Abstract
In this study, the effects of combined stress factors involving light intensity and salinity (NaCl, MgCl2, CaCl2, and their combinations) on the two-stage cultivation of Chlorella vulgaris for simultaneous production of biomass and high-value products, were investigated. The two-stage strategies comprised a 25-day vegetative stage in BG11 medium, followed by a 15-day combined stress stage. During salt stress conditions, the addition of 15 g L−1 CaCl2 or 7.5 g L−1 MgCl2 / 7.5 g L−1 CaCl2 mixture with 140 µmol m−2 s−1 light intensity significantly promoted the growth of C. vulgaris achieving maximum biomass productivity of 50.50 ± 0.50 and 50.25 ± 3.25 mg L−1 d−1, respectively. Cultivation of C. vulgaris in a medium containing 7.5 g L−1 NaCl/ 7.5 g L−1 CaCl2 had remarkably increased the lipid content (31.15 ± 1.18%) and lipid productivity (14.55 ± 1.48 mg L−1 d−1). The saturated fatty acids (SFAs) at 39.52–59.29%, monounsaturated fatty acids (MUFAs) at 27.16–35.47%, and polyunsaturated fatty acids (PUFAs) at 7.18–29.97%, were obtained with palmitic (C16:0), oleic (C18:1), stearic (C18:0), and linolenic (C18:3) acids as predominant fatty acids. Cultures supplemented with 5 g L−1 NaCl / 5 g L−1 MgCl2 / 5 g L−1 CaCl2 and high light intensity exposure attained consistently high carbohydrate content (52.71 ± 2.50%). The combination of 7.5 g L−1 NaCl / 7.5 g L−1 MgCl2 also resulted in a marked increase in the protein content (35.32 ± 2.20%) and total carotenoids (0.31 ± 0.03 μg mL−1) as compared to the Controls. The highest antioxidant activity (86.16%) was achieved with a 7.5 g L−1 NaCl / 7.5 g L−1 CaCl2 combination in the growth stage. The antioxidant activities were attributed to the presence of phenolics, flavonoids, and tannins due to the stressed conditions. One of the key benefits of using a combined stress strategy in this study is that if one factor has a low impact on enhancing target metabolites, other factors can compensate.
Similar content being viewed by others
1 Introduction
Microalgae have great potentials to become the major sources of high-value compounds such as antioxidants, carbohydrates, pigments, lipids, and proteins. This can be attributed to their excellent photosynthetic efficiency, high growth rate, shorter harvesting cycle, and the great prospect for optimization of low-cost medium and cultivation strategy. However, in terms of commercialization, microalgal biodiesel production is still far off from wide and large-scale implementation. These are primarily determined by the biomass productivity and cellular lipid content of microalgae. Microalgae typically had a lipid content of 1–70% but may increase to 90% with optimal growth conditions [1]. Lipid productivity aside, other microalgal biocompounds such as carbohydrates, intracellular proteins, and antioxidants can be commercially exploited as drugs, food additives, or functional food components. These flexibilities make microalgae advantageous as raw materials for the production of biodiesel and other high-value compounds.
Generally, the conditions required to improve biomass productivity may not trigger the formation of targeted metabolites, while the stressed conditions to elevate secondary metabolite production may inhibit cell growth [2, 3]. Single stress factors including nutritional factors (e.g., nitrogen, phosphorus, carbon source) and environmental factors (temperature, light intensities, pH, and salinity) can induce the production of carotenoids, lipids, or carbohydrates [4, 5]. Theoretically, high biomass and high bioproducts could improve the economics of the bioprocess. Two-stage cultivation strategy (TSCS) is best suited to overcome this limitation of using single medium and single-stage cultivation [3]. The TSCS provides optimal conditions for biomass production in the first stage and stress conditions for targeted compound accumulation in the second stage [2, 3]. The transition from the first to the second stage may involve changes in growth mode (photoautotrophy, heterotrophy, mixotrophy), operation (batch, semi-batch, fed-batch, and continuous), physiochemical factors (nutrients, temperature, light intensity, pH and salinity), and cultivation system (open or close systems) [6]. There are several studies on the application of TSCS for microalgal biomass as feedstock for biodiesel production, but limited reports have addressed its potential for high-value compounds [2, 3, 6].
In some microalgae species, integrating two or more types of stressors is not only useful but pertinent [7]. The combination of the two-stress factors has been reported to improve lipid productivity (25–54%), higher than the single-stress factor [8]. Incorporating light intensity and high salinity as stressors affect not only cell growth and lipid accumulation, but also the fatty acid profile [9]. In the stress stage, C. vulgaris vegetative cells exposed to light intensity and high salt stress attain elevated lipid content, with a modified fatty acid profile [10]. Salt stress elevates the reactive oxygen species (ROS) resulting in increased rates of energy production, biopolymer and lipid biosynthesis, and alter membrane permeability due to ion homeostasis disruption [11]. Antioxidant compounds such as phenols, flavonoids, and carotenoids may get accumulated to quench the increased free radicals [10].
The addition of NaCl has been reported to increase lipid accumulation in microalgal species such as C. vulgaris [10], Desmodesmus abundans [9], and C. reinhardtii [12], while salts’ ions such as magnesium (Mg2+) and calcium (Ca2+) could affect lipid accumulation [13, 14]. However, too high amount of stress factors, above the limit of tolerance may inhibit cell growth and induce cell death [15]. There is a dearth of reports on the influence of various salts and/or their combinations on gene expression and lipid accumulation. The application of two salt stresses, NaCl / CaCl2, has resulted in an increase of 3.5-fold in the lipid content (73.4%), and 2.1-fold in productivity (10.9 mg L−1d−1) of C. reinhardtii, as compared to salt-free conditions [16]. To the best of our knowledge, no research has clearly reported the combination of more than two salt stressors and their effects on the simultaneous production of valuable products during TSCS of microalgal culture.
In this study, the effects of combined stress factors involving high light intensity, and a single or a combination of different salt stressors, NaCl, MgCl2, and CaCl2, were investigated based on TSCS for C. vulgaris cultivation. The co-production of lipids for biodiesel, carbohydrates, proteins, carotenoids, and antioxidant compounds was determined. The kinetics of cell growth and lipid production were evaluated, and the fatty acids profile was analyzed.
2 Materials and methods
2.1 Chemicals and reagents
Pure hexane, chloroform, ethanol, toluene, methanol, and sulphuric acid were purchased from Merck Co. (Darmstadt, Germany). Other chemicals were of pure grade and were purchased from Sigma–Aldrich (St. Louis, MO, USA).
2.2 Microalgal culture conditions
C. vulgaris was cultured in BG11 medium [17], under fluorescent white light (Philips, TLD18W/ 54–765) at 40 μmol photons m−2 s −1, 25 ± 1 °C and pH 7 ± 1 with constant bubbling of air (filtered through a 0.22 μm microporous filter) throughout the experiments.
2.3 Combination of salt stress and light in TSCS
The experimental design and culture conditions of the TSCS of C. vulgaris based on combined stress factors (salt stress and light) are shown in Table 1 and Fig. 1. C. vulgaris was inoculated into BG-11 medium (10% of the total growth medium) in 1 L conical flasks, and incubated under the aforementioned conditions for 25 days (vegetative phase). In the salt-stress phase, the vegetative cells were divided into 2 groups: (i) Group 1, the cultures were maintained under the same conditions for 15 days (Control 1). The cultures were moved to grow under 140 μmol photons m−2 s −1 light intensity for 15 days Control 2 (light stress). (ii) Group 2, the cultures were exposed to 140 μmol photons m−2 s −1 with single salt (15 g L−1 NaCl or 15 g L−1 MgCl2 or 15 g L−1 CaCl2) and designated as C1/2/3; or combination of two salts (7.5 g L−1 NaCl / 7.5 g L−1 MgCl2 or 7.5 g L−1 NaCl / 7.5 g L−1 CaCl2 or 7.5 g L−1 MgCl2/7.5 g L−1 CaCl2) and designated as C4/5/6; or combination of three salts (5 g L−1 NaCl/ 5 g L−1 CaCl2/5 g L−1 MgCl2) and designated as C7. All flasks were incubated for 15 days at 25 ± 1 °C and 140 μmol photons m−2 s −1 with constant bubbling of air (filtered through 0.22 μm filter).
2.4 Cell growth measurements
The cell density was measured by OD680 nm spectrophotometrically (T60 UV/Vis spectrophotometer, PG instruments, UK) in both the vegetative and stress stages based on 3 mL sample removal every 5 days. The dry weight (DW) was determined gravimetrically at the beginning and the end of the cultivation period. After centrifugation of 20 mL of culture, the pellets were collected and washed two times using deionized water, dried overnight at 60 °C, and then cooled and weighed. The biomass productivity (BP, mg L−1 day−1) and biomass yield (BY, mg L−1) were determined according to Equations as follows: BP = (Xf − X0)/t, BY = (Xf − X0), where Xf and X0 are the final and initial biomass concentrations (g L−1), respectively; t is the duration of the run (day) [18].
2.5 Biomass composition
Dried C. vulgaris biomass samples were ground using a mortar into powder. The samples were scanned, and recorded in triplicate using Near-infrared (NIR) spectroscopy (DA1650, FOOS, Denmark) located at Central Laboratory, Faculty of Agriculture, Al-Azhar University, Cairo, Egypt. Lipids, total carbohydrates, protein, moisture, fibers, and ash contents of C. vulgaris were determined.
2.6 Lipid extraction and fatty acids profile
Lipids were extracted based on the modified protocol [19]. Dried biomass (0.5 g) was added to a 1:1 ratio (v/v) mixture of chloroform and methanol. Deionized water was added to achieve the final ratio of chloroform, methanol, and water at the 1:1:0.9 ratio (v/v). The lipid-containing chloroform layer was separated, and the solvent was evaporated (40–45 °C). The extracted lipids were weighed to calculate the lipid content on the basis of the cell DW: L = (WL/ WB) *100, where L is the lipid content (%); WL and WB are the weights of the extracted lipids (mg L−1) and the dry biomass (mg L−1), respectively. The lipid productivity (LP) was calculated as follows [20]: LP = BP*L, where LP is the lipid productivity (mg L−1 day−1), BP is the biomass productivity (mg L−1 day−1), and L is the lipid content (% dry weight). The lipid yield was determined as follows [21]: LY = BY *L, where LY is the lipid yield (mg L−1), biomass yield, BY (g L−1), and lipid content, L (% dry weight).
Extracted lipids were transesterified according to a previous study [22]. The fatty acid analysis was performed using Gas Chromatography with an FID detector (300° C) and BPX capillary column (60.0 m × 320 µm) under air (400 mL min−1) and H2 (35.5 mL min−1) (Agilent 6890, Model G1530A, USA). The oven temperature was initially programmed at 120 °C for 1 min, then increased to 210 °C at a rate of 8°/min, then raised to 225 °C at 2°/min and stayed for 5 min. Nitrogen was the carrier gas at a 3.5 mL min−1 flow rate. The injector temperature was 250 °C with a split ratio of 20:1.
2.7 Pigments extraction and quantification
The pigments (chlorophylls and carotenoids) were extracted according to a previous report [23]. Briefly, 10 mL samples were centrifuged at 2,500 rpm for 5 min and the supernatant was discarded. The cells were re-suspended in 10 mL methanol (96%) and were homogenized at 1,000 rpm for 1 min. The homogenate was centrifuged at 3,000 rpm for 10 min. The supernatant was collected and the absorbance was recorded at 653, 666, and 470 nm, the concentration of pigments were calculated using the equations below:
where A653, A666, and A470 nm are the absorbance at the indicated wavelength
2.8 Algal extracts preparation
One gram of freeze-dried microalgal biomass was extracted with a mixture of methanol/ methylene chloride at the 1:1 ratio (v/v). The algal biomass to solvent ratio was 1:50 (w/v), and the extraction was conducted in an ultrasonic bath (SONICS, USA) with a frequency of 20 kHz and ultrasonic power of 400 W. The supernatant was collected, filtered through filter paper, and evaporated using a rotary vacuum evaporator (40–45 ˚C)0.2.9
2.9 Antioxidant activity
The 2,2-Diphenyl-1-picrylhydrazyl (DPPH) solution in methanol (0.16 mM) was used to estimate the free-radical scavenging activity of crude extracts. Two mL of DPPH, considered as a Control, was mixed with 2 mL of crude extracts or the standard antioxidants (Vitamin C and butyl hydroxyl toluene (BHT)) at 250 µg mL−1 concentration. The reaction mixture was held at room temperature for 30 min in the dark [24]. The absorbance was later measured at 517 nm, and the radical scavenging activity was calculated:
where, At and Ac represent the absorbance of the samples and the DPPH control, respectively.
2.10 Determination of total phenolics, flavonoids, and tannins contents
The phenolic contents in crude extract were measured using the method as described before [25] and represented as gallic acid equivalent/g DW (GAE/g DW). One hundred µl aliquot of sample was mixed with 2 mL of 2% Na2CO3 and allowed to stand for 2 min at room temperature. After incubation, 100 µl of 50% Folin-Ciocalteu’s phenol reagent was added, mixed thoroughly, and left to stand for 30 min in the dark. Absorbance was determined at 720 nm.
The flavonoids contents in the algal extracts were determined using the spectrophotometric method [26]. Briefly, 1 mL of 2% AlCl3 solution dissolved in methanol was added to 1 mL extract and incubated for an hour at room temperature. The absorbance was determined at ʎmax = 415 nm. The same procedure was repeated for the standard solution of Rutin and the calibration line was constructed. Based on the measured absorbance, the content of flavonoids in extracts was expressed in terms of Rutin equivalent (mg of rutin/g DW).
The tannin content was determined based on the vanillin hydrochloride method [27]. In brief, 1 mL of algal extract was mixed with 5 mL vanillin hydrochloride reagent. The absorbance was measured at 500 nm after 20 min. The amount of tannic acid was analyzed from the standard curve (20–100 mg tannic acid) and represented as tannic acid equivalent/g DW [27].
2.11 Statistical analysis
The experiments were carried out in three replicates. The significant difference of variables was determined using one-way ANOVA with 95% confidence (probability limit of p < 0.05). Tukey’s test was used to identify the differences between each level of treatment. The statistical analyses were performed using Minitab software (V18, Minitab Inc., USA).
3 Results and Discussion
3.1 The effects of combined salt and light factors on cell growth of C. vulgaris
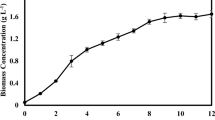
High salinity and light intensity are essential environmental stressors to enhance lipids, carbohydrates, carotenoids, or bioactive chemicals in marine and freshwater microalgae [10, 12]. In this study, the TSCS involved a 25-day vegetative stage without salt stress with light exposure of 40 µmol m−2 s−1, followed by a 15-day stress stage with salt in combination with a high light intensity of 140 µmol m−2 s−1 (Table 2 and Fig. 2). Figure 2 A, B shows that all cultures exhibited significant differences (p < 0.05) in the growth rate under different salt stresses. The growth parameters, as shown in Table 2, were significantly (p < 0.05) affected by the variation of salt type in the medium. The highest biomass productivity and yield were obtained in the cultures supplemented with CaCl2 (C3) or a mixture of MgCl2/CaCl2 (C6) and exposed to 140 μmol m−2 s−1 light intensity, as compared to the Controls. On the other hand, the lowest biomass productivity and yield were those grown in a medium with a combination of NaCl/ MgCl2/ CaCl2 (C7) salts and exposed to high light intensity (Table 2).
The significant accumulation of biomass observed despite the growth rate inhibition could be attributed to the size of microalgal cells and organelles (mitochondria, chloroplast, vacuole, etc.) even under salt stress [28]. Furthermore, the accumulation of osmoprotectants molecules such as glycerol, proteins, proline, etc. regulates the osmotic pressure caused by NaCl and inhibits water losses [29]. For example, the biomass productivity of Chlorella sp. TLD6B increases significantly from 22.0 mg L−1 d−1 to 27.7 mg L−1 d−1 after the addition of 200 mM NaCl [30]. Lower NaCl levels (e.g., 25 mM) can also promote the growth of the freshwater microalgae Chlamydomonas mexicana and Scenedesmus obliquus [31], while the levels of 50 mM or 100 mM NaCl improve the cell growth of C. reinhardtii [16]. The addition of 5 g L−1 MgCl2 to the TSCS of C. vulgaris with exposure to 140 mol m−2 s−1 light intensity for 20 days promotes growth [10]. However, the combinations of NaCl (50–200 mM) and CaCl2 (100 mM) significantly suppress C. reinhardtii cell growth [16].
3.2 Effects on the biomass composition
The composition of C. vulgaris biomass cultivated under combined stress factors (salts and light) in TSCS was analyzed using Near-Infrared Spectroscopy (NIRS) (Table 3). The salt stressors (type and combination effect), and light intensity were clearly shown to significantly affect (p < 0.05) the production of targeted products. The highest lipid content (31.15 ± 1.18% and 30.59 ± 0.60%), and lipid productivity (14.55 ± 1.48 mg L−1 d−1 and 13.72 ± 0.69 mg L−1 d−1) were achieved when the cultures were transferred and exposed to 140 μmol photons m−2 s−1 light intensity, with 7.5 g L−1 NaCl and 7.5 g L−1 CaCl2 (C5) or 15 g L−1 NaCl (C1) addition, respectively, for 15 days (Table 2 and 3).
The lipid content of microalgal cells can be increased by a single inducer, such as high salinity [32], high light intensity [33], or by combining several stress factors [34]. Combining the triggers such as nutrient starvation and physical stressors, during the cultivation period, typically results in hyper-accumulation of lipid content [35, 36]. Furthermore, combined stress has a significant advantage wherein if a particular factor has a low impact on improving desired metabolites, other factors can compensate [3]. The obtained results demonstrated the synergistic effect of salt combinations and light intensity as has also been reported in other microalgal species. In Chlamydomonas reinhardtii, exposure to combinatorial treatment of 100 mM NaCl/100 mM CaCl2 and 80 μmol photons m−2 s −1 resulted in a significant increase in lipid content and productivity, which were 3.5- and 2.1-fold higher, respectively, than the salt-free control conditions. This treatment also upregulated the expression of key enzymes such as glycerol-3-phosphate dehydrogenase (GPDH), lysophosphatidic acid acyltransferase (LPAAT), and diacylglycerol acyltransferase (DAGAT), all of which play a crucial role in lipid accumulation. These findings suggest that combinatorial treatment with NaCl/CaCl2 and light intensity could be a promising strategy for enhancing lipid production in C. reinhardtii [16]. The accumulation of particularly neutral lipids contributes towards preserving membrane integrity in response to salt stress, which could cause a reduction in cell membrane osmotic pressure and fluidity [37]. Salt such as Ca2+ ion regulates numerous metabolic pathways and is involved in various signaling pathways in microalgae [38], which could have promoted cellular lipid production [14], to meet the demand for high energy consumption and storage during stressed conditions.
Table 3 shows the highest carbohydrate content (52.71 ± 2.50%) was obtained in C7 where the culture was subjected to a combination of NaCl, MgCl2, and CaCl2 during the stress stage for 15 days. Carbohydrate accumulation in response to salt stress has been observed in a variety of microalgal strains including Chlorella sp., Dunaliella sp., Chlamydomonas sp., Mesotaenium sp., Scenedesmus quadricauda, and Tetraedron sp. [39]. The semipermeable cell membrane serves as a barrier between the cytoplasm and the external environment, and it is capable of adapting to changes in salinity, particularly in relation to carbohydrates and lipids. Carbohydrates are crucial in regulating the transfer of cations and maintaining a low concentration of Na+ in the cytoplasm when exposed to saline media [39]. However, there was a reduction in the carbohydrate content and an increase in the lipid content of C. vulgaris in the C1 and C5 treatments. This could be a result of the carbohydrates-to-lipid switch. The significant reduction in lipid content (p < 0.05) upon culturing with NaCl / MgCl2 (C4) or MgCl2 / CaCl2 (C6) or NaCl / MgCl2/ CaCl2 (C7) was most probably due to the antagonistic effects between salts. Additionally, high salinity combined with light intensity cause an increase in cellular respiration activity, which leads to the degradation of energy-rich storage molecules such as lipids and starch in some microalgae [40]. During salt stress, microalgae utilize carbohydrates as osmoprotectants to regulate homeostasis and ensure osmotic adjustment [41], and help cells to adapt [15]. Hence, the lipid content of microalgae can be enhanced through a metabolic switch from starch-to-lipid synthesis, in which intracellular energy is consumed utilizing the lipids in the storage synthesized from starch [42].
Unfavorable conditions such as nutrient depletion or excessive salinity may cause microalgae to accumulate more lipids and carbohydrates than proteins [16]. The physiological disorders under high salinity could have removed K+ ions, a crucial element in protein synthesis [13]. The protein content of C. vulgaris in the present study varied from 19.18 to 35.32% under different salt stresses. The highest protein content was observed under combined stress conditions (C4 and C3) compared to single inducer (Control 2) (Table 3). These variations suggest that microalgae may respond to salt stress and high light intensity by increasing the soluble proteins, instead of soluble carbohydrates [43]. Chlorella salina exhibited an increase in both soluble and total carbohydrates with the rise in salinity levels. This was accompanied by a corresponding increase in both soluble and total proteins, which could be due to an improvement in the photosynthesis rates. One of the possible reasons behind this may be under high salinity stress an accumulation in free amino acids and proline contents in C. vulgaris compared to the control was observed. The percent increase was 21.74 and 64.5% over the control, respectively at 0.8 M NaCl [43]. The lowest protein content (19.75 ± 1.40%) was recorded under C1 condition. The physiological disorders under high salinity could have removed K+ ions, a crucial element in protein synthesis [13]. During salinity stress, higher plants and macroalgae have been observed to down-regulate genes that are associated with primary metabolism and protein synthesis, as well as activation of genes related to autophagy and protein degradation [44]. Other cellular components such as ash content indicated that the inorganic matter including the minerals was comparable to a previous report (6.3 -15.8% dry weight) [45]. The highest fiber contents were achieved in C1 and C3 (11.87% and 11.18%, respectively), whilst moisture content ranged from 9.35–14.55% (Table 3).
The profile of the high lipid cultures (C1, C3, C5) showed the presence of 13 identified fatty acids (Table 4). The fatty acid methyl esters (FAMEs) contained saturated (SFAs) (39.52–59.29% of total FAME), monounsaturated (MUFAs) (27.16–345.74%), and polyunsaturated fatty acids (PUFAs) (7.18–29.97%) with the chain lengths from C14 to C24, as shown in Fig. 2. The predominant component is palmitic acid (C16:0, 24.99–35.90%), followed by oleic acid (C18:1, 20.67–29.02%), linolenic acid (C18:2, 4.70–16.34%), and stearic acid (C18:0, 3.07–12.59%). The content of the other fatty acids was relatively low. Oleic acid and palmitic acid produced from aerobic desaturation and chain elongation act as precursors of membrane glycerolipids [20]. Oleic acid serves as the main product of De novo fatty acid synthesis which produces omega 3 such linolenic acid C18:3, eicosapentaenoic acid C20:5 and etc. This class of FA play a major role in biotic and abiotic stress responses [46]. The highest SFAs content (59.29%) was achieved at the end of the vegetative stage, followed by Control 1 (44.62%); and Control 2 (42.76%). Control 2 had similar conditions to Control 1, but the culture was exposed to higher light intensity (Fig. 3). There was a noticeable increase in MUFA for C1 (35.74%), C3 (30.52%), and C5 (30.46%). The PUFAs also recorded a marked increase in all optimal salt stress conditions, reaching a maximum in C5 (29.97%), and C3 (29.85%) as compared to Controls (7.18–28.16%).
Fatty acid components of the highest lipid content from C. vulgaris biomass cultured under salt-stress conditions in a two-stage cultivation strategy. SFAs, saturated fatty acids (C14:0–C 24:0); UFAs, unsaturated fatty acids (C16:1–C 20:1); MUFAs, monounsaturated fatty acids (C16:1–C20:1); and PUFAs, polyunsaturated fatty acids (C18:2–C18:3)
Combining high salinity and light intensity as stressors not only affects cell growth and lipid, but also the fatty acid composition [9]. The increase in UFAs at the expense of SFAs in this study can be considered as a microalgae response to preserve the membrane from salt alteration. Generally, salt stress causes membrane degradation, resulting in the alteration of membrane permeability, integrity, fluidity and ion transport selectivity [47]. Several studies reported the crucial role of UFAs in adaptation and tolerance responses to salt stress by protecting the plasma membrane and the photosynthetic machinery [48]. For biodiesel production, a high level of lipid and triacylglycerol (TAG), with a balanced fatty acid composition should be ideally attained. The long-chain fatty acids (C16–18) are preferable as the increase in carbon chain length leads to an increase in the biodiesel properties such as heat of combustion, cetane number, and viscosity [49]. The C16-C18 content (84–91%) of C. vulgaris under C1, C3, and C5 conditions is markedly higher than in Scenedesmus obliquus CNW-N (67–86%) [50], and Haematococcus pluvialis (76.6%) [51]. Based on the European Standard (EN 14214) for biodiesel, the PUFAs (≥ 3 double bonds) should be 1%, which could affect positively the properties of biodiesel [52]. C. vulgaris is a promising feedstock for biodiesel production as it has demonstrated a considerable amount of C18:2 and C18:3, resulting in low melting points, which are therefore appropriate for low-temperature biodiesel [53].
3.3 Effects on chlorophyll a and carotenoids production
Figure 4 shows the chlorophyll a and carotenoid production in both vegetative and stress stages of all cultures. During the vegetative stage, chlorophyll a and carotenoid concentrations were simultaneously increased. The accumulation of chlorophyll is favored under conditions that are optimal for cell growth, which is consistent with their role in photosynthesis [54]. In the stress stage (carotenogenesis), the total carotenoid concentrations increased under all salt stress and light conditions, while chlorophyll declined in all cases except in Control 1. The reduction of Chl a may be an oxidative stress symptom related to increased chlorophyllase activity promoting Chl a degradation [55]. It may also be related to decrease in Rubisco activity due to the low CO2 uptake [56]. It is reported that salt stress negatively affects carbon fixation and carbon concentrating mechanisms in C. reinhardtii which is required for CO2 availability for Rubisco [56]. Pandit et al. [15] related this chlorophyll reduction to osmotic and toxic ionic stress, which causes a decrease in photosynthetic rate and consequently lowers Chl and protein content.
The highest carotenoid level (0.31 ± 0.03 μg mL−1) was observed for C. vulgaris cultured in a medium supplemented with NaCl/MgCl2 and exposed to high light intensity (C4). The elevation of chlorophyll a and carotenoid levels clearly indicated the synergistic effect of the salt combination. Increased salinity and light intensity are important stimuli for the production of pigment [10, 12, 57]. Higher levels of carotenoids are synthesized as a defence mechanism to counter increased ROS levels under stress conditions. A metabolic pathway mediated by abscisic acid [58], may be enhanced with increased salt content and combination. The higher the abscisic acid content, the greater will be the counter-response to the stressors, resulting in higher carotenoids production [59]. Salt stress has also been reported to down-regulate the photosystem I and II genes in C. reinhardtii after the treatment with 200 mM NaCl [16]. These appear to support the cell growth inhibition and reduction in chlorophyll level in C. vulgaris as observed during the salt stress stage (Fig. 2 and 4).
3.4 Antioxidant activity of C. vulgaris extracts cultured under combined stress factors
As shown in Fig. 5, the highest antioxidant activities were observed, in C5 (86.16 ± 2.32%) and C1 (71.6 ± 1.45%) conditions, and were only slightly lower than the standards BHT (87.63 ± 1.55%) and vitamin C (88.8 ± 1.8%). The C6 and Control 1 conditions exhibited the lowest activities (61.05 ± 0.98%, 63.8 ± 1.98%), and were almost comparable to C2 and C7. The IC50 value (14.23 μg mL−1) of C5 extract which was close to the standard antioxidants (BHT, 11.2 μg mL−1 and vitamin C, 12.9 μg mL−1), suggests the feasibility of attaining strong antioxidant activity with TSCS. Phytochemical screening showed the presence of considerable amounts of phenolic compounds (46.79 ± 0.98 mg Gallic Acid Equivalent/g DW), flavonoids (34.56 ± 1.34 mg of Rutin/ g DW), and tannins (6.12 ± 0.12 mg tannic Acid Equivalent/ g DW) in the extract of C5 (Fig. 6). The abundance of unsaturated bonds and hydroxyl groups in chemical extracts has been attributed to their ability to scavenge free radicals and inhibit oxidation [60, 61]. Cultivation of C. vulgaris in a medium containing 5 g L−1 NaCl or MgCl2, and with exposure to high light intensity, led to enhanced antioxidant activities (65–79%) as well as the production of antioxidant phytochemicals [10].
The results of our study proved the great potential of TSCS of C. vulgaris based on combinations of NaCl, MgCl2, and CaCl2 and light intensity to be further optimized as a cost-effective approach for high microalgal biomass density with simultaneous production of lipid, carbohydrates, protein, carotenoid, and antioxidant compounds. Combining different stimuli during the cultivation of microalgae usually results in the hyper-accumulation of target product. Thus, an evaluation of the synergic effects of the stimuli as well as the impact of each stress factor on the product-induction process is necessary. Furthermore, the feasibility of producing target metabolites through two-stage cultivation would require cost-effective cultivation systems, efficient downstream processes, and the integration of nutrient recovery from waste streams. To assess the feasibility of implementing these strategies, comprehensive techno-economic analysis and life cycle assesemsnt (LCA) studies are necessary on a case-by-case basis.
4 Conclusions
Combination of salinity and light intensity as stressors in the TSCS of C. vulgaris had been shown as a potent strategy for the co-production of high density biomass, lipids for biodiesel, high-value products, and antioxidant compounds. The NaCl/CaCl2 combination with high light intensity markedly increased the lipid yield and accumulated significant amounts of esterifiable lipids, containing mainly C16-C18 fatty acid chains suitable as biodiesel feedstock. The combination of three salts enhanced total carbohydrate content, while the NaCl/MgCl2 combination increased the carotenoids content. The elevation of antioxidant compounds proved the cultural responses to the stresses from the combined effects of two or three salts with high light intensity. The approach formulated was viable for the simultaneous optimal production of bioenergy and valuable products from C. vulgaris.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
References
Ma X, Mi Y, Zhao C, Wei Q (2022) A comprehensive review on carbon source effect of microalgae lipid accumulation for biofuel production. Sci Total Environ 806:151387
Nagappan S, Devendran S, Tsai P-C, Dahms H-U, Ponnusamy VK (2019) Potential of two-stage cultivation in microalgae biofuel production. Fuel 252:339–349
Aziz MMA, Kassim KA, Shokravi Z, Jakarni FM, Liu HY, Zaini N, Tan LS, Islam ABMS, Shokravi H (2020) Two-stage cultivation strategy for simultaneous increases in growth rate and lipid content of microalgae: A review. Renew Sustain Energy Rev 119:109621
De Oliveira ML, Nunes de Mello F, VischiWinck F (2022) Characterization of the nuclear proteome of Chlamydomonas in response to salt stress. Phycology 2:280–296
Schüler LM, Schulze PS, Pereira H, Barreira L, León R, Varela J (2017) Trends and strategies to enhance triacylglycerols and high-value compounds in microalgae. Algal Res 25:263–273
Liyanaarachchi VC, Premaratne M, Ariyadasa TU, Nimarshana PHV, Malik A (2021) Two-stage cultivation of microalgae for production of high-value compounds and biofuels: A review. Algal Res 57:102353
Ho SH, Nakanishi A, Kato Y, Yamasaki H, Chang JS, Misawa N, Hirose Y, Minagawa J, Hasunuma T, Kondo A (2017) Dynamic metabolic profiling together with transcription analysis reveals salinity-induced starch-to-lipid biosynthesis in alga Chlamydomonas sp JSC4. Sci Rep 7:45471
Kwak HS, Kim JYH, Woo HM, Jin E, Min BK, Sim SJ (2016) Synergistic effect of multiple stress conditions for improving microalgal lipid production. Algal Res 19:215–224
Xia L, Rong J, Yang H, He Q, Zhang D, Hu C (2014) NaCl as an effective inducer for lipid accumulation in freshwater microalgae Desmodesmus abundans. Bioresour Technol 161:402–409
Ali HEA, El-fayoumy EA, Rasmy WE, Soliman RM, Abdullah MA (2021) Two-stage cultivation of Chlorella vulgaris using light and salt stress conditions for simultaneous production of lipid, carotenoids, and antioxidants. J Appl Phycol 33:227–239
Udayan A, Pandey AK, Sirohi R, Sreekumar N, Sang BI, Sim SJ, Kim SH, Pandey A (2022) Production of microalgae with high lipid content and their potential as sources of nutraceuticals. Phytochem Rev 23:1–28
Fal S, Aasfar A, Rabie R, Smouni A, Arroussi HEL (2022) Salt induced oxidative stress alters physiological, biochemical and metabolomic responses of green microalga Chlamydomonas reinhardtii. Heliyon 8:e08811
Chen Z, Cuin TA, Zhou M, Twomey A, Naidu BP, Shabala S (2007) Compatible solute accumulation and stress-mitigating effects in barley genotypes contrasting in their salt tolerance. J Exp Bot 58:4245–4255
Srivastava G, Nishchal Goud VV (2017) Salinity induced lipid production in microalgae and cluster analysis (ICCB 16-BR_047). J Exp Bot 242:244–252
Pandit PR, Fulekar MH, Karuna MSL (2017) Effect of salinity stress on growth, lipid productivity, fatty acid composition, and biodiesel properties in Acutodesmus obliquus and Chlorella vulgaris. Environ Sci Pollut Res 24:13437–13451
Hang LT, Mori K, Tanaka Y, Morikawa M, Toyama T (2020) Enhanced lipid productivity of Chlamydomonas reinhardtii with combination of NaCl and CaCl2 stresses. Bioprocess Biosyst Eng 43:971–980
Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G (1971) Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev 35:171–205
Vidyashankar S, VenuGopal KS, Swarnalatha GV, Kavitha MD, Chauhan VS, Ravi R, Bansal AK, Singh R, Pande A, Ravishankar GA, Sarada R (2015) Characterization of fatty acids and hydrocarbons of chlorophycean microalgae towards their use as biofuel source. Biomass Bioenergy 77:75–91
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Hempel N, Petrick I, Behrendt F (2012) Biomass productivity and productivity of fatty acids and amino acids of microalgae strains as key characteristics of suitability for biodiesel production. J Appl Phycol 24:1407–1418
Yang F, Long L, Sun X, Wu H, Li T, Xiang W (2014) Optimization of medium using response surface methodology for lipid production by Scenedesmus sp. Mar drugs 12:1245–1257
Christie WW (1993) Preparation of ester derivatives of fatty acids for chromatographic analysis. Adv lipid Methodol 2:e111
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592
Yen GC, Chen HY (1995) Antioxidant activity of various tea extracts in relation to their antimutagenecity. J Agric Food Chem 43:27–37
Taga MS, Miller EE, Pratt DE (1984) Chia seeds as a source of natural lipid antioxidants. J Am Oil Chem Soc 61:928–931
Quettier-Deleu C, Gressier B, Vasseur J, Dine T, Brunet C, Luyckx M, Trotin F (2000) Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J Ethnopharmacol 72:35–42
Broadhurst RB, Jones WT (1978) Analysis of condensed tannins using acidified vanillin. J Sci Food Agric 29:788–794
Sinetova MA, Sidorov RA, Medvedeva AA, Starikov AY, Markelova AG, Allakhverdiev SI, Los DA (2021) Effect of salt stress on physiological parameters of microalgae Vischeria punctata strain IPPAS H-242, a superproducer of eicosapentaenoic acid. J Biotechnol 331:63–73
Anand V, Kashyap M, Samadhiya K, Ghosh A, Kiran B (2019) Salinity driven stress to enhance lipid production in Scenedesmus vacuolatus: A biodiesel trigger? Biomass Bioenerg 127:105252
Li H, Tan J, Mu Y, Gao J (2021) Lipid accumulation of Chlorella sp TLD6B from the Taklimakan Desert under salt stress. Peer J 9:e11525
El-S S, Kim HC, Abou-Shanab RA, Ji MK, Oh YK, Kim SH, Jeon BH (2013) Biomass, lipid content, and fatty acid composition of freshwater Chlamydomonas mexicana and Scenedesmus obliquus grown under salt stress. Bioprocess Biosyst Eng 36:827–833
Gour RS, Garlapati VK, Kant A (2020) Effect of Salinity Stress on Lipid Accumulation in Scenedesmus sp. and Chlorella sp.: Feasibility of Stepwise Culturing. Curr Microbiol 77:779–785
He Q, Yang H, Hu C (2018) Effects of temperature and its combination with high light intensity on lipid production of Monoraphidium dybowskii Y2 from semi-arid desert areas. Bioresour Technol 265:407–414
Sun X, Cao Y, Xu H, Liu Y, Sun J, Qiao D, Cao Y (2014) Effect of nitrogen-starvation, light intensity and iron on triacylglyceride/carbohydrate production and fatty acid profile of Neochloris oleoabundans HK-129 by a two-stage process. Bioresour Technol 155:204–212
Guedes AC, Amaro HM, Malcata FX (2011) Microalgae as sources of carotenoids. Mar drugs 9:625–644
Singh L, Brennan TA, Russell E, Kim J-H, Chen Q, Johnson FB, Pignolo RJ (2016) Aging alters bone-fat reciprocity by shifting in vivo mesenchymal precursor cell fate towards an adipogenic lineage. Bone 85:29–36
Ji X, Cheng J, Gong D, Zhao X, Qi Y, Su Y, Ma W (2018) The effect of NaCl stress on photosynthetic efficiency and lipid production in freshwater microalga—Scenedesmus obliquus XJ002. Sci Total Environ 633:593–599
Chen H, Zhang Y, He C, Wang Q (2014) Ca2+ signal transduction related to neutral lipid synthesis in an oil-producing green alga Chlorella sp. C2. Plant cell Physiol 55:634–644
Arora N, Laurens LML, Sweeney N, Pruthi V, Poluri KM, Pienkos PT (2019) Elucidating the unique physiological responses of halotolerant Scenedesmus sp. cultivated in sea water for biofuel production. Algal Res 37:260–268
Yao CH, Ai JN, Cao XP, Xue S (2013) Salinity manipulation as an effective method for enhanced starch production in the marine microalga Tetraselmis subcordiformis. Bioresour Technol 146:663–671
Tietel Z, Wikoff WR, Kind T, Ma Y, Fiehn O (2020) Hyperosmotic stress in Chlamydomonas induces metabolomic changes in biosynthesis of complex lipids. Eur J Phycol 55:11–29
Kato Y, Ho S-H, Vavricka CJ, Chang J-S, Hasunuma T, Kondo A (2017) Evolutionary engineering of salt-resistant Chlamydomonas sp. strains reveals salinity stress-activated starch-to-lipid biosynthesis switching. Bioresour Technol 245:1484–1490
Farghl AM, Shaddad MA, Galal HR, Hassan EA (2015) Effect of salt stress on growth, antioxidant enzymes, lipid peroxidation, and some metabolic activities in some freshwater and marine algae. Egypt J Bot 55:1–5
Dittami SM, Gravot A, Renault D, Goulitquer S, Eggert A, Bouchereau A, Tonon T (2011) Integrative analysis of metabolite and transcript abundance during the short-term response to saline and oxidative stress in the brown alga Ectocarpus siliculosus. Plant cell Environ 34:629–642
Wan MWMA, Lorwirachsutee A, Theodoropoulos C, Gonzalez-Miquel M (2019) Polyol-Based Deep Eutectic Solvents for Extraction of Natural Polyphenolic Antioxidants from Chlorella vulgaris. ACS Sustain Chem Eng 7:5018–5026
Chen D, Yuan X, Liang L, Liu K, Ye H, Liu Z, Xue T (2019) Overexpression of acetyl-CoA carboxylase increases fatty acid production in the green alga Chlamydomonas reinhardtii. Biotechnol Lett 41:1133–1145
Gogna M, Choudhary A, Mishra G, Kapoor R, Bhatla SC (2020) Changes in lipid composition in response to salt stress and its possible interaction with intracellular Na+-K+ ratio in sunflower (Helianthus annuus L). Environ Exp Bot 178:104147
Rismani S, Shariati M (2017) Changes of the total lipid and omega-3 fatty acid contents in two microalgae Dunaliella salina and Chlorella vulgaris under salt stress. Braz Arch Biol Technol 60:e17160555
Francisco E, Neves D, Jacob-Lopes E, Franco T (2010) Microalgae as feedstock for biodiesel production: Carbon dioxide sequestration, lipid production and biofuel quality. J Chem Technol Biotechnol 85:395–403
Ho S-H, Chen W-M, Chang J-S (2010) Scenedesmus obliquus CNW-N as a potential candidate for CO2 mitigation and biodiesel production. Bioresour Technol 101:8725–8730
Damiani MC, Popovich CA, Constenla D (2010) Leonardi PI (2010) Lipid analysis in Haematococcus pluvialis to assess its potential use as a biodiesel feedstock. Bioresour Technol 101:3801–3807
Branco-Vieira M, Martin SS, Agurto C, Santos MA, Freitas MAV (2017) Caetano, N.S. Analyzing Phaeodactylum tricornutum lipid profile for biodiesel production. Energy Procedia 136:369–373
Knothe G (2005) Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process Technol 86:1059–1070
Faraloni C, Torzillo G (2017) Synthesis of antioxidant carotenoids in microalgae in response to physiological stress. In: Cvetkovic D, Nikolic G (eds) Carotenoids. IntechOpen, UK, pp 143–157.
Ji X, Cheng J, Gong D, Zhao X, Qi Y, Su Y, Ma W (2018) The effect of NaCl stress on photosynthetic efficiency and lipid production in freshwater microalga, Scenedesmus obliquus XJ002. Sci Total Environ 633:593–599
Hounslow E, Evans CA, Pandhal J, Sydney T, Couto N, Pham TK, Wright PC (2021) Quantitative proteomic comparison of salt stress in Chlamydomonas reinhardtii and the snow alga Chlamydomonas nivalis reveals mechanisms for salt-triggered fatty acid accumulation via reallocation of carbon resources. Biotechnol Biofuels 14:121
Ali HEA, Vorisek F, Dowd SE, Kesner S, Song Y, Qian D, Crocker M (2022) Formation of Lutein, β-Carotene and Astaxanthin in a Coelastrella sp. Isolate Molecules 27:6950
Yoshida K, Igarashi E, Wakatsuki E, Miyamoto K, Hirata K (2004) Mitigation of osmotic and salt stresses by abscisic acid through reduction of stress-derived oxidative damage in Chlamydomonas reinhardtii. Plant Sci 167:1335–1341
Pancha I, Chokshi K, Maurya R, Trivedi K, Patidar SK, Ghosh A, Mishra S (2015) Salinity induced oxidative stress enhanced biofuel production potential of microalgae Scenedesmus sp. CCNM 1077. Bioresour Technol 189:341–348
El-fayoumy EA, Shanab SM, Shalaby EA (2020) Metabolomics and biological activities of Chlorella vulgaris grown under modified growth medium (BG11) composition. Chiang Mai Univ J Nat Sci 19:91–123
Ramadan KM, El-Beltagi HS, Shanab SM, El-fayoumy EA, Shalaby EA, Bendary ES (2021) Potential antioxidant and anticancer activities of secondary metabolites of Nostoc linckia cultivated under Zn and Cu stress conditions. Processes 9:1972
Acknowledgements
The authors acknowledge the Arturo Falaschi ICGEB Smart Fellowship to Dr. Hamdy Elsayed Ahmed Ali by the International Centre for Genetic Engineering and Biotechnology (ICGEB) [(S/EGY20-02] at the Universiti Malaysia Terengganu.
Funding
Open Access funding is provided by the Qatar National Library. This research was funded by International Partnership Research Grant (IPRG), Universiti Malaysia Terengganu-Qatar University, under grant number UMT/IPRG/55303/2021.
Author information
Authors and Affiliations
Contributions
Conceptualization, E.A.E. and H.E.A.A., methodology, H.E.A.A., E.A.E.; investigation E.A.E. and H.E.A.A.; resources, A.E., S.A.M., K.E., H.E.A.A, E.A.E.; writing—original draft preparation, E.A.E., H.E.A.A.; writing—review and editing, A.E., S.A.M., K.E., M.Z.H.R., M.A.A.; visualization, H.E.A.A., E.A.E., and M.A.A.; supervision, A.E., S.A.M., K.E., M.Z.H.R., M.A.A.; project administration, A.E., S.A.M., K.E., M.Z.H.R.; Funding acquisition, A.E., S.A.M., K.E., M.Z.H.R.; All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable. This study did not involve any human or animal subjects, therefore no ethical approval is required.
Competing interests
The authors declare that they have no competing interests, either of a financial or personal nature, which could influence the content of this article.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-fayoumy, E.A., Ali, H.E.A., Elsaid, K. et al. Co-production of high density biomass and high-value compounds via two-stage cultivation of Chlorella vulgaris using light intensity and a combination of salt stressors. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04442-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04442-z