Abstract
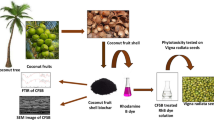
The presence of orthophosphate in streams stimulates eutrophication that brings negative implications to water quality and related economic sectors. This work was aimed at evaluating the removal of orthophosphate from water by iron-loaded adsorbents derived from coconut shells (CS). CS was carbonized and chemically activated with H3PO4 prior to ferric metals loading. The resultant adsorbents, CSA (coconut shell activated carbon), IC-CSA (ferric chloride-loaded), and IS-CSA (ferric sulfate-loaded) were characterized for physicochemical properties. Orthophosphate adsorption was studied under kinetic and equilibrium conditions considering the effects of concentration, solution pH, adsorbent dosage, and contact time. The modified adsorbents showed functional groups of –OH, C = C, and Fe–O, with IC-CSA exhibiting a greater specific surface of 722 m2/g. A maximum orthophosphate removal of 310 mg/g was recorded by IC-CSA in the pH range of 2–3. The Langmuir isotherm describes the equilibrium data well, while the adsorptive rate followed the pseudo-second-order model. Thermodynamic parameters indicate that the adsorption is spontaneous and exothermic. Adsorption was pH-dependent, and desorption was successfully accomplished by alkaline leaching. Iron-loaded coconut shell activated carbon is a promising adsorbent to abate eutrophication due to orthophosphate in water.













Similar content being viewed by others
Data availability
Data will be made available upon request.
References
Kumvimba M, Dzakpasu M, Li X (2020) Potential of invasive watermilfoil (Myriophyllum spp.) to remediate eutrophic waterbodies with organic and inorganic pollutants. J Environ Manag 270:110919
Almanassra WI, Kochkodan V, Mckay G, Atieh MA, Al-Ansari T (2021) Review of phosphate removal from water by carbonaceous sorbents. J of Environ Manag 287:112245
Irvin NB, Irvin EG, Martin JF, Acacena P (2018) Constructed wetlands for water quality improvements: benefit transfer analysis from Ohio. J Environ Manag 206:1063–1071
Quimpo TJR, Ligson CA, Manogan DP, Requilime JNC, Albelda RI, Conaco C, Cabaitan PC (2020) Fish farm effluents alter reef benthic assemblages and reduce coral settlement. Mar Pullut Bull 153:111025
Hongxu Z, Margenot AJ, Li Y, Si B, Wang T, Zhang Y, Li S, Bhattarai R (2020) Phosphorus pollution control using waste-based adsorbents: Material Synthesis, Modification, and Sustainability. Crit Rev Environ Sci Technol. https://doi.org/10.1080/10643389.2020.1866414
Pokhrel MR, Poundel BR, Aryal RL, Paudyal H, Ghimire KN (2019) Removal and recovery of phosphate from water and wastewater using metal-loaded agricultural waste-based adsorbents. J Inst Sci Technol 24:77–89
Qian J, Zhou X, Cai Q, Zhao J, Huang X (2023) The study of optimal adsorption conditions of phosphate on Fe-modified biochar by response surface methodology. Molecules 28:2323. https://doi.org/10.3390/molecules28052323
Li M, Liu J, Xu Y, Qian G (2016) Phosphate adsorption on metal oxides and metal hydroxides: a comparative review. Environ Rev 24:319–332
Loganathan P, Vigneswaran S, Kandasamy J, Nanthi B (2014) Removal and recovery of phosphate from water using sorption. Crit Rev Environ Sci Technol 44:847–907
Yang Q, Wang X, Luo W, Sun J, Xu Q, Chen F, Zhao J, Wang S, Yao F, Wang D, Li X, Zheng G (2017) Effectiveness and mechanisms of phosphate adsorption on iron-modified biochar derived from waste-activated sludge. Biotech 17:1–32
Wang P, Zhi M, Cui G, Chu Z, Wang S (2021) A comparative study on phosphate removal from water using phragmites australis biochars loaded with different metal oxide. R Soc Chem. https://doi.org/10.6084/m9.figshare.c.5433398
Dotto GL, Mckay G (2020) Current scenario and challenges in adsorption for water treatment. J Environ Chem Eng 8(103988):1–6. https://doi.org/10.1016/j.jece.2020.103988
Navarathna CM, Pennission JE, Dewage NB, Reid C, Dotse C, Jazi ME, Rodrigo PM, Zhang X, Farma E, Watson C, Craig DO, Remirez A, Walker M, Madduri S, Mohan D, Mlsna TE (2022) Adsorption of phosphate onto Mg/Al-oxide/Hydroxide/Sulfate-impregnated douglas fir biochar. Processes 11(111):1–15. https://doi.org/10.3390/pr11010111
Hanna S, Artur B, Malgorzata W (2019) Adsorption of phosphate from aqueous solutions on alginate/goethite hydrogel composite. J Water 11:1–13
Kentzer A, Buczkowski R (2005) Application of goethite for immobilisation of phosphorus in lake sediments. AUNC UMK Limnol 24:85–101
Xu W, Liu J, Sun K, Liu Y, Chen C, Wang A, Sun H (2021) Effect of activation temperature on properties of H3PO4-activated carbon. BioResources 16(2):4007–4020
Isah UA, Abdulraheem G, Bala S, Muhammad S, Abdullahi M (2015) Kinetics, equilibrium and thermodynamics studies of C.I. reactive blue 19 dye adsorption on coconut shell based activated carbon. Int Biodeterior Biodegradation 102:265–273
Iqbaldin MN, Khudzir I, MohdAzian MI, Zaid AG, Surani B, Zubri Z (2013) Properties of coconut shell activated carbon. J Trop For Sci 25(4):497–503
Nyamful A, Nyogbe EK, Mohammed L, Zainudeen MN, Darkwa SA, Phiri I (2020) Processing and characterization of activated carbon from coconut shell and palm kernel shell waste by H3PO4 activation. Ghana J Sci 61(2):91–104
Mahmood T, Saddique MT, Naeem A, Westerhoff P, Mustafa S, Alum A (2011) Comparison of different methods for point of zero charge determination of NiO. Ind Eng Res 50:10017–10028
Libourel G, Garino C, Delbo M, Niezgoda M, Remy B, Aranda L, Michel P (2021) Network of thermal cracks in meteorites due to temperature variations: new experimental evidence and implications for steroid surfaces. R Astron Soc 500:1905–1920
Cornelis G, Hund-Rinke K, Brink NWV, Kuhlbusch TAJ (2014) Fate and bioavailability of engineer nanoparticles in soils: a review. Crit Rev Environ Sci Technol. https://doi.org/10.1080/10643389.2013.829767
Yuan J, Zhu Y, Wang J, Liu Z, He M, Zhang T, Li P, Qiu F (2021) Facile modification ofbiochar derived from agricultural straw waste with effective adsorption and removal of phosphorus from domestic sewage. J Inorg Organomet Polym Mater 31:3867–3879. https://doi.org/10.1007/s10904-02-01992-5
Al-wabel MI, Al-Omran A, El-Naggar AH, Nadeem M, Usman ARA (2013) Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from conocarpus wastes. Bioresource Technol 131:374–379
Guo H, Ma L, Shen F, Yang G, Zhang Y, Deng S, Zhang J, Song C, Zeng Y (2017) Effect of La-involvement on biomass pyrolysis behaviors and properties of produced biochar. J Rare Earths 35:593–601
Liu WJ, Jiang H, Tian K, Ding YW, Yu HQ (2013) Mesoporous carbon stabilized MgCl2 preloaded waste biomass for highly efficient CO2 capture. Environ Sci Technol 47:9397–9433. https://doi.org/10.1021/es401286p
Wang Z, Nie E, Li J, Yang M, Zhao Y, Luo X, Zheng Z (2012) Equilibrium and kinetics of adsorption of phosphate onto iron-doped activated carbon. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-012-0799-y
Ponce J, Andrade JGS, Dos Santos LN, Bulla MK, Barros BCB, Favaro SL, Hioka N, Caetano W, Batistela VR (2021) Alkali pre-treated sugarcane bagasse, rice husk, and corn husk waste as lignocellulosic bio sorbent for dyes. J Carbohydr Polym Technol Appl 2:100061. https://doi.org/10.1016/j.carpta.2021.100061
Lalley J, Han C, Li X, Dionysiou DD, Nadaguuda MN (2016) Phosphate adsorption using modified iron oxide-based sorbents in lake water: kinetics, equilibrium, and column tests. Chem Eng J 284:1386–1396. https://doi.org/10.1016/j.cej.2015.08.114
Ndongo GK, Nsami NJ, Mbadcam KJ (2020) Ferromagnetic activated carbon from cassava (Manihot dulcis) peels activated by Iron(III) Chloride: Synthesis and characterization. Bioresources 15(2):2133–2146
Zhang QL, Lin YC, Chen X, Gao NY (2007) A method for preparing ferric activated carbon composites adsorbents to remove arsenic from drinking water. J Hazard Mater 148(3):671–678
Gu Z, Fang J, Deng B (2005) Preparation and evaluation of GAC based iron-containing adsorbents for arsenic removal. Environ Sci Technol 39(10):3833–3843
Gratuito MKB, Panyathanmaporn T, Chumnanklang RA, Sirinuntawittaya N, Dutta A (2008) Production of activated carbon from coconut shell: optimization using response surface methodology. Bioresource Technol 99:4887–4895
Sumathi S, Bhatia S, Lee KT, Mohamed AR (2010) Selection of best impregnated palm shell activated carbon (PSAC) for simultaneous removal of SO2 and NOx. J Hazard Mater 176(1–3):1093–1096
Asaoka S, Yamamoto T (2010) Characteristics of phosphate adsorption onto granulated coal ash in seawater. Mar Pollut Bull 60(8):1188–1192
Mao H, Chen X, Huang R, Chen M, Yang R, Lan P, Zhou M, Zhang F, Yang Y, Zhou X (2018) Fast preparation of carbon spheres from enzymatic hydrolysis lignin: effects of hydrothermal. Sci Rep 8:9501
Yacob A (2008) Comparison of various sources of high surface area carbon prepared by different types of activation. MJAS 12:264–271
Rugayah AF, Astimar AA, Norzita N (2014) Preparation and characterization of Activated carbon from kernel shell by physical activation with steam. J Oil Palm Res 26(3):251–264
Fisseha AB, Evans MNC (2021) Removal of phosphate from contaminated water using activated carbon supported nanoscale zero-valent iron (nZVI) particles. Chem Eng Trans 84:1–6
Patil-Mansing R, Raut PD (2013) Removal of phosphate from sewage effluent by adsorption on laterite. International Journal of Engineering Research & Technology (IJERT) 2(9):551–559
Quakouak AK, Youcef L (2016) Phosphate removal by activated carbon. Sens Lett 14:1–6
Babayem KA, Onukwuli DO (2017) Equilibrium studies and optimization of phosphate adsorption from synthetic effluent using acid modified bio-sorbent. Am J Eng Appl Sci 10(4):980–991
Mondal P, Majumder CB, Mohanty B (2008) Effects of adsorbent dose, its particle size and initial arsenic concentration on the removal of arsenic, iron and manganese from simulated ground water by Fe3+ impregnated activated carbon. J Hazard Mater 150(3):695–702
Lawal S, Zaini MAA (2022) Existing and emerging technologies for the removal of orthophosphate from wastewater by agricultural waste adsorbents: a review. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-021-02203-4
Sharma G, Sharma S, Kumar A, Lai CW, Naushad M, Shehnaz A, Iqbal J, Stadler FJ (2022) Activated carbon as super adsorbent and sustainable material for diverse application. Adsorption Sci Technol. https://doi.org/10.1155/4184809
Xiong JB, Mahmood Q (2010) Adsorptive removal of phosphate from aqueous media by peat. Desalination 259(1–3):59–64
Kuang Y, Zhang X, Zhou S (2020) Adsorption of methylene blue in water onto activated Carbon by surfactant modification. J Water 12:587. https://doi.org/10.3390/w12020587
Junjie Y, Yao Z, Jizhang W, Zhigang L, Meiying H, Tao Z, Pingping L, Fengxian Q (2021) Facile modification of biochar derived from agricultural straw waste with effective adsorption and removal of phosphorus from domestic sewage. J Inorg Organomet Polym Mater 31:3867–3879
Tan KL, Hamee BH (2017) Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J Taiwan Inst Chem Eng 74:25–48. https://doi.org/10.1016/j.jtice.2017.01.024
Wu FC, Tseng RL, Juang RS (2005) Comparisons of porous and adsorption properties of carbons activated by steam and KOH. J Colloid Interface Sci 283(1):49–56
Khalil AKA, Dweiri F, Almanassra IW, Chatla A, Atieh MA (2022) Mg-Al layered double hydroxide doped activated carbon composites for phosphate removal from synthetic water: adsorption and thermodynamics studies. Sustainability 14:6991. https://doi.org/10.3390/su14126991
El Nemr A, Khaled A, Abdelwahab O, El-Sikaily A (2008) Treatment of wastewater containing toxic chromium using new activated carbon developed from date palm seed. J Hazard Mater 152(1):263–275
Li KQ, Zheng Z, Huang XF, Zhao GH, Feng JW, Zhang JB (2009) Equilibrium, kinetic and thermodynamic studies on the adsorption of 2-nitroaniline onto activated carbon prepared from cotton stalk fibre. J Hazard Mater 166(1):213–220
Kim DS (2004) Adsorption characteristics of Fe(III) and Fe(III)-NTA complex on granular activated carbon. J Hazard Mater 106(1):67–84
Yu SM, Chen CL, Chang PP, Wang TT, Lu SS, Wang XK (2008) Adsorption of Th(IV) onto AL-pillared rectorite: Effect of pH, ionic strength, temperature, soil humic acid and fulvic acid. Appl Clay Sci 38(3–4):219–226
Wang Z, Shi M, Li J, Zheng Z (2014) Influence of moderate pre-oxidation treatment on the physical, chemical and phosphate adsorption properties of iron-containing activated carbon. J Environ Sci 26:519–528
Zhan Y, Yang K, Fang Y, Ding J, Zhang H (2022) Removal of phosphate from wastewater with a recyclable La-based particulate adsorbent in a small-scale reactor. Water 14:2326. https://doi.org/10.3390/w14152326
Gu Y, Yang M, Wang W, Han B (2019) Phosphate adsorption from solution by zirconium-loaded carbo nanotubes in batch mode. J Chem Eng Data 64:2849–2858
Shi Z, Lin F, Yao S (2011) Adsorptive removal of phosphate from aqueous solutions using activated carbon loaded with Fe(III) oxide. N Carbon Mater 26:299–306
Zhu X, Yuchen I, Feng Q, Hua S, Xinchao W, Shicheng Z, Chen J, Zhiyong JR (2018) Carbon transmission of CO2 activated nano-MgO carbon composites enhances phosphate immobilization. J Mater Chem 6:3705–3713
Acknowledgements
This work was part of S Lawal’s thesis for the award of PhD.
Funding
The project is funded in part by UTM-ICONIC Grant No. 09G54.
Author information
Authors and Affiliations
Contributions
S Lawal (PhD Candidate): Conceptualization, methodology, experimental work, analysis, first draft.
MAA Zaini (Associate Professor): Grant recipient, supervision, conceptualization, review, validation.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Conflict of interests
All authors declare that they have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Iron-loaded carbons were prepared from coconut shell for orthophosphate removal.
• Ferric chloride loading enhanced the specific surface from 178 to 722 m2/g.
• A maximum orthophosphate capacity of 310 mg/g was recorded.
• Regeneration by alkaline solution showed 89% removal after three cycles.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sirajo, L., Zaini, M.A.A. Iron-loaded coconut shell-activated carbons for orthophosphate adsorption. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04284-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04284-9