Abstract
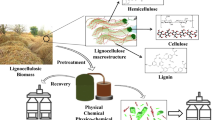
Lignocellulosic biomass is considered a sustainable source for the production of biofuels and platform molecules such as furfural (FAL). In this study, a series of solids with different acidity were tested for the production of FAL from xylose and corn residues. Functionalized Cloisite Na+ (CLOI-SO3H) and Preyssler heteropolyacid (HPA-Preyssler) showed the best catalytic performance in the production of FAL form xylose. Under optimal reaction conditions, the HPA-Preyssler catalyst achieved a maximum yield of 75% in just 15 min and maintained its activity for 5 consecutive reaction cycles, while the CLOI-SO3H catalyst obtained a 97% yield in 15 min, but its activity decreased considerably during reuse. Using techniques such as FTIR, SEM, EDS, and TGA, the possible causes of the decrease in the activity of the catalysts were established. The cellulose, hemicellulose, and lignin contents of different corn residues were determined to determine the most appropriate for the production of FAL. Using the HPA-Preyssler, the temperature and amount of catalyst selected for the dehydration of xylose to FAL, the appropriate time, amount of substrate, and type of solvent were established to obtain FAL directly from yellow corn stalks, reaching a maximum yield of 14% concerning hemicellulose content in 3 h at 180 °C in DMSO without performing any pretreatment to the corn residues, and the catalyst was recovered for subsequent reactions. Therefore, using the HPA-Preyssler catalyst is a new alternative for efficiently converting xylose or residual lignocellulosic biomass into FAL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
A large number of compounds used as feedstock are produced from non-renewable fossil fuels [1]; for this reason, new sources are needed to meet the chemical industry’s demand for these compounds. An alternative for obtaining compounds of industrial interest is the use of residual lignocellulosic biomass [2, 3] since this is produced in millions of tons per year around the world and is considered a sustainable source for the production of biofuels [4] and platform molecules [3,4,5]. Furfural (FAL) was recognized as a competitive bio-based platform molecule by the US Department of Energy because it can be used to produce high-value-added compounds [6], including those used to make novel surfactants and lube oils [7], for the pharmaceutical and agrochemical industry and refinery processes [5]. The FAL produced from fossil resources is not profitable, so production options are sought that improve yields without negatively impacting the environment [8, 9]. The production of FAL at an industrial level uses homogeneous acid catalysts such as H2SO4 and HCl, but these processes generate a large amount of liquid waste and gases leading to equipment corrosion and increasing the production price due to the high maintenance cost [10, 11].
Solid acids have been reported to overcome this common disadvantage of homogenous acids. For example, Toumsri et al. [12] designed a porous carbonaceous acid catalyst (SO3H-MPBG), using sugarcane residues as a base, reaching a yield of 55% and a conversion of 86% at 170 °C, 3 h in γ-valerolactone (GVL) as solvent. Rusanen et al. [13], with the help of iron catalysts supported on carbon (5Fe-AC), obtained a yield of 57% for 3 h at 170 °C in methyl isobutyl ketone (MIBK). Zhiqiang et al. [5] synthesized a new solid carbon-based magnetic acid catalyst (MMCSA), reaching a 79% yield at 190 °C for 10 min using three times the amount of catalyst in relation to the substrate in a biphasic MIBK/water system. Xu et al. [3] synthesized a sulfonated carbonaceous solid acid catalyst, and the maximum yield was 80% with 100% conversion using SP-170 at 170 °C for 1 h; these results varied according to the degree of sulfonation of the catalyst, which influenced the acidity of the catalyst. Wang et al. [4] synthesized a mesoporous catalyst (OMC-SO3H) using biomass lignin as a carbon source, reaching 76% yield and 100% xylose conversion at 200 °C for 45 min in GVL: water (85:1 %v/v). Zhou et al. [14] designed a tin oxide-supported micro-mesoporous carbon catalyst (MC-SnOx) for the production of FAL from xylose at 180 °C for 20 min in a 0.2 M solution of NaCl in a mixture of H2O/2MTHF (1:1 %v/v), reaching a yield of 53%, and the catalyst was reused for five cycles.
Wang et al. [15] prepared and used a sulfonated clay zeolite catalyst (PAL-SO3H), reaching a yield and selectivity of 87% and 96%, respectively, at 180 °C in 60 min using GVL-water (95:5 %v/v), and the catalyst was reused four cycles. Tran et al. [16] synthesized a functionalized mesoporous silica catalyst (SO3H-KIT-6) by varying the molar ratio of tetraethoxysilane and 3-mercaptopropyl(methyl)dimethoxysilane. The best catalyst was synthesized using tetraethoxysilane and 3-mercaptopropyl(methyl)dimethoxysilane at a molar ratio of 0.8:0.2, reaching 92% yield at 170 °C for 2 h. Lu et al. [17] supported tungstophosphoric acid on titanium oxide (TPA-TiO2), obtaining a 96% conversion and a 76% yield for 1 h at 190 °C in MIBK-water. Sajid et al. [18] obtained a 53% yield at 120 °C in 1 h using a mixture of Brönsted and Lewis acids (pTSA-CrCl3·6H2O). Wang et al. [19] used a zeolite modified by dealumination and ion exchange as a catalyst, and the highest FAL yield achieved was 77% with a xylose conversion of 99% in water/n-butanol (1–1.5 %v/v) for 30 min and 180 °C.
Although high yields of FAL have been obtained from xylose, its direct production from lignocellulosic residues is undoubtedly a more difficult and complicated process because it involves the process of breaking down the hemicellulose-cellulose-lignin complex, hydrolysis of hemicellulose, and xylose dehydration [20]. Ionic liquids (IL) have been used for the production of FAL from lignocellulosic biomass, with yields between 1 and 37% and demonstrating important advantages for its production. However, its industrial use in large-scale processes is hampered by economic, technological, and environmental issues [21]. Furthermore, the development of effective methods for IL recovery and recycling is a great challenge. In addition to the use of IL, in recent years, different catalytic systems have been investigated to improve and optimize the production of FAL from lignocellulosic biomass. Liang et al. [22] treated corn lignocellulosic residues with ultrasound and subsequently used a bio-based heterogeneous catalyst (CSUTS-CSW), which obtained a 68% FAL yield at 180 °C in 15 min. Lee et al. [23] also pre-treated oil palm leaves with ultrasound and subsequently carried out a reaction in an aqueous solution of choline-oxalic acid at 120 °C for 60 min, obtaining a 56% yield. Lyu [24] produced 85% of FAL from corn bagasse through a tandem electrochemical route using AlCl3 as a catalyst. Li et al. [25] synthesized a phosphotungstic acid functionalized biochar (PA-FCB) for the production of FAL from corncob using GVL/water as the solvent, resulting in a 96% yield at 205 °C for 120 min. Zhang et al. [26] synthesized a sucrose-based N-doped sulfonated activated carbon (SSUAC) catalyst for obtaining FAL from wheat straw, reaching a maximum yield of 78% at 180 °C for 90 min. Gong et al. [27] from sunflower stem and using a carbonaceous solid acid catalyst SO42−/SnO2-SSXR obtained a yield of 82% in ChCl-MAA/toluene at 180 °C in 15 min. Finally, Li et al. [28], by immobilization of the eutectic solvent betaine: lactic acid on silica, synthesized a heterogeneous catalyst B: LA-SG (SiO2) with which they obtained a 45% FAL yield from corncob for 30 min and 170 °C.
As mentioned above, several works in recent years have focused on obtaining FAL from xylose or residual lignocellulosic biomass. However, there are still several disadvantages such as high temperatures, long reaction times, use of ionic liquids, expensive deep eutectic solvents, use of complex catalysts to obtain, use of technologies difficult to scale up to industrial levels such as microwaves and ultrasound, and the difficulty of recovery and reuse of the catalyst. In addition, when starting from lignocellulosic biomass, the use of pretreatments increases production costs and, in some cases, involves the use of corrosive reagents. In an attempt to efficiently produce a larger amount of FAL, we studied a series of solids with different acidic properties as heterogeneous catalysts for the production of FAL from xylose and corn residues. For the first time, a high yield to FAL is obtained in short periods of time compared with other previous works, using the HPA-Preyssler catalyst. The best reaction conditions (temperature, time, catalyst loading, amount of substrate, and solvent) were established.
2 Materials and methods
2.1 Materials
Reagent grade xylose (CAS: 58-86-6, 99%), dimethyl sulfoxide (DMSO) (CAS: 67-68-5), and γ-valerolactone (CAS: 108-29-2, 98%) were purchased from ACROS ORGANIC. Dichloromethane (75-09-2), ethanol (CAS: 64-17-5), benzene (CAS: 1076-43-3, 99.5%), acetic acid (CAS 64-19-7,96%), sulfuric acid (CAS: 7664-93-9, 95%), methanol (CAS: 67-56-1, 99.8%), and methylisobutylketone MIBK (CAS: 108-10-1) were purchased from MERCK. Sodium hydroxide (CAS: 1310-73-2, 97%) and sodium chlorite (CAS: 7758-19-2, 80%) were purchased from FISHER SCIENTIFIC. The reagents were used without further purification.
2.2 Catalysts tested
Amberlyst® 15 acid resin was purchased from Sigma Aldrich Catalyst. Preyssler heteropolyacid (H14[NaP5W30O110]·44H2O) was prepared according to the method reported in the literature (see Supplementary Information). The sulfonic acid-based catalysts were obtained by direct organosilylation with 2-(4-chlorosulfonylphenyl)ethyltrimethoxysilane (here designated by -CSP) or direct sulfonation using chlorosulfonic acid (here designated by -SO3H) of different materials: a montmorillonite (Cloisite-Na+, here designated by CLOI), halloysite nanotubes (here designated by HNT), multiwalled carbon nanotubes (here designated by CNT), and silica nanoparticles with particles size around 10 nm (here designated by SiO2-10). The previously prepared catalysts (see Supplementary Information) HNT-CSP, SiO2-10-CSP, CLOI-SO3H, and CNT-CSP were provided by the University of Porto, and the synthesis and characterization methodology were previously reported in the literature.
2.3 Catalytic activity
The selection of catalysts was performed in two stages as follows: (a) selection of catalysts using water as solvent. Briefly, 40 mg of xylose was dissolved in 3 mL of water, and the catalyst (25 %w/w) was added, and the reactions were carried out at 140 °C and 600 rpm. (b) The catalysts that showed the highest catalytic performance were evaluated under the same conditions, but using DMSO as solvent. With the catalysts that showed the best catalytic performance in DMSO, reactions were carried out in the range of 150 to 180 °C, monitoring the percentage yield at FAL at 0.08, 0.16, 0.25, 0.5, 1, 2, and 3 h, keeping all other conditions constant. Carrying out the reactions with the time and temperature conditions established in the previous step, different solvents and solvent-mixtures were evaluated as follows: γ-valerolactone (GVL), DMSO, methylisobutylketone (MIBK), water, GVL-water (1:1), DMSO-water (1:1), MIBK-water (1:1), DMSO-GVL (1:1), DMSO-MIBK (1:1), and GVL-MIBK (1:1). All other conditions were maintained constant. With the solvent selected according to the previous step, the optimum amount of catalyst (15 %w/w, 25 %w/w, 35 %w/w, or 45 %w/w) was established. The other reaction conditions were constant.
Once the optimum reaction conditions were established, catalyst reuse tests were carried out as follows: The HPA-Preyssler catalyst was recovered taking as reference the methodology proposed by Pardo et al. [29]. Before each reuse, the catalyst was separated from the reaction mixture by changing the polarity of the reaction medium with 5 mL of CH2Cl2 and later cooling to 4 °C for 30 min. Then, the precipitated catalyst was recovered by centrifugation at 3500 rpm for 10 min, washed with CH2Cl2 (2 × 3 mL), and dried at 50 °C for 10 min.
.The CLOI-SO3H catalyst was recovered by centrifugation at 3400 rpm for 10 min. Each of the catalysts was washed with CH2Cl2 (2 × 3 mL) and oven dried at 50 °C for 10 min before use in each reaction.
The quantification of FAL was performed by high-performance liquid chromatography (HPLC) in an equipment Agilent 1200 equipped with a C18 column (150 mm × 4.6 mm), reference number USKH056756, HP reverse phase, with a UV nm detector. Methanol-water (60:40 %v/v), flow rate 0.4 mL/min (isocratic method) at 40 °C was used as the mobile phase. All samples were filtered with a pore size of 0.45μm. A calibration curve for FAL was performed. The yield and selectivity to FAL and xylose conversion to different reaction types are estimated according to the following equations:
2.4 Catalyst characterization
The solids were prior to and after the catalytic measurements by FT-infrared spectroscopy (FTIR), scanning electron microscopy and energy dispersive X-Ray spectroscopy (SEM-EDS), and thermogravimetric analysis (TGA). FTIR analysis was performed using a Thermo Scientific Nicolet iS50 FT-IR equipment, in a range of 400–4500 cm−1, and the catalysts were placed directly on a Zn-Se plate and measured using the ATR technique. Each sample was scanned 60 times, and the scan resolution was 4 cm−1. SEM-EDS analysis was performed on a ZEIZZ MA10 series scanning electron microscope with SE1 (secondary) and BSD (backscattered) electron detectors. EDS analysis was performed with an Oxford EDS lance. TGA studies were performed on SETARAM equipment. Samples (20–50 mg) were measured under the following conditions: stabilization: 30 °C for 10 min; heating: 10°C/min up to 800 °C; stabilization: 800 °C for 10 min; and cooling: 800 °C up to 30 °C for 40 min. The above conditions were performed in an inert N2 atmosphere.
3 Chemical characterization of yellow corn (Z. mays) wastes
Samples of yellow corn wastes came from the municipality of Santa Sofía, Boyacá-Colombia, and their chemical composition was determined in triplicate, following the methodology of the TAPPI standards (T 210 cm - 13, T 211 om - 16, T 264 cm - 07, T 204 cm - 97, T 222 om - 02, T 9 wd - 75, and T 203 cm - 09).
3.1 Catalytic activity using yellow corn residues
According to the better results of Section Catalytic activity, the best catalyst was used by monitoring a reaction for 6 h varying the amount of corn residue (40, 60, and 80 mg), under the following conditions: 180 °C, 3 mL of DMSO, 45 %w/w of catalyst, and 600 rpm. Posteriorly, it was studied the solvent effect for obtaining furfural from biomass using GVL and GVL: MIBK (1:1) at 180 °C in 3 mL, 45 %w/w of catalyst, and 600 rpm.
4 Results and discussion
4.1 Dehydration of xylose to furfural
The dehydration reactions of xylose to FAL were performed in sealed glass tubes in an oil bath. Six catalysts with different acidity were tested including HPA-Preyssler heteropolyacid, Amberlyst 15 acid resin, acid solids based on functionalized halloysite nanotubes (HNT-CSP), carbon nanotubes (CNT-CSP) montmorillonite clay (CLOI-SO3H), and silica nanoparticles (SiO2-10-CSP). The activity of the catalysts in the dehydration of xylose to FAL in decreasing order was as follows: CLOI-SO3H > HNT-CSP > HPA-Preysseler > SiO2-10-CSP > Amberlyst 15 > CNT-CSP. Overall, the FAL yields obtained with all catalysts tested were below 20% at the maximum reaction time (Fig. 1).
The low yield obtained with HPA-Preyssler is attributed to its instability in aqueous media, changing the acidity of the solutions [29, 30], while the low yields obtained with the other catalysts are attributed to a decrease in acidity in the xylose to FAL reaction because the acid sites (SO3H) are hydrophilic and can absorb water [31]. Although the acidity of the sulfonic groups of HNT-CSP is lower than that of SiO2-10-CSP and CNT-CSP, by XPS analysis, it is possible to observe that these –SO3H active sites are more on the surface and therefore more available to catalytic reaction than those of SiO2-10-CSP and CNT-CSP allowing better activity (Table 1). It is also important to note that amberlyst-15, despite having a very high amount of acid sites 4.7 mmol/g [31], did not give rise to a higher activity compared to the catalysts presented in Table 1.
Based on the above results, the three catalysts with the highest FAL yield were chosen, and their activity was evaluated in DMSO as solvent using the same reaction conditions. The use of DMSO favors the yield to FAL from xylose due to inhibition of FAL degradation reactions. In addition, these solvents increase the stability of the heterogeneous catalysts, improving their activity, unlike aqueous systems where leaching of the acid sites of the catalyst occurs at relatively high temperatures [9], which reduces their catalytic performance.
The activity of the catalysts in DMSO in decreasing order was as follows: CLOI-SO3H > HPA-Preyssler > HNT-CSP. With the catalyst CLOI-SO3H, the yield at FAL remained constant (92%) after 3 h of reaction. With HPA-Preyssler, a maximum yield of 57% was obtained in 3 h and then declined. With the HNT-CSP catalyst, a maximum yield of 16% was reached during 4 h of reaction (Fig. 2). The activity of CLOI-SO3H and HPA-Preyssler is extremely dependent on the solvent used showing significant increases when switching from water to DMSO. HNT-CSP does not show significant improvements comparing with CLOI-SO3H, and this difference can be mainly attributed to their lower acidity (0.82 mmol, H+ g−1) when compared with CLOI-SO3H (1.78 mmol, H+ g−1) (Table 1). The catalytic performance of HPA-preyssler is associated with its percentage of Brönsted and Lowry acid sites (52% Brönsted sites, 22% Lewis sites, and 26% Lewis-Brönsted sites), determined in previous work by our group using pyridine adsorption followed by FTIR spectroscopy [33]. Due to the higher catalytic activity demonstrated by the catalysts, CLOI-SO3H and HPA-Preyssler were selected to continue establishing the best reaction conditions.
Temperature and reaction time are key parameters for energy efficiency and costs during the production processes of any compound. The effect of these reaction conditions was determined using HPA-Preyssler and CLOI-SO3H catalysts in DMSO as solvent by monitoring for 3 h at different temperatures (Fig. 3). The highest yields to FAL using the HPA-Preyssler catalyst were obtained at 170 °C and 180 °C. At 170 °C, the FAL yield increased with time to reach the highest yield (85%) in 2 h, and after this time, the yield decreased by 4%. At 180 °C, the yield reaches a maximum of 75% in 0.5 h, and after this time, the yield started to decrease (Fig. 3a). Figure 3b displays the results of the effect of temperature and reaction time using CLOI-SO3H. It can be observed a readily formation of FAL in short times of reaction at lower temperatures of reaction than Preyssler heteropolyacid; however, as can be seen in Fig. 3b, the yield at FAL increases with time up to a maximum point, after which the yield begins to decay, being most noticeable at 170 °C and 180 °C. This behavior shown by the two catalysts is possibly due to the formation of undesirable reactions of xylose and FAL which promoted to give rise to humins [19, 34]. In both catalysts, as the reaction progresses, the catalyst is saturated probably with humins that decreases the yields to furfural obtained. Thus, 0.25 h of reaction at 180 °C and 160 °C was selected using the HPA-Preyssler and CLOI-SO3H catalysts, respectively.
Table 2 shows the effect of the type of solvent on the dehydration of xylose to FAL with each of the catalysts. With pure solvents as well as with mixtures of solvents, better yields were obtained with the CLOI-SO3H catalyst. The highest yields were achieved with polar aprotic solvents due to the stability of FAL in this type of solvents [35], and within these, the highest yields obtained with GVL (87%) using the CLOI-SO3H catalyst and those obtained using DMSO (76%) with the HPA-Preyssler catalyst stand out (Table 2, entries 1 and 3). Similarly, better yields were obtained when mixtures of aprotic organic solvents were used (Table 2, entries 7–9). The low yield obtained with MIBK (Table 2, entry 5) was due to the low solubility of xylose in this solvent. The low yield obtained with mixtures including water (Table 2, entries 2, 4, and 6) was due to the decreased acidity of the catalysts when mixed with this solvent.
According to the results described above, GVL was selected for reactions using the CLOI-SO3H catalyst and DMSO for reactions using the HPA-Preyssler catalyst as the most suitable solvents for obtaining FAL from xylose. GVL is a promising solvent in the production of FAL due to its high thermal stability, biodegradability, and the fact that it is presented as a renewable chemical compatible with several types of catalysts [9]. DMSO is an aprotic solvent used in multiple organic and biomass transformation reactions due to its high boiling temperature and high polarity [36, 37].
The effect of catalyst loading on the dehydration of xylose to FAL is shown in Fig. 4. The mass of CLOI-SO3H which promotes a better yield to furfural (97%) was 14 mg (35 %w/w) of catalyst. The increase of the catalyst amount to 18 mg (45 %w/w) allowed a decline of 10% in the FAL yield. This behavior is possible because relatively high amounts of catalyst increase the number of acid sites that promote the dehydration of xylose to FAL [38] but at the same time favor the formation of resignification and condensation products, decreasing the amount of FAL, similar to the results reported by Ji et al. [39]. Regarding the effect of using different amounts of HPA-Preyssler catalyst, a linear trend can be easily observed even for every increase of 4–5 mg of catalyst up to 14 mg of catalyst with an increase in yield of ~ 10–15%. However, using 18 mg instead of 14 mg, the increment was smaller (5%) going from 70 to a maximum of 75% FAL yield. Considering that some saturation had already occurred, we selected 18 mg (45 % w/w) as the optimal amount of catalyst to promote a higher FAL yield. The use of DMSO in the absence of the catalyst HPA-Preyssler yields 27% of FAL, possibly through a mechanism similar to that reported for fructose dehydration [40]. Several researchers in recent years have studied the conversion of xylose to FAL using heterogeneous catalysts, but their yields have been limited by the amount of catalyst. Wang et al. [4] used 60% catalyst at 200 °C, while Dai et al. [41] used 100% catalyst loading and 190 °C, obtaining 77 and 74% yields, respectively. Ji et al. [39] used 50% catalyst loading at 170 °C for 4 h of reaction to obtain 70% yield. The CLOI-SO3H and HPA-Preyssler catalysts demonstrated high catalytic performance at short reaction times, relatively low temperatures, and low catalyst loadings, which makes these catalysts promising catalysts for the efficient production of FAL from xylose, using conventional methods and thus reducing production costs in terms of time and catalyst mass. According to our results, 35 %w/w of the CLOI-SO3H catalyst, and 45 %w/w of the HPA-Preyssler catalyst were selected as the most suitable amount for further research.
The yield at FAL in the first reuse of the ClOI-SO3H catalyst decreased by 64%, and in subsequent reuses, it decreased considerably reaching 5% in the fourth reuse (Fig. 5).
The decrease in the activity of the catalyst is attributed to the loss of sulfonic groups (-SO3H) from the clay, as a consequence of its instability at relatively high temperatures due to the type of bond established with the clay. The results by TGA-DTG of the fresh ClOI-SO3H catalyst showed a 34% weight loss between 61 and 169 °C with a maximum desorption peak according to the DTG curve peak at 121 °C, due to physisorbed water the sulfonic acid adsorbed to the surface and a 48% weight loss between 192 and 284 °C with a maximum desorption peak at 262 °C due to the decomposition reactions of the catalyst (Fig. 6a), while the reused ClOI-SO3H showed a weight loss of 7% between 47 and 134 °C with a maximum desorption peak at 89 °C due to physisorbed water originating during xylose dehydration, a 15% loss between 118 and 189 °C with a maximum desorption peak at 144 °C due to loss of sulfonic groups, and two weight losses of 24% and 2% between 190 and 290 °C with a maximum peak at 235 °C and between 572 and 645 °C with a maximum peak at 618 °C, respectively (Fig. 6b), possibly due to catalyst decomposition reactions and desorption of reactants and reaction products. Similar results were reported for sulfonated coals [42].
EDS analysis results established a 4-fold decrease in sulfur content in the reused catalyst (Table 3). In addition, X-ray mapping spectra showed the decrease and change in sulfur distribution in the reused catalyst (Fig. 7). On the other hand, when comparing the microstructure of the fresh catalyst with that of the reused catalyst, it is observed that the particles in the latter tend to aggregate, resulting in a loss of the homogeneous dispersion observed in the former. Moreover, the reused catalyst exhibits a higher number of aggregation clusters compared to the fresh catalyst, which may be attributed to the leaching of acid sites (-SO3H) and subsequent formation of loose particles (Fig. 8). Additionally, the reused catalyst displays the presence of lamellae, a characteristic feature of non-functionalized clays [43].
The HPA-Preyssler catalyst showed more stability in its activity during the reuses (Fig. 5). In the first reuse, the yield to FAL decreased by 14%; in the subsequent three reuses, the yield decreased less until a 45% yield was obtained in the 4th reuse. The decrease in the catalytic activity of the HPA-Preyssler is attributed to the loss of catalyst during the reuse process because, at the end of the 4th reuse, a 33% total catalyst mass loss was obtained, distributed in 5%, 8%, 9%, and 11% during reuses 1, 2, 3, and 4 respectively. However, the calculated turnover number (TON), for the fresh catalyst was 74, while for reuses 1, 2, 3, and 4 were 64, 61, 62, 62, and 63, respectively, indicating that the catalyst activity is maintained after the first reuse.
The decrease in activity after the first reaction cycle could be due to the adsorption of products on the catalyst surface (FAL and humins), [19, 44] which was followed by FTIR analysis of the spent catalysts (Fig. 9). The bands at 3009 and 2933 cm−1 can be assigned at C-H tension vibration attributed to the aldehyde group of FAL and band at 1337 cm−1 attributed to stretching of the C-H bond of the aldehyde group, and a band at 1407 cm−1 is attributed to the C=C bond of the aromatic ring of FAL. These bands are observed after the reuse of catalysts and can indicate some irreversible adsorption of FAL on the surface of the catalyst inhibiting its activity after first reuse. These results were reinforced by the thermal stability showed by TGA-DTG of HPA-Preyssler before and after reuses (Fig. 10). The fresh HPA-Preyssler showed a total weight loss of 9.5% between 67 and 253 °C due to physisorbed water with a maximum desorption peak according to the peak of the DTG curve at 146 °C (Fig. 10a). The reused HPA-Preyssler showed a total weight loss of 9.4% divided into a 2.3% loss between 47 and 129 °C with a maximum desorption peak at 88 °C due to physisorbed water, a 7% loss between 145 and 257 °C with a maximum desorption peak at 208 °C possibly due to adsorption of FAL on the catalyst surface, and a 0.5% between 357 and 435 °C with a maximum desorption peak at 409 °C (Fig. 10b) possibly due to adsorption of humins on the catalyst surface.
4.2 Characterization of yellow corn (Z. mays) residues
Lignocellulosic residues have different compositions according to the source, species, and environmental conditions, among others [45]. The chemical characterization of residues from yellow corn (Z. mays) is shown in Table 4. The cellulose, hemicellulose, and lignin content in yellow corn stalks and corncob was similar to that reported by Isikgor et al. [46]. Hemicellulose content in corncob, leaves, and stalk of yellow corn was similar, being slightly higher in leaves and stalk, while the lowest content was found in corncob obtained from unripe corn. The lignin content was lower in stalks compared to leaves, possibly because the latter have a greater number of tissues (epidermal, photosynthetic, xylem, and phloem) that provide a greater amount of lignin, while the stalk is mostly constituted by vascular tissues since their main function is the transport of water and nutrients between roots and leaves [47]. The yellow corncob obtained from unripe corn presented a higher moisture content compared to the other yellow corn wastes because this sample comes from a lower stage of corn maturation. Considering that it has been established that the amount of lignin present in lignocellulosic wastes directly influences the obtaining of furan derivatives [48] and that the yield to FAL is directly proportional to the amount of hemicellulose, wastes from yellow corn stalks were selected for the production of FAL, using the conditions established in the dehydration of xylose to FAL.
4.3 Obtaining furfural from yellow corn stalks
The amount of substrate is an important variable at the time of transformation of residual lignocellulosic biomass. The yellow corn stalk substrate was screened with a pore size of 0.105 mm since it has been reported that particle sizes smaller than 0.4 mm do not affect the reaction rate and yield [49]. The effect of the amount of substrate for obtaining FAL from corn stalk residues is shown in Fig. 11. It was observed that using 40 mg of yellow corn stalks, the highest yield to FAL (14%) was obtained in less time. As the amount of substrate was increased, the yield decreased, possibly due to diffusional problems since the substrate adhered to the tube walls which did not allow good contact between the substrate and the catalyst, a similar phenomenon was reported by Lyu [24] when obtaining an inverse relationship in the production of FAL with respect to the corncob substrate loading. Moreover, this decrease could also be due to an increased formation of humins that may be trapped in the catalyst. On the other hand, this result may be attributed to the recalcitrance conferred by the polyphenolic structure of lignin, which makes it difficult to access those substrates (hemicellulose and cellulose) retained within the lignocellulosic biomass [50]. Pretreatments could contribute to improved performance at FAL, but some require sophisticated ultrasound or microwave equipment that is expensive and not competitive on a large scale, and others involve high energy costs or include strong acids or bases that corrode the equipment and generate a negative impact on the environment [51]. Different authors have used low substrate concentrations to obtain higher performance results; e.g., Yang T et al. [52] started from an amount of corn stover equal to ours, using GVL as a solvent, 190 °C for 80 min, obtaining a 53% yield at FAL, while Lyu [24] used 2 mg/mL of corncob in cyclopentyl methyl ether as a solvent for 2.5 h at 200 °C obtaining 85% yield. On the other hand, Li X et al. [25] used 10 mg/mL of corncob in a two-phase GVL/water (8:2) system, at 195 °C for 120 min, obtaining 96% yield at FAL. It should be emphasized that in the present work, the direct conversion of residual biomass from yellow corn stalks to FAL was achieved using readily available and environmentally friendly solvents, as well as a low catalyst loading and without high operational costs. Moreover, the use of HPA-Preyssler at relatively high temperatures and in the direct conversion of biomass to FAL has not been reported before.
GVL is one of the most popular solvents for biomass to FAL transformation, as it can solubilize degradation products of both FAL and lignin [9] and help to fractionate biomass, contributing to improved yield [53]. On the other hand, it has been reported that the use of biphasic MIBK: GVL and GVL: water systems can contribute to improving FAL production from lignocellulosic biomass [4, 5, 9] since it is more stable in organic systems. For this reason, reactions were carried out using GVL and a GVL: MIBK mixture (1:1) under the same conditions when DMSO was used as the solvent. Surprisingly, FAL was not obtained using pure GVL or with the biphasic mixture, possibly due to the relatively low temperature established using the HPA-Preyssler catalyst (180 °C) compared to works reported by other authors using this type of solvent [49]. Lignin has been reported to undergo pyrolysis in the range of 200–400 °C where most of the ether bonds are broken [54]. Thus, lower temperatures further complicate the access of the substrate to the catalyst, while the synergistic effect between DMSO and HPA-Preyssler catalyst facilitated the pyrolysis of lignin and thus the interaction between hemicellulose and the catalyst.
5 Conclusions
Among different types of catalysts and solvents, CLOI-SO3H and HPA-Preyssler catalysts were identified with the highest catalytic activities in the conversion of xylose to FAL using GVL and DMSO as solvents, respectively. Within 15 min, 97% yield to FAL at 160 °C was achieved with CLOI-SO3H, while 75% was achieved with HPA-Preyssler at 180 °C. However, during reuses, only HPA-Preyssler maintained its activity and showed higher stability. The decrease in the catalytic activity of HPA-Preyssler after the first reaction cycle was attributed to the adsorption of FAL and humins on the surface of the catalyst; however, its activity was maintained in the following reuses, with a calculated TON value around 62. Stalk corn residues presented concentrations of hemicellulose (27.7%) and lignin (18.4%), which are more appropriate for obtaining FAL. Under optimum reaction conditions, a 14% yield of FAL was obtained from yellow corn stalk using HPA-Preyssler and DMSO as solvent at 180 °C for 3 h, without any pretreatment, and the catalyst was recovered without loss of activity. Finally, it should be noted that a high yield to FAL was obtained in a short time from xylose and that the methodology allowed obtaining an acceptable yield directly from residual biomass under mild reaction conditions using for the first time the HPA-Preyssler.
Data availability
This declaration is not applicable.
References
Forsberg CW, Dale BE, Jones DS, Hossain T, Morais ARC, Wendt LM (2021) Replacing liquid fossil fuels and hydrocarbon chemical feedstocks with liquid biofuels from large-scale nuclear biorefineries. Appl Energy 298:117225. https://doi.org/10.1016/J.APENERGY.2021.117225
Jiang Z, Hu D, Zhao Z, Yi Z, Chen Z, Yan K (2021) Mini-review on the synthesis of furfural and levulinic acid from lignocellulosic biomass. Processes 9:1234. https://doi.org/10.3390/pr9071234
Ntimbani RN, Farzad S, Görgens JF (2021) Furfural production from sugarcane bagasse along with co-production of ethanol from furfural residues. Biomass Conv Bioref 12:5257–5267. https://doi.org/10.1007/s13399-021-01313-3
Wang X, Qiu M, Tang Y, Yang J, Shen F, Qi X, Yu Y (2021) Synthesis of sulfonated lignin-derived ordered mesoporous carbon for catalytic production of furfural from xylose. Int J Biol Macromol 187:232–239. https://doi.org/10.1016/j.ijbiomac.2021.07.155
Qi Z, Wang Q, Liang C, Yue J, Liu S, Ma S, Wang X, Wang Z, Li Z, Qi Z (2020) Highly efficient conversion of xylose to furfural in a Water–MIBK system catalyzed by magnetic carbon-based solid acid. Ind Eng Chem Res 59:17046–17056. https://doi.org/10.1021/acs.iecr.9b06349
Bozell JJ, Petersen GR (2010) Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy’s ‘Top 10’ revisited. Green Chem 12:539. https://doi.org/10.1039/B922014C
Huang R, Chang J, Choi H et al (2022) Furfural upgrading by aldol condensation with ketones over solid-base catalysts. Catal Lett 152:3833–3842. https://doi.org/10.1007/s10562-022-03960-1
Mamman AS, Lee JM, Kim YC, Hwang IT, Park NJ, Hwang YK, Chang JS, Hwang JS (2008) Furfural: hemicellulose/xylose-derived biochemical. Biofuels Bioprod Biorefin 2:438–454. https://doi.org/10.1002/bbb.95
Lee CBTL, Wu TY (2021) A review on solvent systems for furfural production from lignocellulosic biomass. Renew Sust Energ Rev 137:110172. https://doi.org/10.1016/j.rser.2020.110172
Corma A, Iborra S, Velty A (2007) Chemical routes for the transformation of biomass into chemicals. Chem Rev 107:2411–2502. https://doi.org/10.1021/cr050989d
Huang T, Yuan K, Nie XL, Chen J, Zhang HX, Chen JZ, Xiong WM (2021) Preparation of furfural from xylose catalyzed by diimidazole hexafluorophosphate in microwave. Front Chem 9:727382. https://doi.org/10.3389/fchem.2021.727382
Toumsri P, Auppahad W, Panpranot J, Chuenchom L (2020) Facile preparation of monolithic magnetic porous carbon acid catalysts via surface self-assembly method and their applications in conversion of xylose into furfural. In: IOP Conference Series: Materials Science and Engineering, vol 773, p 012008. https://doi.org/10.1088/1757-899X/773/1/012008
Rusanen A, Kupila R, Lappalainen K, Kärkkäinen J, Hu T, Lassi U (2020) Conversion of xylose to furfural over lignin-based activated carbon-supported iron catalysts. Catalysts 10:821. https://doi.org/10.3390/catal10080821
Zhou N, Zhang C, Cao Y, Zhan J, Fan J, Clark JH, Zhang S (2021) Conversion of xylose into furfural over MC-SnOx and NaCl catalysts in a biphasic system. J Clean Prod 311:127780. https://doi.org/10.1016/J.JCLEPRO.2021.127780
Wang R, Liang X, Shen F, Qiu M, Yang J, Qi X (2019) Mechanochemical synthesis of sulfonated palygorskite solid acid catalysts for selective catalytic conversion of xylose to furfural. ACS Sustain Chem Eng 8:1163–1170. https://doi.org/10.1021/acssuschemeng.9b06239
Vi Tran TT, Kongparakul S, Karnjanakom S, Reubroycharoen P, Guan G, Chanlek N (2019) Highly productive xylose dehydration using a sulfonic acid functionalized KIT-6 catalyst. Fuel 236:1156–1163. https://doi.org/10.1016/j.fuel.2018.09.089
Kaifeng L, Yifan L, Caidi W, Robert J, Weihong L, Jun T, Lingjun Y, Shuron Z (2022) Novel approach on developing TiO2-supported heteropolyacids catalyst for the efficient conversion of xylose to furfural. Energy Fuel 36:7599–7607. https://doi.org/10.1021/acs.energyfuels.2c01232
Sajid M, Rizwan Dilshad M, Saif Ur Rehman M, Liu D, Zhao X (2021) Catalytic conversion of xylose to furfural by p-toluenesulfonic acid (pTSA) and chlorides: process optimization and kinetic modeling. Molecules 26:2208. https://doi.org/10.3390/molecules26082208
Wang Y, Dai Y, Wang T, Li M, Zhu Y, Zhang L (2022) Efficient conversion of xylose to furfural over modified zeolite in the recyclable water/n-butanol system. Fuel Process Technol 237:107472. https://doi.org/10.1016/j.fuproc.2022.107472
Zhang T, Li W, An S, Huang F, Li X, Liu J, Pei G, Liu Q (2018) Efficient transformation of corn stover to furfural using p-hydroxybenzenesulfonic acid-formaldehyde resin solid acid. Bioresour Technol 264:261–267. https://doi.org/10.1016/j.biortech.2018.05.081
Peleteiro S, Rivas S, Alonso JL, Santos V, Parajó JC (2016) Furfural production using ionic liquids: a review. Bioresour Technol 202:181–191. https://doi.org/10.1016/j.biortech.2015.12.017
Liang J, Zha J, Zhao N, Tang Z, He Y, Ma C (2021) Valorization of waste lignocellulose to furfural by sulfonated biobased heterogeneous catalyst using ultrasonic-treated chestnut shell waste as carrier. Processes 9:2269. https://doi.org/10.3390/pr9122269
Cornelius L, Wu BTL, Cheng TY, Siow LF, Chew IML (2021) Nonsevere furfural production using ultrasonicated oil palm fronds and aqueous choline chloride-oxalic acid. Ind Crop Prod 166:113397. https://doi.org/10.1016/j.indcrop.2021.113397
Lyu X (2021) Furfural and hydrogen production from corncob via tandem chemical and electrochemical approach. Bioresour Technol Rep 15:100790. https://doi.org/10.1016/j.biteb.2021.100790
Xiaoyu L, Xuebin L, Wenxuan H, Haocheng H, Jingguang C, Jian X, Lefu L, Zhihao Y, Chuanling X (2022) Phosphotungstic acid functionalized biochar for furfural production from corncob. Fuel Process Technol 229:107178. https://doi.org/10.1016/j.fuproc.2022.107178
Zhang T, Wei H, Gao J, Chen S, Jin Y, Deng C, Wu S, Xiao LW (2022) Synthesis of sulfonated hierarchical carbons and their application on the production of furfural from wheat straw. Mol Catal 517:112034. https://doi.org/10.1016/j.mcat.2021.112034
Gong L, Zha J, Pan L, Ma C, He Y (2022) Highly efficient conversion of sunflower stalk-hydrolysate to furfural by sunflower stalk residue-derived carbonaceous solid acid in deep eutectic solvent/organic solvent system. Bioresour Technol 351:126945. https://doi.org/10.1016/j.biortech.2022.126945
Li Q, Ma C, Di J, Ni J, He YC (2022) Catalytic valorization of biomass for furfuryl alcohol by novel deep eutectic solvent-silica chemocatalyst and newly constructed reductase biocatalyst. Bioresour Technol 347:126376. https://doi.org/10.1016/j.biortech.2021.126376
Pardo Cuervo OH, Romanelli GP, Cubillos JA, Rojas HA, Martínez JJ (2020) Selective catalytic dehydration of xylose to furfural and fructose and glucose to 5-hydroximethylfurfural (HMF) using Preyssler heteropolyacid. ChemistrySelect 5:4186–4193. https://doi.org/10.1002/slct.202000657
Timofeeva MN (2003) Acid catalysis by heteropoly acids. Appl Catal A Gen 256:19–35. https://doi.org/10.1016/S0926-860X(03)00386-7
Zhong Y, Deng Q, Zhang P, Wang J, Wang R, Zeng Z, Deng S (2019) Sulfonic acid functionalized hydrophobic mesoporous biochar: design preparation and acid-catalytic properties. Fuel 240:270–277. https://doi.org/10.1016/j.fuel.2018.11.152
Pardo Cuervo OH, Simeonov SP, Peixoto AF, Popova MD, Lazarova HI, Romanelli GP, Martínez JJ, Freire C, Afonso CAM (2019) Efficient continuous production of the biofuel additive 5-(t-butoxymethyl) furfural from 5-hydroxymethylfurfural. Energ Technol 7:1900780. https://doi.org/10.1002/ente.201900780
Páez A, Rojas HA, Portilla O, Sathicq G, Afonso CA, Romanelli GP, Martínez JJ (2017) Preyssler heteropolyacids in the self-etherification of 5-hydroxymethylfurfural to 5,5′-[oxybis (methylene)]bis-2-furfural under mild reaction conditions. ChemCatChem 9:3322–3329. https://doi.org/10.1002/cctc.201700457
Cousin E, Namhaed K, Pérez Y, Cognet P, Delmas M, Hermansyah H, Gozan M, Alaba PA, Aroua MK (2022) Towards efficient and greener processes for furfural production from biomass: a review of the recent trends. Sci Total Environ 847:157599. https://doi.org/10.1016/j.scitotenv.2022.157599
Ricciardi L, Verboom W, Lange JP, Huskens J (2021) Selectivity switch by phase switch–the key to a high-yield furfural process. Green Chem 23:8079–8088. https://doi.org/10.1039/D1GC01752G
Lu Q, Yan D, Wu P, Chen L, Yagoub AEA, Ji Q, Yu X, Zhou C (2022) Ultrasound-NATDES/DMSO system for corn straw biomass conversion into platform compounds. Renew Energy 190:675–683. https://doi.org/10.1016/j.renene.2022.03.154
Tashrifi Z, Khanaposhtani MM, Larijani B, Mahdavi M (2020) Dimethyl sulfoxide: yesterday’s solvent, today’s reagent. Adv Synth Catal 362:65–86. https://doi.org/10.1002/adsc.201901021
Wang Y, Len T, Huang Y, Taboada AD, Boa A, Ceballos C, Delbecq F, Mackenzie G, Len C (2017) Sulfonated sporopollenin as an efficient and recyclable heterogeneous catalyst for dehydration of d-xylose and xylan into furfural. ACS Sustain Chem Eng 5:392–398. https://doi.org/10.1021/acssuschemeng.6b01780
Ji R, Jiang L, Yin D, Lv F, Yu S, Li L, Liu S, Wu Q, Liu Y (2022) Core-shell catalyst WO3@mSiO2-SO3H interfacial synergy catalyzed the preparation of furfural from xylose. Mol Catal 530:112592. https://doi.org/10.1016/j.mcat.2022.112592
Amarasekara AS, Williams LD, Ebede CC (2008) Mechanism of the dehydration of d-fructose to 5-hydroxymethylfurfural in dimethyl sulfoxide at 150 C: an NMR study. Carbohydr Res 343(18):3021–3024. https://doi.org/10.1016/j.carres.2008.09.008
Dai Y, Yang S, Wang T, Tang R, Wang Y, Zhang L (2022) High conversion of xylose to furfural over corncob residue-based solid acid catalyst in water-methyl isobutyl ketone. Ind Crop Prod 180:114781. https://doi.org/10.1016/j.indcrop.2022.114781
Zailai X, Siyuan T, Fan L, Dan W, Dang Sheng S, Wei Q (2018) Hydration of phenylacetylene on sulfonated carbon materials: active site and intrinsic catalytic activity. RSC Adv 8:38150–38156. https://doi.org/10.1039/C8RA07966H
Obando M, Cardenas V, Montaño D, Casanova H, Giraldo L, Cardona W, Rosero-Moreano M (2016) Arcillas naturales funcionalizadas con líquidos iónicos para microextracción de Ocratoxina A por membrana hueca empacada. Sci Chromatogr 8:129–136. https://doi.org/10.4322/sc.2016.024
Alekseev SA, Zaitsev VN, Fraissard J (2006) Organosilicas with covalently bonded groups under thermochemical treatment. Chem Matt 18:1981–1987. https://doi.org/10.1021/cm052776a
Cai J, He Y, Yu X, Banks SW, Yang Y, Zhang X, Bridgwater AV (2017) Review of physicochemical properties and analytical characterization of lignocellulosic biomass. Renew Sust Energ Rev 76:309–322. https://doi.org/10.1016/j.rser.2017.03.072
Isikgor FH, Becer CR (2015) Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers. Polym Chem 6:4497–4559. https://doi.org/10.1039/C5PY00263J
Nabors MW (2006) Introducción a la Botánica. Pearson Educación, Madrid-España
Banu Jamaldheen S, Kurade MB, Basak B, Yoo CG, Oh KK, Jeon B, Kim TH (2022) A review on physico-chemical delignification as a pretreatment of lignocellulosic biomass for enhanced bioconversion. Bioresour Technol 346:126591. https://doi.org/10.1016/j.biortech.2021.126591
Baruah J, Nath BK, Sharma R, Kumar S, Deka RC, Baruah DC, Kalita E (2018) Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front Energ Res 6:141. https://doi.org/10.3389/fenrg.2018.00141
Zoghlami A, Paës G (2019) Lignocellulosic biomass: understanding recalcitrance and predicting hydrolysis. Front Chem 7:874. https://doi.org/10.3389/fchem.2019.00874
Yiin CL, Yap KL, Ku AZE, Chin BLF, Lock SSM, Cheah KW, Loy ACM, Chan YH (2021) Recent advances in green solvents for lignocellulosic biomass pretreatment: potential of choline chloride (ChCl) based solvents. Bioresour Technol 333:125195. https://doi.org/10.1016/j.biortech.2021.125195
Yang T, Li W, Ogunbiyi AT, An S (2021) Efficient catalytic conversion of corn stover to furfural and 5-hydromethylfurfural using glucosamine hydrochloride derived carbon solid acid in Ƴ-valerolactone. Ind Crop Prod 161:113173. https://doi.org/10.1016/j.indcrop.2020.113173
Lê HQ, Ma Y, Borrega M, Sixta H (2016) Wood biorefinery based on γ-valerolactone/water fractionation. Green Chem 18:5466–5476. https://doi.org/10.1039/C6GC01692H
Chio C, Sain M, Qin W (2019) Lignin utilization: a review of lignin depolymerization from various aspects. Renew Sustain Energy Rev 107:232–249. https://doi.org/10.1016/j.rser.2019.03.008
Acknowledgements
We thank Vicerrectoría de Investigaciones-Universidad Pedagógica y Tecnológica de Colombia for the financial support. We extend our thanks to the Departamento de Química e Bioquímica, Faculdade de Ciências, Universidade do Porto, for providing us with some tested catalysts and technical advice.
Funding
Open Access funding provided by Colombia Consortium. The partial financial support was received from Vicerrectoría de Investigaciones-Universidad Pedagógica y Tecnológica de Colombia under internal Project SGI 3313.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Andreía Peixoto contributed with the synthesis and characterization of some catalysts. Material preparation, data collection, and analysis were performed by Oscar H. Pardo Cuervo, Cristian F. González, Hugo A. Rojas, José J. Martínez, and Gustavo P. Romanelli. The first draft of the manuscript was written by Oscar H. Pardo Cuervo, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
This declaration is not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 25 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pardo Cuervo, O.H., Gonzalez, C.F., Rojas, H.A. et al. Increasing furfural production from xylose and directly obtaining it from corn residues using Preyssler heteropolyacid. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04707-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04707-7