value of 15.90 μg/mL and in the β-carotene with an IC50 value of 20.21 μg/mL. The ethanolic extract from leaves and stems showed excellent antibacterial activity against Candida albicans, with inhibition diameters of 13 mm and 12 mm, respectively. This work comprises a preliminary investigation, and as such, it suggests Oudneya africana R. as a potential candidate for having antibacterial and antioxidant properties and also draws attention to the potential value of using this plant as a traditional treatment.




Similar content being viewed by others
Data availability
Not applicable
References
Frecska E, Bokor P, Winkelman M (2016) The therapeutic potentials of ayahuasca: possible effects against various diseases of civilization. Front Pharmacol 7:35
Anand U, Jacobo-Herrera N, Altemimi A, Lakhssassi N (2019) A comprehensive review on medicinal plants as antimicrobial therapeutics: potential avenues of biocompatible drug discovery. Metabolites 9(11):258
Ekor M (2014) The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol 4:177
Miara MD, Bendif H, Rebbas K, Rabah B, Hammou MA, Maggi F (2019) Medicinal plants and their traditional uses in the highland region of Bordj Bou Arreridj (Northeast Algeria). J Herb Med 16:100262
Fagbule OF, Emenyonu U, Idiga E, Oni OO, Ijarogbe OA, Osuh ME, Lawal FB, Owoaje TO, Ibiyemi O (2023) Using traditional rhyme (folk song) as a tool for oral hygiene promotion (UTRATOHP) among children in rural communities in Nigeria: a protocol for a randomised controlled trial. PLoS One 18:e0280856
Courric E, Brinvilier D, Couderc P, Ponce-Mora A, Méril-Mamert V, Sylvestre M, Pelage JH, Vaillant J, Rousteau A, Bejarano E (2023) Medicinal plants and plant-based remedies in Grande-Terre: an ethnopharmacological approach. Plants 12(3):654
Hamza N, Berke B, Umar A, Cheze C, Gin H, Moore N (2019) A review of Algerian medicinal plants used in the treatment of diabetes. J Ethnopharmacol 238:111841
Telli A, Esnault MA, El Hadj O, Khelil A (2016) An ethnopharmacological survey of plants used in traditional diabetes treatment in south-eastern Algeria (Ouargla province). J Arid Environ 127:82–92
Hammami R, Hamida JB, Vergoten G, Lacroix JM, Slomianny MC, Mohamed N, Fliss I (2009) A new antimicrobial peptide isolated from Oudneya africana seeds. Microbiol Immunol 53(12):658–666
Nabti LZ, Belhattab R (2016) In vitro antioxidant activity of Oudneya africana R. Br aerial parts. IBSPR 4:58–64
Djeridane A, Yousfi M, Nadjemi B, Boutassouna D, Stocker P, Vidal N (2006) Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem 97:654–660
Tiwari P, Bajpai M, Sharma A (2023) Antimicrobials from medicinal plants: key examples, success stories and prospects in tackling antibiotic resistance. Lett Drug Des Discovery 20(4):420–438
Larbi BAM, Naima B, Elsharkawy ER, Neghmouche NS (2018) Phytochemical characterization, in-vitro cytotoxic and antibacterial activity of Cotula cinerea (Delile) Vis essential oil. J Nat Remedies 18(3):107–112
Bouhadjera K, Kebir T, Baba-Ahmed A, Bendahou M (2005) Antimicrobial activity of the sterols and steroids extracted from the Algerian Oudneya Africana R.Br. Pakistan. J Biol Sci 8:834–838
Altwaty NH, El-Sayed OE, Aly NA, Baeshen MN, Baeshen NA (2016) Molecular and cytogenetic assessment of Dipterygium glaucum genotoxicity. An Acad Bras Cienc 88:623–634
Talbi S, Rojas JA, Sahrawy M, Rodríguez-Serrano M, Cárdenas KE, Debouba M, Sandalio LM (2020) Effect of drought on growth, photosynthesis and total antioxidant capacity of the Saharan plant Oudeneya africana. Environ Exp Bot 176:104099
Khedher O, Rigane G, Riguene H, Ben Salem R, Moussaoui Y (2021) Phenolic profile (HPLC-UV) analysis and biological activities of two organic extracts from Echinops spinosissimus Turra roots growing in Tunisia. Nat Prod Res 35:5786–5793
Yahyaoui A, Khedher O, Rigane G, Ben Salem R, Moussaoui Y (2018) Chemical analysis of essential oil from Echinops Spinosus L. roots: antimicrobial and antioxidant activities. Rev Roum Chim 63:199–204
Mechaala S, Bouatrous Y, Adouane S (2022) Traditional knowledge and diversity of wild medicinal plants in El Kantara's area (Algerian Sahara gate): an ethnobotany survey. Acta Ecol Sin 42(1):33–45
Ramdane F, Essid R, Fares N, El Ouassis D, Aziz S, Hadj Mahammed M, Ould Hadj MD, Limam F (2017) Antioxidant antileishmanial cytotoxic and antimicrobial activities of a local plant Myrtus nivellei from Algeria Sahara. Asian Pac J Trop Biomed 7(8):702–707
Nithya M, Ragavendran C, Natarajan D (2018) Antibacterial and free radical scavenging activity of a medicinal plant Solanum xanthocarpum. Int J Food Prop 21(S1):S313–S327
Halla N, Boucherit K, Boucherit-Otmani Z, Touati FZ, Rahmani N, Aid I (2019) Ammodaucus leucotrichus and Citrullus colocynthis from algerian Sahara: Ethnopharmacological application, phytochemical screening, polyphenols content and antioxidant activity of hydromethanolic extracts. J King Saud Univ Sci 31(4):541–548
Taïbi K, Aït Abderrahim L, Boussaid M, Taibi F, Achir M, Souana K, Benaissa T, Farhi KH, Naamani FZ, Nait Said K (2021) Unraveling the ethnopharmacological potential of medicinal plants used in Algerian traditional medicine for urinary diseases. Eur J Intern Med 44:101339
Aydi S, Sassi Aydi S, Rahmani R, Bouaziz F, Souchard JP, Merah O, Abdelly C (2023) Date-palm compost as soilless substrate improves plant growth, photosynthesis, yield and phytochemical quality of greenhouse melon (Cucumis melo L.). Agronomy 13(1):212
Santhosh S, Manivannan N, Ragavendran C, Mathivanan N, Natarajan D, Hemalatha N, Dhandapani R (2019) Growth optimization, free radical scavenging and antibacterial potential of Chlorella sp. SRD3 extracts against clinical isolates. J Appl Microbiol 127(2):481–494
Aissani F, Grara N, Bensouici C, Bousbia A, Ayed H, Idris MHM, Teh LK (2022) Algerian Sonchus oleraceus L.: a comparison of different extraction solvent on phytochemical composition, antioxidant properties and anti-cholinesterase activity. Adv Trad Med 22:383–394
Hechaichi FZ, Bendif H, Bensouici C, Alsalamah SA, Zaidi B, Bouhenna MM, Souilah N, Alghonaim MI, Benslama A, Medjekal S, Qurtam AA, Miara MD, Boufahja F (2023) Phytochemicals, antioxidant and antimicrobial potentials and LC-MS analysis of Centaurea parviflora Desf. Extracts. Moleculess 28:2263
Pietrzak W, Nowak R, Olech M (2014) Effect of extraction method on phenolic content and antioxidant activity of mistletoe extracts from Viscum album subsp. abietis. Chem Pap 68:976–982
Sánchez-Vioque R, Rodríguez-Conde M, Reina-Ureña J, Escolano-Tercero M, Herraiz-Peñalver D, Santana-Méridas O (2012) In vitro antioxidant and metal chelating properties of corm, tepal and leaf from saffron (Crocus sativus L.). Ind Crop Prod 39:149–153
Stucki JW, Anderson W (1981) The quantitative assay of minerals for Fe2+ and Fe3+ using 1, 10-phenanthroline: I. Sources of variability. Soil Sci Soc Am J 45(3):633–637
Stocker P, Yousfi M, Salmi C, Perrier J, Brunel J, Moulin A (2005) Maackiain 3-O-(6′-O-malonyl-β-D-glucopyranoside) from Oudneya africana, a powerful inhibitor of porcine kidney acylase I. Biochimie 87(6):507–512
Stocker P, Yousfi M, Djerridane O, Perrier J, Amziani R, El Boustani S, Moulin A (2004) Effect of flavonoids from various Mediterranean plants on enzymatic activity of intestinal carboxylesterase. Biochimie 86(12):919–925
Hajlaoui H, Arraouadi S, Mighri H, Chaaibia M, Gharsallah N, Ros G, Nieto G, Kadri A (2019) Phytochemical constituents and antioxidant activity of Oudneya africana L. leaves extracts: evaluation effects on fatty acids and proteins oxidation of beef burger during refrigerated storage. Antioxidants 8(10):442
Othman A, Ismail A, Ghani NA, Adenan I (2007) Antioxidant capacity and phenolic content of cocoa beans. Food Chem 100(4):1523–1530
Rashid M, Akter M, Uddin J, Islam S, Rahman M, Jahan K, Sarker M, Rahman M, Sadik G (2023) Antioxidant, cytotoxic, antibacterial and thrombolytic activities of Centella asiatica L.: possible role of phenolics and flavonoids. Clin Phytoscience 9(1):1–9
Silva A, Silva V, Igrejas G, Aires A, Falco V, Valentão P, Poeta P (2023) Phenolic compounds classification and their distribution in winemaking by-products. Eur Food Res Technol 249(2):207–239
Haque M, Khaliduzzaman A, Asaduzzaman M, Pattadar S, Hasan M (2023) Dietary food antioxidants and their radical scavenging activity: a review. Int Food Res J 30(1):63–78
Wu Y-T, Wu S-B, Wei Y-H (2014) Metabolic reprogramming of human cells in response to oxidative stress: implications in the pathophysiology and therapy of mitochondrial diseases. Curr Pharm Des 20(35):5510–5526
Radha P, Sumathi S (2022) Biomolecular protective effects of Bacopa Monnieri (L.) Pennell leaf extracts against oxidative damage. Bangladesh J Bot 51(1):123–129
Suriyaprom S, Mosoni P, Leroy S, Kaewkod T, Desvaux M, Tragoolpua Y (2022) Antioxidants of fruit extracts as antimicrobial agents against pathogenic bacteria. Antioxidants 11:602
Khalil AM, Saleh AM, Abo El-Souad SM, Mohamed MS (2023) Plants from a semi-arid environment as a source of phytochemicals against Fusarium crown and foot rot in zucchini. AMB Express 13(1):6
Domán A, Dóka É, Garai D, Bogdándi V, Balla G, Balla J, Nagy P (2023) Interactions of reactive sulfur species with metalloproteins. Redox Biol 60:102617
Osonga FJ, Akgul A, Miller RM, Eshun GB, Yazgan I, Akgul A, Sadik OA (2019) Antimicrobial activity of a new class of phosphorylated and modified flavonoids. ACS Omega 4(7):12865–12871
Shamsudin NF, Ahmed QU, Mahmood S, Ali Shah SA, Khatib A, Mukhtar S, Alsharif MA, Parveen H, Zakaria ZA (2022) Antibacterial effects of flavonoids and their structure-activity relationship study: a comparative interpretation. Molecules 27(4):1149
Edeas M (2007) Les polyphénols et les polyphénols de thé. Phytothérapie 5(5):264–270
Caesar LK, Cech NB (2019) Synergy and antagonism in natural product extracts: when 1+ 1 does not equal 2. Nat Prod Rep 36(6):869–888
He F, Wang W, Wu M, Fang Y, Wang S, Yang Y, Ye C, Xiang F (2020) Antioxidant and antibacterial activities of essential oil from Atractylodes lancea rhizomes. Ind Crop Prod 153:112552
Khameneh B, Eskin NAM, Iranshahy M, Bazzaz BSF (2021) Phytochemicals: a promising weapon in the arsenal against antibiotic-resistant bacteria. Antibiotics 10:1044
Wang X, Shen Y, Thakur K, Han J, Zhang J-G, Hu F, Wei Z-J (2020) Antibacterial activity and mechanism of ginger essential oil against Escherichia coli and Staphylococcus aureus. Molecules 25:3955
Johnson JB, Mani JS, Naiker M (2022) Development and validation of a 96-well microplate assay for the measurement of total phenolic content in ginger extracts. Food Anal Methods 15(2):413–420
Tian W, Chen G, Gui Y, Zhang G, Li Y (2021) Rapid quantification of total phenolics and ferulic acid in whole wheat using UV–Vis spectrophotometry. Food Control 123:107691
El Aanachi S, Gali L, Nacer SN, Bensouici C, Dari K, Aassila H (2020) Phenolic contents and in vitro investigation of the antioxidant, enzyme inhibitory, photoprotective, and antimicrobial effects of the organic extracts of Pelargonium graveolens growing in Morocco. Biocatal Agric Biotechnol 29:101819
Wang B, Peng L, Zhu L, Ren P (2007) Protective effect of total flavonoids from Spirodela polyrrhiza (L.) Schleid on human umbilical vein endothelial cell damage induced by hydrogen peroxide. Colloids Surf B 60(1):36–40
Öztürk M, Duru ME, Kivrak Ş, Mercan-Doğan N, Türkoglu A, Özler MA (2011) In vitro antioxidant, anticholinesterase and antimicrobial activity studies on three Agaricus species with fatty acid compositions and iron contents: a comparative study on the three most edible mushrooms. Food Chem Toxicol 49(6):1353–1360
Rokkarukala S, Cherian T, Ragavendran C, Mohanraju R, Kamaraj C, Almoshari Y, Albariqi A, Sultan MH, Alsalhi A, Mohan S (2023) One-pot green synthesis of gold nanoparticles using Sarcophyton crassocaule, a marine soft coral: assessing biological potentialities of antibacterial, antioxidant, anti-diabetic and catalytic degradation of toxic organic pollutants. Heliyon 9:e14668
Shahidi A, Ellner PD (1969) Effect of mixed cultures on antibiotic susceptibility testing. Appl Microbiol 18(5):766–770
Acknowledgments
This research has been funded by Scientific Research Deanship at University of Ha’il - Saudi Arabia through project number <<RG-23 014>>.
Funding
This research has been funded by Scientific Research Deanship at University of Ha’il - Saudi Arabia through project number <<RG-23 014>>.
Author information
Authors and Affiliations
Contributions
Conceptualization: S.N.N., M.I.K. and C.B.; methodology: A.Z. and A.H.; software: M.L.B.A., N.E., and D.G.; validation: A.H., C.B. M.I.K., and Y.M.; formal analysis: N.E., F.L., and C.B.; investigation: S.N.N. and N.E.; resources: A.Z., R.B.S., and S.N.N.; data curation: S.N.N.; writing—original draft preparation: N.E, S.N.N., C.B.; writing—review and editing: D.G., S.N.N., and Y.M.; visualization: R.B.S. and A.Z.; supervision: N.E. and F.L. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Institutional review board statement
Not applicable
Informed consent statement
Not applicable
Conflict of interest
The authors declare no conflict of interest.
Sample availability
Samples of all the compounds are available from corresponding author.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nacer, S.N., Zobeidi, A., Bensouici, C. et al. In vitro antioxidant and antibacterial activities of ethanolic extracts from the leaves and stems of Oudneya Africana R. growing in the El Oued (Algeria). Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04856-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04856-9