Abstract
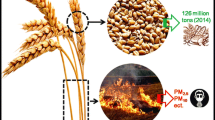
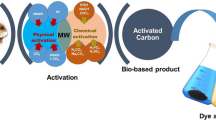
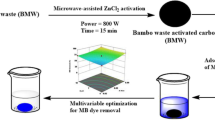
The carbon content of biomass waste makes it usable as a precursor in the production of activated carbon. The purpose of this work was to investigate the influence of lignocellulosic composition (i.e., lignin, cellulose, hemicellulose) in biomass waste on the microstructure and dye adsorption characteristics of microwave-assisted ZnCl2 activated carbon. Three biomass wastes with significant differences in lignocellulosic composition (i.e., pineapple leaf, cocoa shell, and coconut shell) were used as model substances. Carbonization experiments were conducted at 500 °C for two hours in a muffle furnace while the activation process was carried out at 600 W for 6 min in a microwave oven. The results showed that the composition of hemicellulose, cellulose, and lignin in biomass has an effect on the character of activated carbon. It was also found that cellulose is responsible for the formation of porosity so the high cellulose content in the biomass increases the specific surface area of the activated carbon obtained. It is in contrast with lignin which is responsible for the presence of functional groups and pore size. Activation using ZnCl2 coupled with microwave heating creates a template effect, resulting in uniform pores in activated carbon. As a result, the adsorption of dye increases with an increase in the impregnation ratio. The best adsorption ability was found in activated carbon with the highest cellulose content. Under the conditions of pH 3, an adsorption time of 180 min, and an adsorbent dose of 0.3 g per 50 mm−3 dye solution, the adsorption ability of all activated carbons was the best (adsorption rate reached 99% for the initial concentration of methyl violet dye of 100 mg.dm−3). All activated carbons exhibited the best fit with the Langmuir isotherm model.
Graphical abstract











Similar content being viewed by others
Availability of data and materials
This declaration is not applicable for this study.
References
Mbarki F, Selmi T, Kesraoui A, Seffen M, Gadonneix P, Celzard A, Fierro V (2019) Hydrothermal pre-treatment, an efficient tool to improve activated carbon performances. Ind Crops Prod 140:111717. https://doi.org/10.1016/j.indcrop.2019.111717
Astuti W, Chafidz A, Al-Fatesh AS, Fakeeha AH (2021) Removal of lead (Pb(II)) and zinc (Zn(II)) from aqueous solution using coal fly ash (CFA) as a dual-sites adsorbent. Chin J Chem Eng 34:289–298. https://doi.org/10.1016/j.cjche.2020.08.046
Suhaimi A, Abdullah AS, Jawad AH, Yousef TA, Al Duaji OK, Othman ZA, Wilson LD (2022) Production of large surface area activated carbon from a mixture of carrot juice pulp and pomegranate peel using microwave radiation-assisted ZnCl2 activation: An optimized removal process and tailored adsorption mechanism of crystal violet dye. Diam Relat Mater 130:109456. https://doi.org/10.1016/j.diamond.2022.109456
Chafidz A, Astuti W, Hartanto D, Mutia AS, Sari PR (2018) Preparation of activated carbon from banana peel waste for reducing air pollutant from motorcycle muffler. MATEC Web Conf 154:1–5. https://doi.org/10.1051/matecconf/201815401021
Livani MJ, Ghorbani M, Mehdipour H (2018) Preparation of an activated carbon from hazelnut shells and its hybrids with magnetic NiFe2O4 nanoparticles. New Carbon Mater 33(6):578–586. https://doi.org/10.1016/S1872-5805(18)60358-0
Enniya I, Rghioui L, Jourani A (2018) Adsorption of hexavalent chromium in aqueous solution on activated carbon prepared from apple peels. Sustain Chem Pharm 7:9–16. https://doi.org/10.1016/j.scp.2017.11.003
Siddique A, Nayak AK, Singh J (2020) Synthesis of FeCl3-activated carbon derived from waste Citrus limetta peels for removal of fluoride: An eco-friendly approach for the treatment of groundwater and bio-waste collectively. Groundw Sustain Dev 10:100339. https://doi.org/10.1016/j.gsd.2020.100339
Wang S, Nam H, Nam H (2020) Preparation of activated carbon from peanut shell with KOH activation and its application for H2S adsorption in confined space. J Environ Chem Eng 8(2):103683. https://doi.org/10.1016/j.jece.2020.103683
Niksiar A, Nasernejad B (2018) Modeling of gasification reaction to produce activated carbon from pistachio shells in a spouted bed. Biomass Bioenerg 119:97–108. https://doi.org/10.1016/j.biombioe.2018.09.008
Akbayrak S, Özçifçi Z, Tabak A (2020) Activated carbon derived from tea waste: a promising supporting material for metal nanoparticles used as catalysts in hydrolysis of ammonia borane. Biomass Bioenerg 138:105589. https://doi.org/10.1016/j.biombioe.2020.105589
Pallarés J, González-Cencerrado A, Arauzo I (2018) Production and characterization of activated carbon from barley straw by physical activation with carbon dioxide and steam. Biomass Bioenerg 115:64–73. https://doi.org/10.1016/j.biombioe.2018.04.015
Neme I, Gonfa G, Masi C (2022) Preparation and characterization of activated carbon from castor seed hull by chemical activation with H3PO4. Results Mater 15:100304. https://doi.org/10.1016/j.rinma.2022.100304
Rangabhashiyam S, Balasubramanian P (2019) The potential of lignocellulosic biomass precursors for biochar production: performance, mechanism and wastewater application-A review. Ind Crops Prod 128:405–423. https://doi.org/10.1016/j.indcrop.2018.11.041
Danish M, Ahmad T (2018) A review on utilization of wood biomass as a sustainable precursor for activated carbon production and application. Renew Sustain Energy Rev 87:1–21. https://doi.org/10.1016/j.rser.2018.02.003
Boundzanga HM, Cagnon B, Roulet M, de Persis S, Vautrin-Ul C, Bonnamy S (2022) Contributions of hemicellulose, cellulose, and lignin to the mass and the porous characteristics of activated carbons produced from biomass residues by phosphoric acid activation. Biomass Convers Biorefin 12(8):3081–3096. https://doi.org/10.1007/s13399-020-00816-9
Jawad AH, Surip SN (2022) Upgrading low rank coal into mesoporous activated carbon via microwave process for methylene blue dye adsorption: Box Behnken Design and mechanism study. Diam Relat Mater 127:109199. https://doi.org/10.1016/j.diamond.2022.109199
Teimouri Z, Salem A, Salem S (2019) Clean and new strategy for catalytic conversion of agriculture waste shells to activated carbon via microwave-assisted impregnation: Applied and eco-friendly aspect for decoloration of industrial corn syrup and process identifications. J Environ Chem Eng 7(3):103161. https://doi.org/10.1016/j.jece.2019.103161
Jawad AH, Malek NNA, Khadiran T, ALOthman ZA, Yaseen ZM (2022) Mesoporous high-surface-area activated carbon from biomass waste via microwave-assisted-H3PO4 activation for methylene blue dye adsorption: an optimized process. Diam Relat Mater 128:109288. https://doi.org/10.1016/j.diamond.2022.109288
Yousef TA, Sahu UK, Jawad AH, AbdMalek NN, Al Duaij OK, ALOthman ZA (2022) Fruit peel-based mesoporous activated carbon via microwave assisted K2CO3 activation: Box Behnken design and desirability function for methylene blue dye adsorption. Int J Phytoremediation 1–13. https://doi.org/10.1080/15226514.2022.2137102
Razali NS, Abdulhameed AS, Jawad AH, ALOthman ZA, Yousef TA, Al-Duaji OK, Alsaiari NS (2022) High-surface-area-activated carbon derived from mango peels and seeds wastes via microwave-induced ZnCl2 activation for adsorption of methylene blue dye molecules: statistical optimization and mechanism. Molecules 27(20). https://doi.org/10.3390/molecules27206947
Hong D, Zhou J, Hu C, Zhou Q, Mao J, Qin Q (2019) Mercury removal mechanism of AC prepared by one-step activation with ZnCl2. Fuel 235:326–335. https://doi.org/10.1016/j.fuel.2018.07.103
Astuti A, Sulistyaningsih T, Maksiola M (2016) Chemically modified kapok sawdust as adsorbent of methyl violet dye from aqueous solution. J Teknol 78(9):35–42. https://doi.org/10.11113/jt.v78.6524
Ali NS, Jabbar NM, Alardhi SM, Majdi HS, Albayati TM (2022) Adsorption of methyl violet dye onto a prepared bio-adsorbent from date seeds: isotherm, kinetics, and thermodynamic studies. Heliyon 8(8):e10276. https://doi.org/10.1016/j.heliyon.2022.e10276
Hussain OA, Hathout AS, Abdel-Mobdy YE, Rashed MM, Abdel Rahim EA, Fouzy ASM (2023) Preparation and characterization of activated carbon from agricultural wastes and their ability to remove chlorpyrifos from water. Toxicol Rep 10:146–154. https://doi.org/10.1016/j.toxrep.2023.01.011
Jiang F, Cao D, Hu S, Wang Y, Zhang Y, Huang X, Zhao H, Wu C, Li J, Ding Y, Liu K (2022) High-pressure carbon dioxide-hydrothermal enhance yield and methylene blue adsorption performance of banana pseudo-stem activated carbon. Bioresour Technol 354:127137. https://doi.org/10.1016/j.biortech.2022.127137
Parada MS, Fernández K (2017) Modelling the hydrophilic extraction of the bark of Eucalyptus nitens and Eucalyptus globulus: Adsorption isotherm and thermodynamic studies. Ind Crops Prod 109:558–569. https://doi.org/10.1016/j.indcrop.2017.08.059
Duan YT, Yao Y, Ameta RK (2023) Removal and recovering of anionic and cationic dyes using neem leaf ash prepared at 250, 500 and 750 °C: analyzed by adsorption isotherm and physicochemical parameters. J Mol Liq 370:121012. https://doi.org/10.1016/j.molliq.2022.121012
Leng E, Guo Y, Chen J, Liu S, Jiaqiang E, Xue Y (2022) A comprehensive review on lignin pyrolysis: mechanism, modeling and the effects of inherent metals in biomass. Fuel 309:122102. https://doi.org/10.1016/j.fuel.2021.122102
Ji W, Richter F, Gollner MJ, Deng S (2022) Autonomous kinetic modeling of biomass pyrolysis using chemical reaction neural networks. Combust Flame 240:111992. https://doi.org/10.1016/j.combustflame.2022.111992
Chen D, Cen K, Zhuang X, Gan Z, Zhou J, Zhang Y, Zhang H (2022) Insight into biomass pyrolysis mechanism based on cellulose, hemicellulose, and lignin: evolution of volatiles and kinetics, elucidation of reaction pathways, and characterization of gas, biochar and bio-oil. Combust Flame 242:112142. https://doi.org/10.1016/j.combustflame.2022.112142
Jayasuriya WJ, Mulky TC, Niemeyer KE (2022) Smouldering combustion in cellulose and hemicellulose mixtures: examining the roles of density, fuel composition, oxygen concentration, and moisture content. Combust Theory Model 26(5):831–855. https://doi.org/10.1080/13647830.2022.2071170
Ma Y, Wang Q, Wang X, Sun X, Wang X (2015) A comprehensive study on activated carbon prepared from spent shiitake substrate via pyrolysis with ZnCl2. J Porous Mater 22(1):157–169. https://doi.org/10.1007/s10934-014-9882-8
Pang YX, Yan Y, Foo DCY, Sharmin N, Zhao H, Lester E, Wu T, Pang CH (2021) The influence of lignocellulose on biomass pyrolysis product distribution and economics via steady state process simulation. J Anal Appl Pyrolysis 158:104968. https://doi.org/10.1016/j.jaap.2020.104968
Spagnoli AA, Giannakoudakis DA, Bashkova S (2017) Adsorption of methylene blue on cashew nut shell based carbons activated with zinc chloride: the role of surface and structural parameters. J Mol Liq 229:465–471. https://doi.org/10.1016/j.molliq.2016.12.106
Beltrame KK, Cazetta AL, de Souza PSC, Spessato L, Silva TL, Almeida VC (2018) Adsorption of caffeine on mesoporous activated carbon fibers prepared from pineapple plant leaves. Ecotoxicol Environ Saf 147:64–71. https://doi.org/10.1016/j.ecoenv.2017.08.034
Astuti W, Sulistyaningsih T, Kusumastuti E, Thomas GYRS, Kusnadi RY (2019) Thermal conversion of pineapple crown leaf waste to magnetized activated carbon for dye removal. Bioresour Technol 287:121426. https://doi.org/10.1016/j.biortech.2019.121426
Astuti W, Handayani AD, Wulandari DA (2018) Adsorpsi methyl violet oleh karbon aktif dari limbah tempurung kelapa dengan aktivator ZnCl2 menggunakan pemanasan gelombang mikro. J Rekayasa Kim Lingkung 13(2):189–199. https://doi.org/10.23955/rkl.v13i2.11945
Mbarki F, Selmi T, Kesraoui A, Seffen M (2022) Low-cost activated carbon preparation from corn stigmata fibers chemically activated using H3PO4, ZnCl2 and KOH: study of methylene blue adsorption, stochastic isotherm and fractal kinetic. Ind Crops Prod 178:114546. https://doi.org/10.1016/j.indcrop.2022.114546
Movasaghi Z, Yan B, Niu C (2019) Adsorption of ciprofloxacin from water by pretreated oat hulls: equilibrium, kinetic, and thermodynamic studies. Ind Crops Prod 127:237–250. https://doi.org/10.1016/j.indcrop.2018.10.051
Chahinez HO, Abdelkader O, Leila Y, Tran HN (2020) One-stage preparation of palm petiole-derived biochar: Characterization and application for adsorption of crystal violet dye in water. Environ Technol Innov 19:100872. https://doi.org/10.1016/j.eti.2020.100872
Nabih MH, El Hajam M, Boulika H, Chiki Z, Tahar SB, Kandri NI, Zeroule A (2023) Preparation and characterization of activated carbons from cardoon ‘Cynara Cardunculus’ waste: application to the adsorption of synthetic organic dyes. Mater Today Proc 72:3369–3379. https://doi.org/10.1016/j.matpr.2022.07.414
Musyoka SM, Mittal H, Mishra SB, Ngila JC (2014) Effect of functionalization on the adsorption capacity of cellulose for the removal of methyl violet. Int J Biol Macromol 65:389–397. https://doi.org/10.1016/j.ijbiomac.2014.01.051
Astuti W, Sulistyaningsih T, Maksiola M (2017) Equilibrium and kinetics of adsorption of methyl violet from aqueous solutions using modified ceiba pentandra sawdust. Asian J Chem 29(1):133–138. https://doi.org/10.14233/ajchem.2017.20158
Samal K, Raj N, Mohanty K (2019) Saponin extracted waste biomass of sapindus mukorossi for adsorption of methyl violet dye in aqueous system. Surf Interfaces 14:166–174. https://doi.org/10.1016/j.surfin.2018.12.009
Sarkar S, Tiwari N, Behera M, Chakrabortty S, Jhingran K, Sanjay K, Banerjee S, Tripathy SK (2022) Facile synthesis, characterization and application of magnetic Fe3O4-coir pith composites for the removal of methyl violet from aqueous solution: kinetics, isotherm, thermodynamics and parametric optimization. J Indian Chem Soc 99(5):100447. https://doi.org/10.1016/j.jics.2022.100447
Alavinia S, Ghorbani-Vaghei R, Asadabadi S, Atrian A (2023) Sodium alginate/diethyleneamine-triazine-sulfonamide nanocomposite for adsorptive removal of Pb(II) and methyl violet from aqueous solutions. Mater Chem Phys 293:126915. https://doi.org/10.1016/j.matchemphys.2022.126915
Foroutan R, Mohammadi R, Ahmadi A, Bikhabar G, Babaei F, Ramavandi B (2022) Impact of ZnO and Fe3O4 magnetic nanoscale on the methyl violet 2B removal efficiency of the activated carbon oak wood. Chemosphere 286:131632. https://doi.org/10.1016/j.chemosphere.2021.131632
Sulyman M, Kucinska-Lipka J, Sienkiewicz M, Gierak A (2021) Development, characterization and evaluation of composite adsorbent for the adsorption of crystal violet from aqueous solution: Isotherm, kinetics, and thermodynamic studies. Arab J Chem 14(5):103115. https://doi.org/10.1016/j.arabjc.2021.103115
Funding
This work was supported by the Directorate of Research, Technology and Community Service, Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia [Contract Number: 038/E5/PG.02.00.PT/2022].
Author information
Authors and Affiliations
Contributions
Widi Astuti: Conceptualization, design of research, supervision, original draft preparation, revision; Triastuti Sulistyaningsih: Analysis, revision; Dhidik Prastiyanto: Review and editing of the draft; Rusiyanto: Review and editing of the draft. Lanjar, Anis Wiji Astuti, Angelita Dwi Handayani, Diah Ayu Wulandari: Material preparation and data collection; Fatma Indah Riayanti: data collection, original draft preparation; Wahyu Tri Wibowo: data presentation and analysis; All authors commented on previous versions of the manuscript, read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
This declaration is not applicable for this study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Astuti, W., Sulistyaningsih, T., Prastiyanto, D. et al. Influence of lignocellulosic composition in biomass waste on the microstructure and dye adsorption characteristics of microwave-assisted ZnCl2 activated carbon. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04281-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04281-y