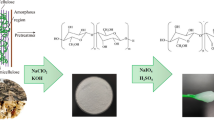
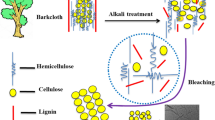
Abstract
Recently, eutectic solvents (ESs) green treatment of lignocellulose biomasses attracted vast attention due to their properties. Concerning the subject, this paper aims to apply choline chloride lactic acid-based ESs for delignification and cellulose isolation optimization from extractive-free natural cotton pods (EFNCPs) to achieve the highest cellulose extraction and purity and also, consumption of ES-treated cotton pods (ESTCPs) for nano-fibrillation. The structure of each step product was characterized by applying Fourier transform infrared (FTIR), X-ray diffraction (XRD), thermogravimetric (TG), derivative thermogravimetric (DTG), field emission scanning electron microscopy (Fe-SEM), and transmission electron microscopy (TEM) methods. As the FTIR diagram shows, respectively decreasing the strength around 1515 and 1740 cm−1 and increasing the intensity of bands at 890, 1030, and 1210–1490 cm−1 are attributed to lignin removal and increasing the cellulose amount during isolation. XRD analysis results show in the extraction and nano-fibrillation process the small peaks, which were related to the lignin and other impurities eliminated and the crystallinity enhanced. The TG and DTG results indicate that during the cellulose isolation process, the narrowness of peak at 200–400 °C increased which is due to the lignin removal. Also, the results of TG and DTG show that during the isolation process by ES, the structure of cellulose fibers is almost unchanged. Also, Fe-SEM and TEM images show that during the isolation process, due to the removal of the non-cellulosic layer surface, roughness increased. The result of this study can be used for cellulose isolation optimization with unique chemical and mechanical properties.








Similar content being viewed by others
Data availability
All datasets are available by corresponding author agreement.
Abbreviations
- AIL:
-
Acid-insoluble lignin
- ANOVA:
-
Analysis of variance
- CNFs:
-
Cellulose nanofibers
- CCD:
-
Central composite design
- CPC:
-
Chemically purified cellulose
- CV:
-
Coefficient of variation
- CrI:
-
Crystallinity index
- DOE:
-
Design of the experiment
- ES:
-
Eutectic solvent
- ESTCPs:
-
Eutectic solvent-treated cotton pods
- EFNCPs:
-
Extractive free natural cotton pods
- Fe-SEM:
-
Field emission scanning electron microscopy
- FTIR:
-
Fourier transform infrared
- HBA:
-
Hydrogen bond acceptor
- HBD:
-
Hydrogen bond donor
- ILs:
-
Ionic liquids
- NCPs:
-
Natural cotton pods
- RSM-CCD:
-
Response surface-Central Composite Design
- TEM:
-
Transmission electron microscopy
- TGA:
-
Thermogravimetric analysis
- DTGA:
-
Derivative thermogravimetric analysis
- XRD:
-
X-Ray Diffraction
References
Zhao D, Zhu Y, Cheng W, Chen W, Wu Y, Yu H (2021) Cellulose-based flexible functional materials for emerging intelligent electronics. Adv Mater 33(28):2000619
Karimian A, Parsian H, Majidinia M, Rahimi M, Mir SM, Kafil HS, Shafiei-Irannejad V, Kheyrollah M, Ostadi H, Yousefi B (2019) Nanocrystalline cellulose: Preparation, physicochemical properties, and applications in drug delivery systems. Int J Biol Macromol 133:850–859
Rajeswari A, Christy EJS, Pius A (2021) Biopolymer blends and composites: processing technologies and their properties for industrial applications. Elsevier, Biopolymers and their industrial applications, pp 105–147
Bilal M, Iqbal HM (2019) Naturally-derived biopolymers: Potential platforms for enzyme immobilization. Int J Biol Macromol 130:462–482
Hon DN-S (2017) Chemical modification of cellulose. Routledge, Chemical modification of lignocellulosic materials, pp 97–127
Brinchi L, Cotana F, Fortunati E, Kenny J (2013) Production of nanocrystalline cellulose from lignocellulosic biomass: technology and applications. Carbohyd Polym 94(1):154–169
Fortunati E, Puglia D, Monti M, Peponi L, Santulli C, Kenny J, Torre L (2013) Extraction of cellulose nanocrystals from Phormium tenax fibres. J Polym Environ 21(2):319–328
Carels N (2011) The challenge of Bioenergies-an overview. Biofuel’s Eng Process Technol 23–64
Schubert S, Schlufter K, Heinze T (2011) Configurations, structures, and morphologies of cellulose, Polysaccharides in medicinal and pharmaceutical applications. Shrewsbury iSmithers 1–55
Bicu I, Mustata F (2011) Cellulose extraction from orange peel using sulfite digestion reagents. Biores Technol 102(21):10013–10019
Shen X-J, Wen J-L, Mei Q-Q, Chen X, Sun D, Yuan T-Q, Sun R-C (2019) Facile fractionation of lignocelluloses by biomass-derived deep eutectic solvent (DES) pretreatment for cellulose enzymatic hydrolysis and lignin valorization. Green Chem 21(2):275–283
Shrestha S, Khatiwada JR, Sharma HK, Qin W (2021) Bioconversion of fruits and vegetables wastes into value-added products. Springer, Sustainable Bioconversion of Waste to Value Added Products, pp 145–163
Das H, Singh SK (2004) Useful byproducts from cellulosic wastes of agriculture and food industry—a critical appraisal. Crit Rev Food Sci Nutr 44(2):77–89
Rajinipriya M, Nagalakshmaiah M, Robert M, Elkoun S (2018) Importance of agricultural and industrial waste in the field of nanocellulose and recent industrial developments of wood based nanocellulose: a review. ACS Sustain Chem Eng 6(3):2807–2828
Ramakrishnan A, Ravishankar K, Dhamodharan R (2019) Preparation of nanofibrillated cellulose and nanocrystalline cellulose from surgical cotton and cellulose pulp in hot-glycerol medium. Cellulose 26(5):3127–3141
Satlewal A, Agrawal R, Bhagia S, Sangoro J, Ragauskas AJ (2018) Natural deep eutectic solvents for lignocellulosic biomass pretreatment: recent developments, challenges and novel opportunities. Biotechnol Adv 36(8):2032–2050
Khan MA, Wahid A, Ahmad M, Tahir MT, Ahmed M, Ahmad S, Hasanuzzaman M (2020) World cotton production and consumption: An overview, Cotton production and uses: Agronomy, crop protection, and postharvest technologies 1–7
Ng H-M, Sin LT, Tee T-T, Bee S-T, Hui D, Low C-Y, Rahmat A (2015) Extraction of cellulose nanocrystals from plant sources for application as reinforcing agent in polymers. Compos B Eng 75:176–200
Chen H-M, Fu X, Luo Z-G (2015) Properties and extraction of pectin-enriched materials from sugar beet pulp by ultrasonic-assisted treatment combined with subcritical water. Food Chem 168:302–310
Kumar B, Bhardwaj N, Agrawal K, Chaturvedi V, Verma P (2020) Current perspective on pretreatment technologies using lignocellulosic biomass: An emerging biorefinery concept. Fuel Process Technol 199:106244
Florindo C, Branco LC, Marrucho IM (2019) Quest for green-solvent design: from hydrophilic to hydrophobic (deep) eutectic solvents. Chemsuschem 12(8):1549–1559
Torres LAZ, Woiciechowski AL, de AndradeTanobe VO, Karp SG, Lorenci LCG, Faulds C, Soccol CR (2020) Lignin as a potential source of high-added value compounds: A review. J Clean Prod 263:121499
H. Soleimanzadeh, F.M. Bektashi, S.Z. Ahari, D. Salari, A. Olad, A. Ostadrahimi (2022) Optimization of cellulose extraction process from sugar beet pulp and preparation of its nanofibers with choline chloride–lactic acid deep eutectic solvents. Biomass Convers Biorefin 1–13
Francisco M, Van Den Bruinhorst A, Kroon MC (2012) New natural and renewable low transition temperature mixtures (LTTMs): screening as solvents for lignocellulosic biomass processing. Green Chem 14(8):2153–2157
Mallakpour S, Dinari M (2012) Ionic liquids as green solvents: progress and prospects. Green Solvents I I:1–32
Halder P, Kundu S, Patel S, Setiawan A, Atkin R, Parthasarthy R, Paz-Ferreiro J, Surapaneni A, Shah K (2019) Progress on the pre-treatment of lignocellulosic biomass employing ionic liquids. Renew Sustain Energy Rev 105:268–292
Bhatia SK, Jagtap SS, Bedekar AA, Bhatia RK, Patel AK, Pant D, Banu JR, Rao CV, Kim Y-G, Yang Y-H (2020) Recent developments in pretreatment technologies on lignocellulosic biomass: effect of key parameters, technological improvements, and challenges. Biores Technol 300:122724
Usmani Z, Sharma M, Gupta P, Karpichev Y, Gathergood N, Bhat R, Gupta VK (2020) Ionic liquid based pretreatment of lignocellulosic biomass for enhanced bioconversion. Biores Technol 304:123003
Mohan M, Goud VV, Banerjee T (2015) Solubility of glucose, xylose, fructose and galactose in ionic liquids: Experimental and theoretical studies using a continuum solvation model. Fluid Phase Equilib 395:33–43
Mohan M, Banerjee T, Goud VV (2016) Solid liquid equilibrium of cellobiose, sucrose, and maltose monohydrate in ionic liquids: experimental and quantum chemical insights. J Chem Eng Data 61(9):2923–2932
Scott M, Deuss PJ, de Vries JG, Prechtl MH, Barta K (2016) New insights into the catalytic cleavage of the lignin β-O-4 linkage in multifunctional ionic liquid media. Catal Sci Technol 6(6):1882–1891
Abbott AP, Boothby D, Capper G, Davies DL, Rasheed RK (2004) Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids. J Am Chem Soc 126(29):9142–9147
Capolupo L, Faraco V (2016) Green methods of lignocellulose pretreatment for biorefinery development. Appl Microbiol Biotechnol 100(22):9451–9467
Pinkert A, Goeke DF, Marsh KN, Pang S (2011) Extracting wood lignin without dissolving or degrading cellulose: investigations on the use of food additive-derived ionic liquids. Green Chem 13(11):3124–3136
Hassan SS, Williams GA, Jaiswal AK (2018) Emerging technologies for the pretreatment of lignocellulosic biomass. Biores Technol 262:310–318
Hansen BB, Spittle S, Chen B, Poe D, Zhang Y, Klein JM, Horton A, Adhikari L, Zelovich T, Doherty BW (2020) Deep eutectic solvents: A review of fundamentals and applications. Chem Rev 121(3):1232–1285
Zhang Q, Vigier KDO, Royer S, Jérôme F (2012) Deep eutectic solvents: syntheses, properties and applications. Chem Soc Rev 41(21):7108–7146
Tang X, Zuo M, Li Z, Liu H, Xiong C, Zeng X, Sun Y, Hu L, Liu S, Lei T (2017) Green processing of lignocellulosic biomass and its derivatives in deep eutectic solvents. Chemsuschem 10(13):2696–2706
Xu P, Zheng G-W, Zong M-H, Li N, Lou W-Y (2017) Recent progress on deep eutectic solvents in biocatalysis. Bioresources Bioprocessing 4(1):1–18
Bystrzanowska M, Tobiszewski M (2021) Assessment and design of greener deep eutectic solvents–A multicriteria decision analysis. J Mol Liq 321:114878
Elhamarnah Y, Qiblawey H, Nasser MS, Benamor A (2020) Thermo-rheological characterization of malic acid based natural deep eutectic solvents. Sci Total Environ 708:134848
Cicco L, Dilauro G, Perna FM, Vitale P, Capriati V (2021) Advances in deep eutectic solvents and water: applications in metal-and biocatalyzed processes, in the synthesis of APIs, and other biologically active compounds. Org Biomol Chem 19(12):2558–2577
Fanali C, Della Posta S, Dugo L, Gentili A, Mondello L, De Gara L (2020) Choline-chloride and betaine-based deep eutectic solvents for green extraction of nutraceutical compounds from spent coffee ground. J Pharma Biomed Anal 189:113421
Rashid T, Sher F, Rasheed T, Zafar F, Zhang S, Murugesan T (2021) Evaluation of current and future solvents for selective lignin dissolution–A review. J Mol Liq 321:114577
Tan YT, Chua ASM, Ngoh GC (2020) Deep eutectic solvent for lignocellulosic biomass fractionation and the subsequent conversion to bio-based products–A review. Biores Technol 297:122522
Bjelić A, Hočevar B, Grilc M, Novak U, Likozar B (2022) A review of sustainable lignocellulose biorefining applying (natural) deep eutectic solvents (DESs) for separations, catalysis and enzymatic biotransformation processes. Rev Chem Eng 38(3):243–272
Xu H, Kong Y, Peng J, Song X, Che X, Liu S, Tian W (2020) Multivariate analysis of the process of deep eutectic solvent pretreatment of lignocellulosic biomass. Ind Crops Prod 150:112363
Massayev S, Lee KM (2022) Evaluation of deep eutectic solvent pretreatment towards efficacy of enzymatic saccharification using multivariate analysis techniques. J Clean Prod 360:132239
Jablonsky M, Haz A, Majova V (2019) Assessing the opportunities for applying deep eutectic solvents for fractionation of beech wood and wheat straw. Cellulose 26:7675–7684
Li A-L, Hou X-D, Lin K-P, Zhang X, Fu M-H (2018) Rice straw pretreatment using deep eutectic solvents with different constituents molar ratios: Biomass fractionation, polysaccharides enzymatic digestion and solvent reuse. J Biosci Bioeng 126(3):346–354
Álvarez A, Cachero S, González-Sánchez C, Montejo-Bernardo J, Pizarro C, Bueno JL (2018) Novel method for holocellulose analysis of non-woody biomass wastes. Carbohyd Polym 189:250–256
Sai YW, Lee KM (2019) Enhanced cellulase accessibility using acid-based deep eutectic solvent in pretreatment of empty fruit bunches. Cellulose 26:9517–9528
Maran JP, Manikandan S, Thirugnanasambandham K, Nivetha CV, Dinesh R (2013) Box-Behnken design based statistical modeling for ultrasound-assisted extraction of corn silk polysaccharide. Carbohyd Polym 92(1):604–611
Soleimanzadeh H, Niaei A, Salari D, Tarjomannejad A, Penner S, Grünbacher M, Hosseini SA, Mousavi SM (2019) Modeling and optimization of V2O5/TiO2 nanocatalysts for NH3-Selective catalytic reduction (SCR) of NOx by RSM and ANN techniques. J Environ Manage 238:360–367
Mali S, Debiagi F, Grossmann MV, Yamashita F (2010) Starch, sugarcane bagasse fibre, and polyvinyl alcohol effects on extruded foam properties: A mixture design approach. Ind Crops Prod 32(3):353–359
Panahi PN, Salari D, Niaei A, Mousavi SM (2015) Study of M-ZSM-5 nanocatalysts (M: Cu, Mn, Fe, Co…) for selective catalytic reduction of NO with NH3: Process optimization by Taguchi method. Chin J Chem Eng 23(10):1647–1654
Jung S-J, Kim S-H, Chung I-M (2015) Comparison of lignin, cellulose, and hemicellulose contents for biofuels utilization among 4 types of lignocellulosic crops. Biomass Bioenerg 83:322–327
Ruiz-Aquino F, González-Peña MM, Valdez-Hernández JI, Revilla US, Romero-Manzanares A (2015) Chemical characterization and fuel properties of wood and bark of two oaks from Oaxaca, Mexico. Ind Crops Prod 65:90–95
Thi S, Lee KM (2019) Comparison of deep eutectic solvents (DES) on pretreatment of oil palm empty fruit bunch (OPEFB): Cellulose digestibility, structural and morphology changes. Biores Technol 282:525–529
Kumar AK, Shah E, Patel A, Sharma S, Dixit G (2018) Physico-chemical characterization and evaluation of neat and aqueous mixtures of choline chloride+ lactic acid for lignocellulosic biomass fractionation, enzymatic hydrolysis and fermentation. J Mol Liq 271:540–549
Soares B, da Costa Lopes AM, Silvestre AJ, Pinto PCR, Freire CS, Coutinho JA (2021) Wood delignification with aqueous solutions of deep eutectic solvents. Indus Crops Prod 160:113128
Lamaming J, Hashim R, Sulaiman O, Leh CP, Sugimoto T, Nordin NA (2015) Cellulose nanocrystals isolated from oil palm trunk. Carbohyd Polym 127:202–208
Johar N, Ahmad I, Dufresne A (2012) Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind Crops Prod 37(1):93–99
Oh SY, Yoo DI, Shin Y, Seo G (2005) FTIR analysis of cellulose treated with sodium hydroxide and carbon dioxide. Carbohyd Res 340(3):417–428
Hou X-D, Li A-L, Lin K-P, Wang Y-Y, Kuang Z-Y, Cao S-L (2018) Insight into the structure-function relationships of deep eutectic solvents during rice straw pretreatment. Biores Technol 249:261–267
Chieng BW, Lee SH, Ibrahim NA, Then YY, Loo YY (2017) Isolation and characterization of cellulose nanocrystals from oil palm mesocarp fiber. Polymers 9(8):355
Sun X, Xu F, Sun R, Fowler P, Baird M (2005) Characteristics of degraded cellulose obtained from steam-exploded wheat straw. Carbohyd Res 340(1):97–106
Zhao C, Jiang E, Chen A (2017) Volatile production from pyrolysis of cellulose, hemicellulose and lignin. J Energy Inst 90(6):902–913
Meda RS, Jain S, Singh S, Verma C, Nandi U, Maji PK (2022) Novel Lagenaria siceraria peel waste based cellulose nanocrystals: Isolation and rationalizing H-bonding interactions. Ind Crops Prod 186:115197
Lynam JG, Kumar N, Wong MJ (2017) Deep eutectic solvents’ ability to solubilize lignin, cellulose, and hemicellulose; thermal stability; and density. Biores Technol 238:684–689
Haafiz MM, Eichhorn S, Hassan A, Jawaid M (2013) Isolation and characterization of microcrystalline cellulose from oil palm biomass residue. Carbohyd Polym 93(2):628–634
Wang Z, Yao Z, Zhou J, Zhang Y (2017) Reuse of waste cotton cloth for the extraction of cellulose nanocrystals. Carbohyd Polym 157:945–952
Kaushik A, Singh M, Verma G (2010) Green nanocomposites based on thermoplastic starch and steam exploded cellulose nanofibrils from wheat straw. Carbohyd Polym 82(2):337–345
Mtibe A, Linganiso LZ, Mathew AP, Oksman K, John MJ, Anandjiwala RD (2015) A comparative study on properties of micro and nanopapers produced from cellulose and cellulose nanofibres. Carbohyd Polym 118:1–8
Fareez IM, Ibrahim NA, Wan Yaacob WMH, MamatRazali NA, Jasni AH, Abdul Aziz F (2018) Characteristics of cellulose extracted from Josapine pineapple leaf fibre after alkali treatment followed by extensive bleaching. Cellulose 25(8):4407–4421
Gong J, Li J, Xu J, Xiang Z, Mo L (2017) Research on cellulose nanocrystals produced from cellulose sources with various polymorphs. RSC Adv 7(53):33486–33493
Sofla MRK, Brown R, Tsuzuki T, Rainey T (2016) A comparison of cellulose nanocrystals and cellulose nanofibres extracted from bagasse using acid and ball milling methods. Adv Nat Sci Nanosci Nanotech 7(3):035004
Wang H, Li J, Zeng X, Tang X, Sun Y, Lei T, Lin L (2020) Extraction of cellulose nanocrystals using a recyclable deep eutectic solvent. Cellulose 27:1301–1314
Chen W, Yu H, Liu Y, Hai Y, Zhang M, Chen P (2011) Isolation and characterization of cellulose nanofibers from four plant cellulose fibers using a chemical-ultrasonic process. Cellulose 18(2):433–442
Martins MA, Teixeira EM, Corrêa AC, Ferreira M, Mattoso LH (2011) Extraction and characterization of cellulose whiskers from commercial cotton fibers. J Mater Sci 46(24):7858–7864
Shen D, Gu S, Bridgwater A (2010) The thermal performance of the polysaccharides extracted from hardwood: Cellulose and hemicellulose. Carbohyd Polym 82(1):39–45
Xie J, Hse C-Y, Cornelis F, Hu T, Qi J, Shupe TF (2016) Isolation and characterization of cellulose nanofibers from bamboo using microwave liquefaction combined with chemical treatment and ultrasonication. Carbohyd Polym 151:725–734
Zhao J, Zhang W, Zhang X, Zhang X, Lu C, Deng Y (2013) Extraction of cellulose nanofibrils from dry softwood pulp using high shear homogenization. Carbohyd Polym 97(2):695–702
Ferreira F, Mariano M, Rabelo S, Gouveia R, Lona L (2018) Isolation and surface modification of cellulose nanocrystals from sugarcane bagasse waste: from a micro-to a nano-scale view. Appl Surf Sci 436:1113–1122
Chundawat SP, Donohoe BS, da Costa Sousa L, Elder T, Agarwal UP, Lu F, Ralph J, Himmel ME, Balan V, Dale BE (2011) Multi-scale visualization and characterization of lignocellulosic plant cell wall deconstruction during thermochemical pretreatment Energ. Environ Sci 4(3):973–984
Author information
Authors and Affiliations
Contributions
H.Soleimanzadeh, D.Salari, A.Olad, and A.Ostadrahimi contributed to the design and implementation of the research, to the analysis of the results and writing of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Hereby, we assure you that for this manuscript, all the material are the authors’ own original work that reflects research and analysis results in a truthful and complete manner. This manuscript has not been previously published elsewhere and also, is not currently being considered for publication elsewhere.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Soleimanzadeh, H., Salari, D., Olad, A. et al. Optimization of delignification and cellulose isolation process from Natural cotton pods and preparation of its nanofibers with choline chloride–lactic acid eutectic solvents. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04141-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04141-9