Abstract
The isolation of lipid-rich cellulolytic fungi was targeted to be investigated as bioflocculant agents for microalgae harvesting. The fungal isolate coded MD1 was selected based on its lipid content, cellulolytic activity, and its harvesting efficiency for the freshwater oleaginous microalga Chlorella sp. The selected fungus which was molecularly identified as Aspergillus terreus has been applied as bioflocculant after solid state cultivation on pre-treated rice straw (as abundant agro-cellulosic waste). Optimization of harvesting efficiency of Chlorella microalga using A. terreus/rice straw biomass as the “bioflocculant” was investigated. The optimization conditions included microalga/bioflocculant ratio, microalgal age, contact time between the bioflocculant and the microalga, pH of microalgal culture at harvesting time, and cell density of microalgal culture. The obtained results revealed that the harvesting efficiency could reach 97.6% due to 24 h as contact time at 30% flocculant/microalga ratio and pH 7. While after 2 h contact time, 93.3% harvesting efficiency could be obtained using the same bioflocculant:microalga ratio at pH 6. The lipid extracted from harvested Chlorella/A. terreus mixture was applied to produce biodiesel (fatty acid methyl ester) after methylation. The resulted biodiesel contains high percentage (67.2%) of C18:1,2 unsaturated fatty acids which is considered a suitable fraction for biodiesel production. Obtained results revealed the suitability of the novel A. terreus strain as sustainable bioflocculation agent to harvest microalga(e) for biofuel production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The need for microalgal biomass to produce biofuels like biodiesel is growing, especially in light of the clear adverse impacts of excessive fossil fuel usage. However, there are still several technological and cost-related obstacles to the fully commercial production of microalgae [1,2,3]. A major challenge is harvesting microalgae, especially with their small cell size and negative charges on their surfaces that keep them suspended in their relatively dilute cultures. According to the species and growing circumstances of the microalgae, the cost of harvesting microalgae can often be as high as 60% of the total production cost. Therefore, a high-efficiency and economical harvesting method must be created in order to commercialize microalgae-based technologies [4, 5]. There are several harvesting methods for microalgae that include sedimentation (by centrifugation or gravity), mechanical screening, membrane filtration, air flotation, and flocculation methods. It is worth to mention that, no one harvesting method can be regarded as technically or financially viable for all microalga species and/or purposes. Flocculation is considered one of the most widely used harvesting techniques for various large and commercial scale microalgae applications due to its relatively low cost and efficiency with many species. Flocculation means the accumulation of fine/unstable particles through “surface charge neutralization,” “electrostatic patching,” and/or “bridging” due to the addition of “flocculating materials” in the form of sedimentary flocs [6]. Flocculating materials (flocculants) can be physical particles, organic or inorganic chemicals, or biological materials (bioflocculants). Bioflocculants are a good alternative to inorganic and chemically synthesized flocculants for microalgal harvest because of their biodegradable and nontoxic qualities [7].
Bio-flocculants can be bacteria, filamentous fungi, yeasts, or certain types of self-flocculating microalgae as well as “their exudate-rich culture supernatants” [5]. Filamentous fungi are among the most important microorganisms used in microalgae bioflocculation harvesting [8,9,10]. Many species of filamentous fungi are known for their ability to self-pelletize, allowing algal cells to be captured by fungal pellets and adsorbed onto their surfaces. The application of such fungi as bio-flocculating agents for microalgae harvesting can be through co-culturing the fungus with the microalga(e) or by adding ready-grown fungal pellets (or biomass) to the target microalgal culture [11].
There are many factors that could affect the interaction between the microalgae and fungi which causes the algae-fungi coagulation/flocculation such as the electrostatic interaction, hydrophobic interaction, and specific components on cell wall. The negatively charged functional groups on algal cells can be protonated and deprotonated depending on the surrounding pH to create charges and potentials on the surfaces of algal cells [12]. Moreover, filamentous fungi are known to secrete diverse organic acids (e.g., citric acid, gluconic acid, and acetic acid) into the culture medium [13, 14]. Mainly, due to the acidic conditions in fungal media, the surface functional (carboxylic and amine) groups of mycelia remain protonated, leading to the net positive charges of the fungal hyphae [9, 14]. Therefore, charge neutralization can be established and harvesting happens when positively charged fungus adequately interact with negatively charged algae cells, whereas the hydrophobic interaction between the hydrophobic and hydrophilic parts of the outer cell wall proteins can lead to the formation of an amphipathic membrane, and thus can help fungi attach to other microbial surfaces [14, 15]. Based on this amphipathic property, the hydrophobins from filamentous fungi can be utilized to immobilize suspended algae cells on surfaces via adhesive force. The hydrophobic parts of microalgae can contact with filamentous fungi to initiate hydrophobic interactions; meanwhile, the amphipathic film from fungal hydrophobins may regulate the surface property of algae cells, subsequently making it easier to form co-pellets [16]. Moreover, specific components on fungal cell wall (glucans, lipids, chitin, polysaccharides, and proteins) contribute to interactions with the external environment and adhesion to other microbial cells such as microalgae [17].
On the other hand, the mass of fungi used for harvesting may represent a significant portion of the final biomass when harvesting algae. Therefore, the lipid content of fungi used to harvest oleaginous algae may be important (quantitatively and qualitatively) when the purpose of harvesting is biodiesel production. In addition, the ability of fungi to degrade cellulosic residues and use them for growth is an important ecological and economic advantage. Hence, the current work aimed to isolate lipid-rich cellulolytic fungi capable of causing flocculation harvesting of the oleaginous microalga Chlorella sp. as biodiesel feedstock. Such lipid-rich cellulolytic fungi represent a sustainable exploitation of the abundant agricultural residues in the production of bioflocculant for different microalgae to be used in biofuel production. The work also was aimed to investigate the influence of different conditions in order to optimize the harvesting efficiency of the targeted microalga. The optimization conditions included microalga/bioflocculant ratio, microalgal age, contact time between the bioflocculant and the microalga, pH of microalgal culture at harvesting time, and cell density of microalgal culture.
2 Materials and methods
2.1 Samples collection
Nine soil samples have been collected as fungi isolation sources from different locations in the Nile Delta region, in Northern Egypt. Among obtained samples, one sample was from animal manure-enriched soil and one from soil contaminated by urban solid wastes (from Menoufia governorate). The rest of the samples were collected from the rhizosphere of different agricultural crops in the governorates of Gharbia, Sharkia, Qalyubia, and Kafr El-Sheikh. The collected soil samples were transported in sterile plastic bags to laboratory under proper aseptic environment and kept in a refrigerator (at 4 °C) until use.
2.2 Isolation of lipid-rich cellulolytic fungi
The isolation strategy targeted the isolation of lipid-rich cellulolytic fungi with the ability to cause flocculation and sedimentation (harvesting) of the targeted microalgae. Different fungal isolates were obtained from soil samples by direct isolation according to [18, 19] with minor modifications. In brief, 10 g of individual samples was suspended in sterile tab water (90 mL) and shacked (140 rpm for 30 min) at room temperature, and left static for another 10 min. Tenfold serial dilutions were then prepared in sterilized tap water, and 100µL of the 5th dilution were spread on agar plates of carboxymethylcellulose (CMC) medium (CMC, 10 gL−1; peptone, 5 gL−1; KH2PO4, 1 gL−1; MgSO4·7H2O, 0.5 gL−1; Rose Bengal, 0.033 gL−1; and streptomycin, 0.03 gL−1; pH 5.5–6.0). The inoculated plates were incubated at 30 °C for 5–7 days to allow the growth of cellulolytic fungi in colonies. Colonies that displayed distinction morphological appearance were repeatedly cultured on the same medium until obtaining pure fungal isolates. The purified isolates were cultivated on slants of potato dextrose agar, kept at 4 °C, and re-cultured on proper intervals [20].
Because one of the objectives of the study is the production of biodiesel, so the lipid percentage of biomass in the fungi used for harvesting is important in the whole process. Hence, the obtained cellulolytic fungal isolates were screened for their lipid contents according to Bligh and dyer method described by [21] with some modifications. In brief, fungal isolates were cultivated in liquid CMC medium for 5 days on rotary orbital shaker (30 °C and 140 rpm). After growth, produced fungal biomass was collected, dried, weighted, and grounded. The lipid content of fungal biomass was extracted (from 0.5 g dry biomass) with chloroform–methanol (2:1, v/v) in capped glass tubes and then methanol and water were added to cause the separation of chloroform layer (the final solvent ratio became chloroform:methanol:water of 1:1:0.9). The chloroform/lipid layer was pippeted in new tube and washed with 20 mL of 5% NaCl solution to remove impurities. The purified chloroform/lipid was transferred into pre-weighted vials, evaporated to dryness, and the weight of the crude lipid obtained from each sample was measured gravimetrically. The lipid content was calculated and represented as a percentage of the dry weight of fungal biomass.
2.3 Microalga and cultivation conditions
The oleaginous freshwater microalga Chlorella sp. used in this study (as model biofuel microalga) was previously isolated and stored in the Department of Agricultural Microbiology, National Research Centre, and Egypt. The bold basal medium was used in this study for the cultivation of the targeted microalga [22]. Unless otherwise stated, cultivation was carried out in 5-L conical glass flasks aerated with air bubbles for 2 weeks under constant white fluorescent illumination.
2.4 Screening the fungal isolates for microalgae harvesting
The selected lipid-rich cellulolytic fungi have been screened as bio-flocculating agents to harvest the microalga Chlorella sp. The evaluation was performed in two stages: the first was based on the direct utilization of fungal pellets in microalga harvesting, while the second selection stage was based on the solid-state cultivation of the most potent cellulolytic fungi on rice straw (as common lignocellulosic waste) and utilization of this mixture as bioflocculation agents for Chlorella microalga.
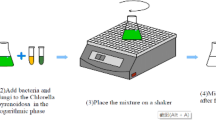
For pellet-assisted harvesting, the mycelium pellets of the selected cellulolytic fungi have been examined for their bioflocculation capabilities of Chlorella sp. The influence of fungal cultivation medium on the bioflocculation efficiency was including also in this experiment. The fungi were individually cultivated on liquid malt extract medium and CMC in an orbital rotary shaker (140 rpm) at ambient temperature to allow the formation of fungal pellets. The incubation period in case of malt extract culture was 3 days while CMC-based cultures were incubated for 7 days. After incubation, the resulted fungal pellets were collected by filtration (using a 200-mesh sieve) and washed with sterilized deionized water to remove the excess of cultivation medium. A volume of 15-mL fungal pellets (from each fungus separately) was transferred to a 100-mL screw-caped glass bottle containing 50 mL of the microalgal culture. The pH of tested microalga culture was adjusted at the value of 7 just before the harvesting experiment. The fungal pellets/microalga mixture was continuously mixed at 140 rpm on a rotary shaker for 1 and 2 h at ambient temperature. The mixtures were left to stand for 10 min to allow the precipitate of flocculated microalgal cells and the microalga-pellets aggregates. Samples (5 mL) for measuring harvesting efficiency were taken from settled bottles at a constant distant (from the middle point of the mixture). A control treatment of Chlorella microalga has been applied without using any fungal addition for comparison. The harvesting efficiency (%) was calculated by comparing the “optical density” (OD) measured spectrophotometrically at 680 nm of control treatment and the collected samples according to the following equation [1]:
where FE refers to flocculation efficiency at time t, ODi refers to initial optical density, and ODf refers to final optical density at 680 nm. Additional calculation for HE was performed by measuring the chlorophyll content (according to [21] of samples compared to the control treatment).
The second screening was based on the uses of rice straw-based fungal growth as biological flocculants. In brief, the rice straw was washed, cut into 1–2-cm pieces, dried, and ground into a fine powder then subjected to alkaline pre-treatment before it is used for fungi cultivation [23]. The dried and grounded rice straw was immersed in 1 M sodium hydroxide solutions and placed for 10 min in an autoclaved at 121 °C. After cooling, the rice straw-alkaline solution was subjected to filtration with filter paper (Whatman No. 1), and the rice straw was washed several times with sterile water until neutralization [24]. Finally, the powder rice straw was dried at 60 °C overnight before being used as a carbon source for fungi cultivation in addition to untreated rice straw as a control. Fungal cultivation on the pre-treated rice straw was performed as solid state on 250-mL conical flasks as follows: 10 g of the pre-treated rice straw and 15 mL of mineral medium were autoclaved and inoculated with three fungal discs (from 7-day-old agar plates), and incubated at ambient temperature for 15 days (without shaking). The whole fungal-rice straw cultures (10 g rice straw and fungal growth) was suspended with 50 mL DW and used as “the bioflocculant” for Chlorella harvesting trials. Similar to harvesting experiment with microalgal pellets, a volume of 15 mL of fungal-rice straw mixture (the bioflocculant) was used to harvest a volume of 50 mL Chlorella culture. FE of different fungi-rice straw bioflocculants was calculated as previously described compared with microalga culture harvested by the pre-treated rice straw without fungal growth.
2.5 Optimization of Chlorella harvesting using fungal-rice straw as bioflocculant
The most potent lipid-rich cellulolytic fungus that exhibited efficiency in harvesting Chlorella microalga was applied as a bioflocculant agent after being grown on pre-treated rice straw. Harvesting optimization of the targeted microalga using the selected fungus was optimized. Optimization factors included microalgal cultural density, pH value, fungal/microalgal ratio, microalgae age, and contact time. The bioflocculant was prepared by cultivation of the selected fungus on alkaline treated rice straw at solid-state condition and suspended in DW (10 g/50 mL) according to steps described in the previous section and used in further harvesting experiments.
The influence of cultural density of microalga has been investigated at three levels of optical densities, i.e., OD680nm at 1, 2, and 3. To perform this experiment, the optical density of a 2-week old culture of Chlorella sp. was adjusted at the specific values either by centrifugation or dilution by cell-free cultural filtrate of the microalga culture.
The pH value of microalgal culture differs according to growth phase, type of microalgae, and type of cultivation medium as well as cultivation conditions. For this reason, it is important to study if the cultural pH influences the efficiency of the targeted microalga using the specific bioflocculant. Hence, the microalgal flocculation efficiency using the prepared fungal/rice straw bioflocculant was investigated over different pH values that commonly occurs in microalgal cultures, i.e., 6, 7, 8, and 9 [25]. To perform this experiment, the cultural pH (after 2 weeks cultivation) was adjusted to the specific pH values using either HCl or NaOH solutions (1N). After adjusting the cultural pH to the specific value, the bioflocculant was added and the microalgal flocculation efficiency was measured as previously mentioned.
Different bioflocculant:microalgal ratio as a volume was investigated to specify the ideal ration for efficient bioflocculation and harvesting process [11]. In this regard, the bioflocculant volume was added to the targeted microalgal culture at 10, 20, and 30% v/v ratios.
The effect of microalgal cultural age on FE using the applied fungi/rice straw bioflocculant was investigated. Microalgal cultures at 1, 2, and 3 weeks old were collected and used for flocculation harvesting experiments.
In order to get the best results, it is necessary to know when to harvest microalgae, since harvest time affects the process [26]. Therefore, different contact times were investigated to determine the efficiency of microalgal flocculation using the prepared fungal/rice straw bioflocculant, i.e., 1, 2, and 24 h. All flocculation steps and calculations were performed as previously described.
2.6 Identification of the selected fungal isolates
The identification of selected fungal isolate has been performed at two levels; the first was done based on the morphological microscopic characteristics (using Olympus cx41 microscope, Japan) up to the genus level. Meanwhile, the second level of identification was based on the molecular identification of the fungal species through sequencing of the internal transcribed spacer (ITS) region. Briefly, the total DNA of harvested fungal mycelium (cultivation in PDB medium for 3 days) was extracted using CTAB protocol [27]. The polymerase chain reaction (PCR) was performed to the extracted DNA in the presence of ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) primers. The contiguous DNA sequence was compared with data from the reference and type strains available in public GenBank databases using the BLAST program (National Centre for Biotechnology Information) (http://www.ncbi.nlm.nih.Gov/BLAST). The obtained sequences were aligned using Jukes Cantor Model [28] and used to identify the fungal species. The obtained sequences were deposited in the GenBank to record an accession number.
2.7 Determination of chlorophyll contents
The chlorophyll content was determined according to the method described by Eida et al. [29] with minor modifications. In brief, 1 mL of the microalgal mixture (with or without fungal bioflocculant) was subjected to centrifugation for 10 min at 10000 rpm (K3 Series, Centurion Scientific, UK). The collected biomass was re-suspended in 1 mL of DMSO and sonicated for 15 min in an ultrasonic water bath at maximum power. The biomass/DMSO mixture was vortexed for 5 min before centrifugation, and the supernatant extract (contains chlorophyll) was collected in a new graduated tube. Second extraction steps were performed by adding another 1 mL of DMSO to the extraction tubes, vortexing, and centrifugation to collect most of the chlorophyll contents. After the second extraction, the mixture was centrifuged again (to spin down the debris) and the supernatant/chlorophyll has been compiled with the previously extracted part. At the end, the total volume was adjusted to 2 mL using DMSO, and the extract was spectrophotometrically measured at 675 nm. The chlorophyll content was calculated and used to estimate the harvesting efficiency of dark-colored microalga cultures due to the presence of rice straw/fungus mixture.
2.8 Biodiesel production and GC analysis of lipid profile
The oleaginous microalga Chlorella sp. (> 30% lipid of dry biomass) [30] was harvested via the fungal bioflocculant under the optimum conditions. The lipid extracted from microalga/fungus bioflocculant flocks mixture was extracted according to the previously described Bligh and dyer modified method [21]. Extracted lipid was used as biodiesel (fatty acid methyl ester (FAME)) feedstock according to the method of [31] with minor modifications. Briefly, around 100 mg of the lipid extracted from the biomass was dissolved in toluene (0.2 mL) and placed in glass test tubes with screw cap. The mixture was mixed with 1.8 mL methanolic HCl (8% w/v) and heated for 1 h in a water bath at 100 °C. The tubes were allowed to cool to room temperature, then 1 mL of hexane was added and vortexed for 5 min to extract the produced FAMEs. One milliliter of water was added to the FAMEs/hexane tubes, vortexed, and centrifuged at 2000 rpm to enable hexane layer separation.
The FAME/hexane layer was transferred to new tube, purified through a membrane filter, and the produced FAME was analyzed using gas chromatography (GC) to investigate the suitability of its fatty acid profile as fuel. The FAMEs of the extracted lipid were analyzed by a gas chromatography system (Hewlett Packard, HP 6890 series, USA) equipped with an Alltech BPX70 Capillary Column (60 m × 320 μm × 0.25 μm) coated with 70% poly silphenyl-siloxane supplied with flame ionizing detector. Fatty acid profile was expressed as a percentage of the total fatty acids identified in the extracted lipid.
2.9 Statistical analysis
All experiments were performed in triplicates and the results were presented as average ± standard error. The averages of data for each experiment were statistically analyzed using analysis of variance by using IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp., and means were compared by using Duncan Multiple Range Test at 0.05% probability.
3 Results and discussion
3.1 Isolation of lipid-rich cellulolytic fungi
The present work aimed to use fungi as a bioflocculant to harvest microalgae as biofuel feedstock. The lipid content of flocculants (fungi) can be critical if they make up a sizeable fraction of the final biomass, much as the lipid content of target microalgae is crucial for the synthesis of biodiesel. Additionally, a variety of fungi can be grown using agricultural cellulosic wastes as a promising, affordable, and sustainable feedstock (cellulolytic fungi). Hence, isolation of lipid-rich cellulolytic fungi was targeted to be investigated as bioflocculant agents for microalgae harvesting. To this end, nine soil samples collected from the rhizosphere of different agricultural crops were used as fungi isolation sources. After serial dilution and direct isolation on CMC plates, a total of 50 fungal colonies were selected. The selection of colonies was based on their good growth taking into account the morphological diversity of the colonies as well as the diversity at the sample source. The selected colonies were screened (after purification on malt age plates) for growth on liquid CMC medium. Out of the screened isolates, only 38 fungal isolates exhibited a good pellet growth on the liquid CMC medium. The ability of such fungal isolates to utilize CMC as the sole carbon source of the liquid medium demonstrated its ability to degrade cellulose and considered as “cellulolytic fungi” [32]. The selected cellulolytic fungi were further screened for their lipid contents, where the results (as in Fig. 1) revealed that the lipid contents of all fungi were ranged between 3.75 and 14.69%. However, the three isolates, i.e., H25, H27, and H35, significantly recorded the highest lipid contents as they exceed 10% of their dry biomass. And twenty-nine isolates, i.e., H1, H2, H3, H4, H5, H6, H8, H9, H10, H11, H12, H13, H14, H16, H19, H20, H21, H 22, H23, H24, H26, H28, H29, H30, H31, H32, H33, H34, and H37, recorded percentage of lipid between 6 and 10%, while the isolates H7, H15, H17, H18, H36, and H38 recorded a significant low lipid percentage (less than 6%). According to [33], there was diversity in the percentage of lipids present in different fungi, and it ranged between 1.5 and 14.1%; for example, Volvariella volvacea had 1.5–4.5% while Alternaria sp. had 14.1%.
3.2 Screening for bioflocculation capability
All fungal isolates were screened for their ability to cause flocculation and sedimentation of Chlorella microalga to be used as biodiesel feedstock. Microalgae harvesting from culture broth are energy intensive and expensive owing to their small size in addition to the low cell density [34]. Hence, it is urgent to develop alternative harvesting technologies to enhance the efficiency of harvesting systems. An efficient and low-cost bioflocculation strategy was developed and evaluated for harvesting relatively diluted microalgal cells in culture broth using filamentous fungi. The evaluation was performed in two stages; the first was based on utilization of the filamentous fungal pellets, while the second one relied on growing cellulolytic fungi on rice straw (as a common lignocellulosic waste) and utilizing the mixture of fungi/partially decomposed rice straw as bioflocculating agent for the freshwater microalga Chlorella sp.
3.2.1 Fungal pellet–assisted harvesting of Chlorella sp.
The selected 38 fungal isolates that exhibited cellulolytic activity on liquid CMC medium have been cultivated on liquid malt extract and CMC media to allow pellets to be formed. The formed fungal pellets of different isolates have been collected by filtration and applied for harvesting Chlorella microalga. Mixing microalga with the filamentous fungal pellets for 2 h resulted in the formation of microalga/pellet flocks. The formed floccus allowed to sedimentation and the efficiency of sedimentation/harvesting was evaluated and is presented in Fig. 2. While the same fungus produced varying harvesting rates due to its growing medium, different fungi’s pellets showed different harvesting efficiencies. The fungal isolate nos. H5, H6, H7, H10, H12, H20, H21, H22, H25, H27, H30, and H32 showed significant high microalgal harvesting efficiency when cultivated on either malt or CMC media. However, the percentages of harvesting efficiencies were higher in case of malt extract medium, as it ranged between 61 and 85% in the case of fungal cultivated in malt and between 54.9 and 60.4% for CMC medium. It is worth noting that the diameter of fungal pellets for the same fungus was smaller when grown in the CMC medium than that grown on malt medium. The efficiency of pellet-assisted flocculation may vary (for the same fungus and the same microalga) according to the diameter of the fungal pellets, the difference in charges on their surfaces, or differences in the extra-materials on their surfaces [5, 14].
The percentages of harvesting efficiencies (HE) (%) for Chlorella sp. using the pellets of isolated fungi are incompatible with that recorded for different fungi in literatures. For instance, [35] recorded microalgal removal percentages between 73 and 93% using pellets of different white-rot fungi after 24 h of contacting. Another study was conducted on A. fumigatus (pellets) as a bioflocculant, the percentage of Chlorella vulgaris harvesting reached 90% after 24 h [24].
3.2.2 Rice straw–based fungal growth as bioflocculant
Based on Chlorella microalga harvesting results using fungal pellets as flocculant as well as lipid and cellulolytic activity, 12 fungal isolates namely H5, H6, H7, H10, H12, H20, H21, H22, H25, H27, H30, and H32 were selected for further investigations. The aforementioned fungal isolates were cultivated as solid state on alkaline-pretreated rice straw (as a carbon source instead of CMC). After sufficient fungal growth, the rice straw/fungus biomass mixture was considered the “fungal flocculants” which was applied to harvest Chlorella sp. in the rest of the following experiments. The harvesting process took place as previously mentioned with 30% v/v bioflocculant/culture, and results of the harvesting process after 2 h were recorded as shown in Fig. 3. The HE results of Chlorella microalga were ranged between 78.9 and 95% without significant change between different isolates except isolate H22 which was significantly lower than all other isolates. It worth to mention that the highest harvesting efficiencies (95.2%) was occurred using H12 isolate; hence, it was used for further studies in this research.
3.3 Identification of the most potent fungus for Chlorella harvesting
The morphological examination of the selected fungus (isolate H12) suggests that it belongs to the genus Aspergillus. The molecular identification to species level was performed by the amplification and sequencing of the ITS genes followed by comparing with the available sequences in GenBank using BLAST program (http://www.ncbi.nlm.nih.Gov/BLAST). Based on obtained sequence and GenBank data, the phylogenetic tree showed that the targeted strain was highly similar to the type strains of A. terreus (Fig. 4). Therefore, this isolate was identified as A. terreus isolate MD1 with accretion number OR425012. A. terreus is considered oleaginous fungus that could accumulate more than 20% of their dry weight lipid [36, 37]. The assortment of lipids found in the biomass of yeasts and fungi is excellent. These lipids are comparable to vegetable oils and may be used to make biodiesel, which can replace fossil fuels in internal combustion engines and electricity generation [38]. Moreover, this fungal species is considered an efficient cellulolytic fungus [39] as well as its ability for co-pelletizing harvesting of microalgae [5, 40].
3.4 Harvesting optimization using A. terreus/rice straw bioflocculant
According to previously harvesting experiments of Chlorella microalga using different fungal isolates, A. terreus was selected for further harvesting optimization experiments. The harvesting of the green, single-celled microalga was optimized using A. terreus/rice straw mixture as the “bioflocculant.” Optimization conditions included the microalga/bioflocculant ratio, microalgal age, contact time between the bioflocculant and microalga, pH of microalga at harvesting time, and cell density of the microalga. Unless otherwise mentioned, the ratio of the bioflocculant to the microalgal culture was 30% (v/v), the pH of the culture was 7, and the age of the algae culture was 2 weeks.
3.4.1 Contact time
The contact time between the flocculant agent and the targeted microorganism is a critical factor that affects the flocculation and harvesting efficiency [20, 41]. Hence, this experiment was conducted to examine the effect of contact time between the “bioflocculant” and the targeted microalga “Chlorella sp.” on the bioflocculation harvesting efficiency. The freshwater microalga Chlorella sp. was subjected for harvesting using A. terreus grown on pretreated rice straw with contact time of 1, 2, and 24 h (at pH 7). The results illustrated in Fig. 5 revealed that the longer contact time between the bioflocculant and the microalga resulted in significant higher harvesting efficiencies. The highest harvesting efficiency (97.6%) was recorded due to 24 h contact time between the microalga and the bioflocculant, while a percentage of 82.7% was recorded for 2 h contact time. In a similar study, the harvesting efficiency of Chlorella sp. was increased from 45 to 90% due to increasing the contact time between the microalga and the pellets of Penicillium sp. from 24 to 48 h [26]. Although the harvest rate after 24 h is higher than 2 h, the economic factor must be taken into account when determining the appropriate contact time in semi-industrial and industrial algae harvesting operations. Although the detailed interaction mechanism between fungi and microalgae still needs to be further studied, the higher contact time seems to allow more adhesion and trapping of microalgal cells on the surface of flocculating fungi. However, the “hyphal surface adsorption and chemisorption from extracellular proteins and exopolysaccharides” seems to be the main reason for the flocculation of microalgae using fungi [42].
3.4.2 Microalgal pH
The cultural pH is an important factor that influences the processes of flocculation and stability of suspended cells [43]. Among the several studied pHs of Chlorella cultures, i.e., 5, 6, 7, 8, and 9, harvesting results obtained from pH 6 treatment were significantly the highest (Fig. 6). The percentage of harvesting was above 91% when harvested by A. terreus/pre-treated rice straw after 2 h. It worth to mention that the autoflocculation of Chlorella microalgal cells increased due to the increase of cultural pH value. However, the lowest auto-flocculation was recorded at pH 5 (less than 22%) while the maximum auto-flocculation of Chlorella after 2 h was 36.6% at pH 9. In a similar biofouling study of Chlorella sp. using fungal pellets of A. terreus, [14] recorded harvest efficiencies of less than 90% when pHs were between 5 and 7. The finding that acidic conditions support the highest bioflocculation of microalgae using fungal growth was also concluded by [44]. The acidic pH of the fungus’ medium causes the surface functional (carboxylic and amine) groups of mycelia to remain protonated, which results in the net positive charges of the fungus’ hyphae in the pellet-assisted mode [9, 14]. Other studies supported such observation using chitosan where the highest harvesting efficiency for Chlorella sp. and Scenedesmus sp. were obtained at pH 6.0 [4, 45].
3.4.3 Effect of microalga age
The effect of microalgal cultural age on FE using the applied A. terreus/rice straw bioflocculant was investigated. Chlorella sp. cultures at 1, 2, and 3 weeks old were used for flocculation harvesting experiments. The lowest HE of Chlorella was observed for 1-week-old culture (45.8%); this percentage was significantly increased as 87.1 and 94.7% for 2- and 3-week-old cultures, respectively (Fig. 7). Although the cell concentration and cultural pH were adjusted to be similar in 1-, 2-, and 3-week-old algae cultures, older cells were able to be harvested and sediment by bio-clustering than fresh cells. Similarly, the autoflocculation was increased due to the aging of the microalgae cells from around 10% at 1-week-old culture to 36.9% in 3-week-old culture.
Autoflocculation increased in aging microalgal cultures due to the increased secretion of extracellular polymeric substances (EPS) that can lead to bridging of cells together. The EPS attached to the surface of algal cells and could cause changes in the outer charges of microalgal cells. These changes in outer charges and the presence of EPS could play a critical role in the flocculation harvesting of microalgal cells using fungal bioflocculants [5, 41].
However, it should be considered that the pH of microalgal cultures usually increased up to 9 and sometimes 10 at the late stationary phase which led to the precipitation of microalgal cells (autoflocculation) [4].
3.4.4 Effect of microalgal cultural density
The microalgal cultures differ in their cell densities due to different cultural and environmental factors. The influence of cultural density of microalga on FE using the fungal bioflocculant has been investigated at three levels of cultural optical densities, i.e., OD680 nm at 1, 2, and 3 values. In this experiment, the optical density of the 2-week-old Chlorella culture was adjusted at the specific values either by centrifugation or by dilution by cell-free cultural filtrate of the same microalga. According to data illustrated in Fig. 8, the FE of Chlorella culture with OD680 nm of 1 was 81.3%. This percentage was significantly increased to 86.4 and 91.2% by increasing the cultural cell density up to OD680 nm of 2 and 3, respectively. These results showed that the same amount of bioflocculant is capable of causing more microalgae biomass flocculation in denser cultures. This conclusion is consistent with the conclusion of [46], who reported that the higher the density of microalgae biomass concentration, the lower the amount of flocculant required per unit amount of biomass. Similarly, [47] reported a linear correlation between the concentration of initial microalgae and the increase in harvest efficiency with the same amount of flocculant.
3.4.5 Effect of fungal algal ratio
The ration between flocculant substance and the targeted particles (microalgae for instance) is a critical factor that could affect the whole performance of the flocculation process [1, 4, 5, 11, 48]. Hence, the ration of A. terreus/rice straw bioflocculant to the Chlorella culture was investigated to optimize its harvesting efficiency for biofuel production. The bioflocculant was added to the targeted microalgal culture at 10, 20, and 30% v/v ratios for 2 h and left half an hour for sedimentation. According to the results of HE presented in Fig. 9, the maximum HE, i.e., 93.2%, was obtained due to application of 30% bioflocculant. However, the percentage of sedimentation was significantly lower as 89 and 83.1% for 20 and 10% bioflocculant:microalga (v/v), respectively. In a similar study [14], obtained around 99% HE due to five h application of A. oryzae fungal pellets to at 1:1 to C. vulgaris culture. In another study, around 97% HE was obtained for C. vulgaris by A. nomius pellets when fungal mycelium to microalgae biomass ratio 1:2 (w/w) was established after 3-h agitation [8].
3.5 Production of biodiesel as renewable fuel source
The biomass of oleaginous microalgae is considered a renewable feed stock for sustainable biodiesel production [21, 49]. The biomass of Chlorella sp. (as oleaginous microalga) was harvested under optimized conditions by the lipid-rich cellulolytic fungus A. terreus grown on rice straw and applied for biodiesel production (Fig. 1S, 2S). The total lipids of microalga/bioflocculant biomass mixture have been extracted (30% of dry weight) and subjected to transesterification reaction with methanol to produce biodiesel fuel. The produced fatty acid methyl ester (biodiesel) was subjected to characterization using GC–MS to determine its suitability as biofuel. The characterization of biodiesel was performed based on the fatty acid profile resulted by GC analysis. According to obtained results (Fig. 3S, Table 1S), the percentage of unsaturated fatty acids was around 67.2% of the total identified fatty acids. Linoleic acid (18:2) and oleic acid (C18:1) fatty acids represent 29.2 and 38% of total lipids, which indicates the suitability of produced biodiesel as fuel. The structural characteristics of fatty acids, such as their chain length, degree of unsaturation, and chain branching, have an impact on the physiochemical properties of biofuel. Accordingly, the best fraction for the production of biodiesel is C16-C18 fatty acids [50]. Generally, bioflocculation using fungi is applicable and an ecofriendly approach to harvest microalgal biomass as feedstock for biofuel production [5].
4 Conclusion
Many fungi have the ability to cause flocculation harvesting of microalgal cells, with the possibility of using the obtained biomass in the production of biodiesel, if its fat content and composition are suitable. Fungi capable of degrading cellulose present an advantage in microalgae harvesting, as their production cost can be reduced by growing them on cheap agricultural cellulose waste such as rice straw. Obtained results of this study revealed the applicability of the lipid rich cellulolytic fungus A. terreus grown on rice straw as bioflocculating agent for microalgae harvesting. The lipid profile of the obtained fungus/microalga biomass represents a suitable fraction for biodiesel properties. Microalgal pH, age, and cell density as well as bioflocculant/culture ratio and contact time are important factors affecting the efficiency of the bio-harvesting process.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Matter IA, Darwesh OM, Eida MF (2018) Harvesting of microalgae Scenedesmus obliquus using chitosan-alginate dual flocculation system. Biosci Res 15:540–548
Lane TW (2022) Barriers to microalgal mass cultivation. Curr Opin Biotechnol 73:323–328. https://doi.org/10.1016/j.copbio.2021.09.013
Lu Z, Beal CM, Johnson ZI (2022) Comparative performance and technoeconomic analyses of two microalgae harvesting systems evaluated at a commercially relevant scale. Algal Res 64:102–667. https://doi.org/10.1016/j.algal.2022.102667
Matter IA, Darwesh OM, Eida MF (2018) Harvesting of Scenedesmus obliquus by bioflocculation: appropriate chitosan concentrations with various pH values at different growth stages. Jordan J Biol Sci 11:475–481
Matter IA, Bui VKH, Jung M, Seo JY, Kim YE, Lee YC, Oh YK (2019) Flocculation harvesting techniques for microalgae: a review. Appl Sci 9:30–69. https://doi.org/10.3390/app9153069
Al Hattab M, Ghaly A, Hammouda A (2015) Microalgae harvesting methods for industrial production of biodiesel: critical review and comparative analysis. J Renew Energy 5:1–54. https://doi.org/10.4172/2090-4541.1000154
Li Y, Xu Y, Liu L, Li P, Yan Y, Chen T, Wang H (2017) Flocculation mechanism of Aspergillus niger on harvesting of Chlorella vulgaris biomass. Algal Res 25:402–412. https://doi.org/10.1016/j.algal.2017.06.001
Talukder MMR, Das P, Wu JC (2014) Immobilization of microalgae on exogenous fungalmycelium: a promising separation method to harvest both marine and freshwater microalgae. Biochem Eng J 91:53–57. https://doi.org/10.1016/j.bej.2014.07.001
Bhattacharya A, Mathur M, Kumar P, Prajapati SK, Malik A (2017) A rapid method for fungal assisted algal flocculation: critical parameters & mechanism insights. Algal Res 21:42–51. https://doi.org/10.1016/j.algal.2016.10.022
Jaiswal KK, Kumarn V, Gururani P, Vlaskin MS, Parveen A, Nanda M, Kurbatova A, Gautam P, Grigorenko AV (2022) Bio-flocculation of oleaginous microalgae integrated with municipal wastewater treatment and its hydrothermal liquefaction for biofuel production. Environ Technol Innov 26:102–340. https://doi.org/10.1016/j.eti.2022.102340
Srinuanpan S, Chawpraknoi A, Chantarit S, Cheirsilp B, Prasertsan P (2018) A rapid method for harvesting and immobilization of oleaginous microalgae using pellet-forming filamentous fungi and the application in phytoremediation of secondary effluent. Int J Phytoremediation 20:1017–1024. https://doi.org/10.1080/15226514.2018.1452187
Ndikubwimana T, Zeng X, He N, Xiao Z, Xie Y, Chang JS, Lin L, Lu Y (2015) Microalgae biomass harvesting by bioflocculation-interpretation by classical DLVO theory. Biochem Eng J 101:160–167. https://doi.org/10.1016/j.bej.2015.05.010
Yang L, Lübeck M, Lübeck PS (2017) Aspergillus as a versatile cell factory for organic acid production. Fungal Biol Rev 31:33–49. https://doi.org/10.1016/j.fbr.2016.11.001
Chu R, Li S, Yin Z, Hu D, Zhang L, Xiang M, Zhu L (2021) A fungal immobilization technique for efficient harvesting of oleaginous microalgae: key parameter optimization, mechanism exploration and spent medium recycling. Sci Total Environ 790:148–174. https://doi.org/10.1016/j.scitotenv.2021.148174
Wessels JGH (1994) Developmental regulation of fungal cell wall formation. Annu Rev Phytopathol 32:413–437. https://doi.org/10.1146/annurev.py.32.090194.002213
Garg S, Wang L, Schenk PM (2014) Effective harvesting of low surface-hydrophobicity microalgae by froth flotation. Biores Technol 159:437–441. https://doi.org/10.1016/j.biortech.2014.03.030
Garcia-Rubio R, de Oliveira HC, Rivera J, Trevijano-Contador N (2020) The fungal cell wall: Candida, cryptococcus, and Aspergillus species. Front Microbiol 10:1–13. https://doi.org/10.3389/fmicb.2019.02993
dos Reis CJ, de Carvalho LE, da Paz LM, Lima AM, Ogusku MM, de Souza JVB (2014) Bioprospecting of Amazon soil fungi with the potential for pigment production. Process Biochem 49:569–575. https://doi.org/10.1016/j.procbio.2014.01.018
Cruz-Lachica I, Marquez-Zequera I, Allende-Molar R, Sañudo-Barajas JA, Leon-Felix J, Ley-Lopez N, Garcia-Estrada RS (2018) Diversity of mucoralean fungi in soils of papaya (Carica papaya L.) producing regions in Mexico. Fungal Biol 122:810–816. https://doi.org/10.1016/j.funbio.2018.04.008
Al-Kashef AS, Nooman MU, Rashad MM, Hashem AH (2023) Abdelraof M (2023) Production and optimization of novel Sphorolipids from Candida parapsilosis grown on potato peel and frying oil wastes and their adverse effect on Mucorales fungal strains. Microb Cell Factories 22:79. https://doi.org/10.1186/s12934-023-02088-0
Taher ZA, Mahfouz AY, Elhenawy SB, Rabea HA, Sidkey NM (2022) Optimization of cultural and nutritional conditions for selection of the most promising microalgae intended for wastewater treatment and biodiesel production by direct transesterification method. Egypt J Chem 65:895–907. https://doi.org/10.21608/ejchem.2022.132259.5818
Barsanti L, Gualtieri P (2005) Algae: anatomy, biochemistry, and biotechnology, 1st edn. CRC press, pp320. https://doi.org/10.1201/9780203492598
Abdel-Mohdy FA, Abdel-Halim ES, Abu-Ayana YM, El-Sawy SM (2009) Rice straw as a new resource for some beneficial uses. Carbohydr Polym 75:44–51. https://doi.org/10.1016/j.carbpol.2008.06.002
Wrede D, Taha M, Miranda AF, Kadali K, Stevenson T, Ball AS, Mouradov A (2014) Co-cultivation of fungal and microalgal cells as an efficient system for harvesting microalgal cells, lipid production and wastewater treatment. PLoS ONE 9:113–497. https://doi.org/10.1371/journal.pone.0113497
Maji G, Choudhury S, Hamid S, Prashanth R, Sibi G (2018) Microalgae harvesting via flocculation: impact of pH, algae species and biomass concentration. MMMB 1:106. https://doi.org/10.31021/mmmb.20181106
Chen J, Leng L, Ye C, Lu Q, Addy M, Wang J, Zhou W (2018) A comparative study between fungal pellet-and spore-assisted microalgae harvesting methods for algae bioflocculation. Bioresour Technol 259(181–190):10. https://doi.org/10.1016/j.biortech.2018.03.040
El-Naggar AA (2019) PCR-based assay for detection of Harpophora maydis the causal agent of late wilt disease in maize plant parts. Egypt J Pathol 47:277–295. https://doi.org/10.21608/ejp.2019.125538
Mostafa SO, Hussain AA, Roushdy MM, Shehabeldine AM, Hasanin MS (2022) Preliminary study for cellulolytic microorganism isolation from different resources, characterization and identification: green convert of microcrystalline cellulose to nanofibers. Egypt J Chem 65:1265–1273. https://doi.org/10.21608/ejchem.2022.135890.5986
Eida MF, Darwesh OM, Matter IA (2018) Cultivation of oleaginous microalgae Scenedesmus obliquus on secondary treated municipal wastewater as growth medium for biodiesel production. Ecol Eng 19:38–51. https://doi.org/10.12911/22998993/91274
Amin NF, Khalafallah MA, Ali MA, Abou-Sdera SA, Matter IA (2013) Effect of some nitrogen sources on growth and lipid of microalgae Chlorella sp. for biodiesel production. J Appl Sci Res 9:4845–4855
Ichihara KI, Fukubayashi Y (2010) Preparation of fatty acid methyl esters for gas-liquid chromatography. J Lipid 51:635–640. https://doi.org/10.1194/jlr.D001065
Khokhar I, Haider MS, Mushtaq S, Mukhtar I (2012) Isolation and screening of highly cellulolytic filamentous fungi. JASEM 16:223–226. https://doi.org/10.1007/978-1-4939-7877-9_3
Athenaki M, Gardeli Diamantopoulou P, Tchakouteu SS, Sarris D, Philippoussis A, Papanikolaou S (2018) Lipids from yeasts and fungi: physiology, production and analytical considerations. J Appl Microbiol 124:336–367. https://doi.org/10.1111/jam.13633
Vandamme D (2013). Flocculation based harvesting processes for microalgae biomass production. https://doi.org/10.31021/mmmb.20181106
Civzele A, Mezule L (2022) Microalgae harvesting after tertiary wastewater treatment with white-rot fungi. J Fungus 8:12–32. https://doi.org/10.3390/jof8111232
Shafiq SA, Abdulkareem AF, Shafi FAA (2021) Influence of the different carbon and nitrogen sources on the production of biodiesel by oleaginous fungi Aspergillus terreus, Aspergillus fumigatus. Baghdad Sci J 18:0225–0225. https://doi.org/10.21123/bsj.2021.18.2.0225
Srinivasan N, Thangavelu K, Uthandi S (2022) Lovastatin production by an oleaginous fungus, Aspergillus terreus KPR12 using sago processing wastewater (SWW). Microb Cell Factories 21:1–14. https://doi.org/10.1186/s12934-022-01751-2
Alrubayae IM, Kadhim KF (2020) November. Determination of oleaginous from non-oleaginous fungi using enzymatic and microscopic techniques. In IOP Conference Series: MSEB 928:20–11. https://doi.org/10.1088/1757-899X/928/6/062011
Sohail M, Ahmad A, Khan SA (2016) Production of cellulase from Aspergillus terreus MS105 on crude and commercially purified substrates 3:1–8. https://doi.org/10.1007/s13205-016-0420-z
Prajapati SK, Kumar P, Malik A, Choudhary P (2014) Exploring pellet forming filamentous fungi as tool for harvesting on-flocculating unicellular microalgae. BioenergyRes 7:1430–1440. https://doi.org/10.1007/s12155-014-9481-1
Salim S, Bosma R, Vermuë MH, Wijffels RH (2011) Harvesting of microalgae by bio-flocculation. J Appl Phycol 23:849–855. https://doi.org/10.1007/s10811-010-9591-x
Nie Y, Wang Z, Wang W, Zhou Z, Kong Y, Ma J (2022) Bio-flocculation of Microcystis aeruginosa by using fungal pellets of Aspergillus oryzae: Performance and mechanism. J Hazard Mater 439:129606
Das P, Thaher MI, Hakim MAQMA, Al-Jabri HMS, Alghasal GSH (2016) Microalgae harvesting by pH adjusted coagulation-flocculation, recycling of the coagulant and the growth media. Bioresour Technol 216:824–829. https://doi.org/10.1016/j.biortech.2016.06.014
Zhou W, Min M, Hu B, Ma X, Liu Y, Wang Q, Shi J, Chen P, Ruan R (2013) Filamentous fungi assisted bio-flocculation: a novel alternative technique for harvesting heterotrophic and autotrophic microalgal cells. Sep Purif Technol 107:158–165. https://doi.org/10.1016/j.seppur.2013.01.030
Rashid N, Rehman SU, Han JI (2013) Rapid harvesting of freshwater microalgae using chitosan. Process Biochem 48:1107–1110. https://doi.org/10.1016/j.procbio.2013.04.018
Huo S, Wang Z, Zhu S, Cui F, Zou B, You W, Yuan Z, Dong R (2014) Optimization of alkaline flocculation for harvesting of Scenedesmus quadricauda# 507 and Chaetoceros muelleri# 862. Energies 7:6186–6195. https://doi.org/10.3390/en7096186
Behera B, Balasubramanian P (2019) Natural plant extracts as an economical and ecofriendly alternative for harvesting microalgae. Biores Technol 283:45–52. https://doi.org/10.1016/j.biortech.2019.03.070
Abdel-Raouf N, Al-Homaidan AA, Ibraheem I (2012) Microalgae and wastewater treatment. Saudi J Biol Sci 19:257–275. https://doi.org/10.1016/j.sjbs.2012.04.005
Saleh DG, Ibrahim MM, El-Sayed AB, Mostafa E (2022) Phycoremediation of slaughterhouse wastewater using microalgae for nutrient recovery and biodiesel production. Egypt J Chem 65:1283–1289. https://doi.org/10.21608/ejchem.2022.138609.6094
Islam MA, Ayoko GA, Brown R, Stuart D, Heimann K (2013) Influence of fatty acid structure on fuel properties of algae derived biodiesel. Procedia Eng 56:591–596. https://doi.org/10.1016/j.proeng.2013.03.164
Acknowledgements
The authors would like to thank National Research Centre (NRC) and Faculty of Science, Menoufia University, Egypt, for providing the necessary facilities to carry out the research work.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
The study was conceptualized and planned by all of the authors; the microbiological experiments were carried out by H.I.A, O.M.D., and I.A.M., who also made contributions to the data analysis, discussion of the findings, and manuscript review. The first copy of the manuscript was written and edited by H.I.A., O.M.D., and I.A.M. The final manuscript was revised and consolidated by I.A.M., O.M.D., and M.M.G. The final copy of the manuscript was approved by all authors after they had read and revised it.
Corresponding author
Ethics declarations
Ethical approval
Ethics approval was not required for this study
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ayad, H.I., Matter, I.A., Gharieb, M.M. et al. Bioflocculation harvesting of oleaginous microalga Chlorella sp. using novel lipid-rich cellulolytic fungus Aspergillus terreus (MD1) for biodiesel production. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04822-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04822-5