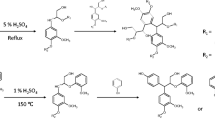
Abstract
In this work, levulinic acid (LLA) was used as a new bio-based solvent for solvolysis liquefaction of wood under mild conditions (temperature 160 ± 5 °C and atmospheric pressure). The liquefaction of sugar maple (Acer saccharum Marsh.) wood at a high rate (up to 93%) was obtained under these conditions. The liquefied product was divided into water-insoluble (WILW) and water-soluble (LArF) fractions. LArFs were mixtures of low-molecular weight compounds including unreacted LLA and furfuryl alcohol, as shown by electrospray ionization-mass spectrometry (ESI–MS) analysis. WILW has shown a high acid number (205–211 mg KOH/g) and a low hydroxyl number (< 23 mg KOH/g), which is in contrast with the conventional hydroxylated liquefied products reported in the literature so far. WILW was cured with LArF, a glycerol-liquefied wood, hexamine, and methylene diphenyl diisocyanate for the preparation of wood coatings. Cured coatings showed good pull-off adhesion strengths to the surface of beech wood, resistance to water, and low ΔE* color change when exposed to ultra-violet (UV) light. Liquefying wood in LLA provides a reactive product containing carboxylic acid groups that could be used to produce cleaner, petroleum-free materials.




Similar content being viewed by others
Data availability
The data are available from the corresponding author upon reasonable request.
References
Mohan D, Pittman CU Jr, Steele PH (2006) Pyrolysis of wood/biomass for bio-oil: a critical review. Energy Fuels 20(3):848–889. https://doi.org/10.1021/ef0502397
Wang G, Dai Y, Yang H, Xiong Q, Wang K, Zhou J, Zhou J, Li Y, Wang S (2020) A review of recent advances in biomass pyrolysis. Energy Fuels 34(12):15557–15578. https://doi.org/10.1021/acs.energyfuels.0c03107
Jindal MK, Jha MK (2016) Hydrothermal liquefaction of wood: a critical review. Rev Chem Eng 32(4):459–488. https://doi.org/10.1515/revce-2015-0055
Honglu X, Tiejun S (2006) Wood liquefaction by ionic liquids. Holzforschung 60:509–512. https://doi.org/10.1515/HF.2006.084
Lin L, Yoshioka M, Yao Y, Shiraishi N (1994) Liquefaction of wood in the presence of phenol using phosphoric acid as a catalyst and the flow properties of the liquefied wood. J Appl Polym Sci 52(11):1629–1636. https://doi.org/10.1002/app.1994.070521111
Budija F, Tavzes Č, Zupančič-Kralj L, Petrič M (2009) Self-crosslinking and film formation ability of liquefied black poplar. Bioresour Technol 100(13):3316–3323. https://doi.org/10.1016/j.biortech.2009.02.004
Kobayashi M, Tukamoto K, Tomita B (2000) Application of liquefied wood to a new resin system—synthesis and properties of liquefied wood/epoxy resins. Holzforshung 54(1):93–97. https://doi.org/10.1515/HF.2000.014
Han S, Cui J, Gan L, Zhou X (2019) Effects of reaction conditions on the phenol liquefaction of peanut shells. BioRes 14(1):1899–1914
Pan H, Shupe TF, Hse C-Y (2008) Synthesis and cure kinetics of liquefied wood/phenol/formaldehyde resins. J Appl Polym Sci 108:1837–1844. https://doi.org/10.1002/app
El Hassan B, Kim M, Wan H (2009) Phenol–formaldehyde-type resins made from phenol-liquefied wood for the bonding of particleboard. J Appl Polym Sci 112(3):1436–1443. https://doi.org/10.1002/app.29521
Yue D, Oribayo O, Rempel GL, Pan Q (2017) Liquefaction of waste pine wood and its application in the synthesis of a flame retardant polyurethane foam. RSC Adv 7:30334–30344. https://doi.org/10.1039/C7RA03546B
Kurimoto Y, Koizumi A, S, Tamura Y, Ono H, (2001) Wood species effects on the characteristics of liquefied wood and the properties of polyurethane films prepared from the liquefied wood. Biomass Bioenergy 21(5):381–390. https://doi.org/10.1016/S0961-9534(01)00041-1
Cheumani-Yona AM, Budija F, Hrastnik D, Kutnar A, Pavlič M, Pori P, Tavzes Č, Petrič M (2015) Preparation of two-component polyurethane coatings from bleached liquefied wood. Biores 10(2):3347–3363
Ugovšek A, Budija F, Kariž M, Šernek M (2011) The influence of solvent content in liquefied wood and of the addition of condensed tannin on bonding quality. DRVNA INDUSTRIJA 62(2):87–95. https://doi.org/10.5552/drind.2011.1039
Signoretto M, Taghavi S, Ghedini E, Menegazz F (2019) Catalytic production of levulinic acid (LA) from actual biomass. Molecules 24(15):2760. https://doi.org/10.3390/molecules24152760
Kang S, Fu J, Zhang G (2018) From lignocellulosic biomass to levulinic acid: a review on acid-catalysed hydrolysis. Renew Sustain Energy Rev 94:340–362. https://doi.org/10.1016/j.rser.2018.06.016
Liu C, Lu X, Yu Z, Xiong J, Bai H, Zhang R (2020) Production of levulinic acid from cellulose and cellulosic biomass in different catalytic systems. Catalysts 10:1006. https://doi.org/10.3390/catal10091006
Girisuta B, Heeres HJ (2017) Levulinic acid from biomass: synthesis and applications. In: Fang Z., Smith, Jr. R., Qi X. (eds) Production of platform chemicals from sustainable resources. biofuels and biorefineries. Springer, Singapore. https://doi.org/10.1007/978-981-10-4172-3_5
Xuan W, Hakkarainen M, Odelius K (2019) Levulinic acid as a versatile building block for plasticizer design. ACS Sustainable Chem Eng 7:12552–12562. https://doi.org/10.1021/acssuschemeng.9b02439
ASTM D 4274–16 (2016) Standard test methods for testing polyurethane raw materials: determination of hydroxyl numbers of polyols
ASTM D974–14 (2014) Standard test method for acid and base number by color-indicator titration
ISO 4624–2016 Paints and varnishes — pull-off test for adhesion
EN ISO 1518–1 paint and varnishes-scratch test
EN 12720:2009 Furniture - Assessment of surface resistance to cold liquids
Kumar A, Sethy A, Chauhan S (2018) Liquefaction behaviour of twelve tropical hardwood species in phenol. Maderas Cienc Tecnol 20(2):211–220. https://doi.org/10.4067/S0718-221X2018005002501
Wang Q, Tuohedi N (2020) Polyurethane foams and bio-polyols from liquefied cotton stalk agricultural waste. Sustainability 12:4214. https://doi.org/10.3390/su12104214
Kunaver M, Jasiukaityte E, Čuk N, Guthrie JT (2010) Liquefaction of wood, synthesis, and characterization of liquefied wood polyester derivatives. J Appl Polym Sci 115:1265–1271. https://doi.org/10.1002/app.31277
Koumba-Yoya G, Stevanovic T (2017) Study of organosolv lignins as adhesives in wood panel production. Polymers 9:46. https://doi.org/10.3390/polym9020046
Zheng X, Gu X, Ren Y, Zhi Z, Lu X (2016) Production of 5-hydroxymethyl furfural and levulinic acid from lignocellulose in aqueous solution and different solvents. Biofuels Bioprod Biorefin 10:917–931. https://doi.org/10.1002/bbb.1720
Perez RF, Fraga MA (2014) Hemicellulose-derived chemicals: one-step production of furfuryl alcohol from xylose. Green Chem 16:3942–3950. https://doi.org/10.1039/c4gc00398e
Shinde SD, Meng X, Kumar R, Ragauskas AJ (2018) Recent advances in understanding the pseudo-lignin formation in lignocellulosic biorefinery. Green Chem 20:2192–2205. https://doi.org/10.1039/C8GC00353J
Zhang H, Zhao M, Zhao T, Li L, Zhu Z (2016) Hydrogenative cyclization of levulinic acid into γ-valerolactone by photocatalytic intermolecular hydrogen transfer. Green Chem 18:2296–2301. https://doi.org/10.1039/C5GC02971F
Barnés MC, Oltvoort J, Kersten SRA, Lange J-P (2017) Wood liquefaction: role of solvent. Ind Eng Chem Res 56:635–644. https://doi.org/10.1021/acs.iecr.6b04086
Woestenborghs PL (2015) Process for the separation of levulinic acid. Patent number WO 2015/063033 Al
Traoré M, Kaal J, Martínez Cortizas A (2018) Differentiation between pine woods according to species and growing location using FTIR-ATR. Wood Sci Technol 52:487–504. https://doi.org/10.1007/s00226-017-0967-9
Zhuang J, Li M, Pu Y, Ragauskas AJ, Yoo CG (2020) Observation of potential contaminants in processed biomass using Fourier transform infrared spectroscopy. Appl Sci 10:4345. https://doi.org/10.3390/app10124345
Lu Y, Lu Y-C, Hu H-Q, Xie F-J, Wei X-Y, Fan X (2017) Structural characterisation of lignin and its degradation products with spectroscopic methods. J Spectrosc (Hindawi) Article ID 8951658 https://doi.org/10.1155/2017/8951658
Zhang H, Yang H, Guo H, Huang C, Xiong L, Chena X (2014) Kinetic study on the liquefaction of wood and its three-cell wall component in polyhydric alcohols. Appl Energy 113:1596–1600. https://doi.org/10.1016/j.apenergy.2013.09.009
Cheng Z, Everhart JL, Tsilomelekis G, Nikolakis V, Saha B, Vlachos DG (2018) Structural analysis of humins formed in the Brønsted acid catalyzed dehydration of fructose. Green Chem 20:997. https://doi.org/10.1039/c7gc03054a
Shi N, Liu Q, Ju R, He X, Zhang Y, Tang S, Ma L (2019) Condensation of α-carbonyl aldehydes leads to the formation of solid humins during the hydrothermal degradation of carbohydrates. ACS Omega 4:7330–7343. https://doi.org/10.1021/acsomega.9b00508
Patil SKR, Heltzel J, Lund CRF (2012) Comparison of structural features of humins formed catalytically from glucose, fructose, and 5-hydroxymethylfurfuraldehyde. Energy Fuels 26(8):5281–5293. https://doi.org/10.1021/ef3007454
Zhao Y, Wang S, Lin H, Chena J, Xua H (2018) Influence of a Lewis acid and a Brønsted acid on the conversion of microcrystalline cellulose into 5-hydroxymethylfurfural in a single-phase reaction system of water and 1,2-dimethoxyethane. RSC Adv 8:7235–7242. https://doi.org/10.1039/c7ra13387a
Kumar A, Petrič M, Kričej B, Žigon J, Tywoniak J, Hajek P, Sever Škapin A, Pavlič M (2015) Liquefied-wood-based polyurethane–nanosilica hybrid coatings and hydrophobization by self-assembled monolayers of orthotrichlorosilane (OTS). ACS Sustain Chem Eng 3(10):2533–2541. https://doi.org/10.1021/acssuschemeng.5b00723
Gürtler C, Danielmeier K (2004) A catalyst system for the reaction of carboxylic acids with aliphatic isocyanates. Tetrahedron Lett 45(12):2515–2521. https://doi.org/10.1016/j.tetlet.2004.02.012
Stanzione M, Russo V, Oliviero L, Verdolotti L, Sorrentino A, Di Serio M, Tesser R, Iannace S, Lavorgna M (2018) Characterisation of sustainable polyhydroxyls, produced from bio-based feedstock, and polyurethane and copolymer urethane-amide foams. Data Brief 21:269–275. https://doi.org/10.1016/j.dib.2018.09.077
Coates J (2000) Interpretation of infrared spectra, a practical approach in encyclopedia of analytical chemistry R.A. Meyers (Ed) pp. 10815–10837, John Wiley & Sons Ltd, Chichester
Wong CS, Badri KH (2012) Chemical analyses of palm kernel oil-based polyurethane prepolymer. Mater Sci Appli 3:78–86. https://doi.org/10.4236/msa.2012.32012
Funding
This research was funded by the Slovenian National Research Agency (ARRS) (“SilWoodCoat” N4-0117 and “Wood and lignocellulosic composites” P4-0015).
Author information
Authors and Affiliations
Contributions
AMCY: Conceptualization and methodology, analysis and data curation, writing—original draft, funding acquisition. DŽ: Analyses and interpretation of the data, writing—original draft. JŽ: Analyses, writing—original draft. AGK: Writing—review and editing original draft. MPa: Writing—review and editing original draft. SD: Writing—review and editing original draft. MPe: Supervision, funding acquisition, writing—original draft.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cheumani Yona, A.M., Žigon, D., Žigon, J. et al. Thermochemical conversion of wood in levulinic acid and application in the preparation of wood coatings. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-03858-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-03858-x